Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108
Abstract
1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Isolation and Manipulation of DNA
2.3. Metabolite Extraction
2.4. Mass Spectrometry (MS) Metabolite Analysis
2.5. Purification
2.6. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.7. Genome Mining and Bioinformatic Analysis
3. Results and Discussion
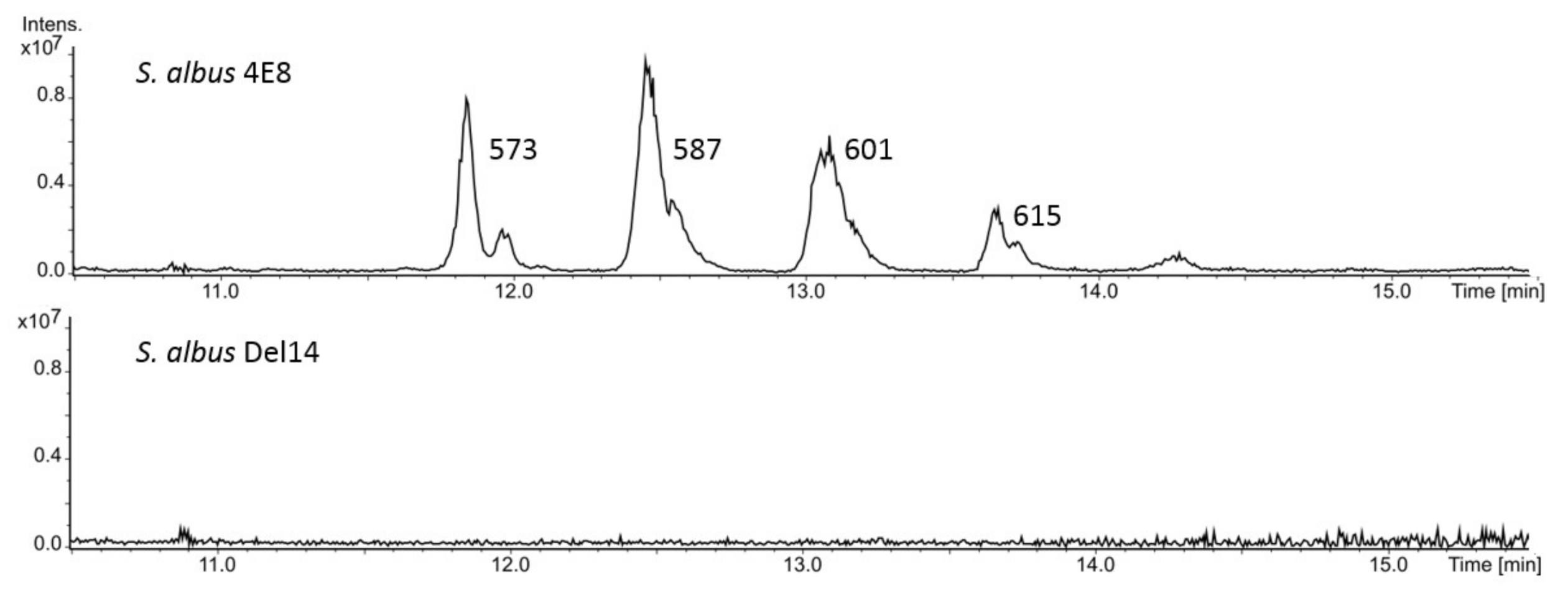
3.1. Identification and Expression of the NRPS Gene Cluster
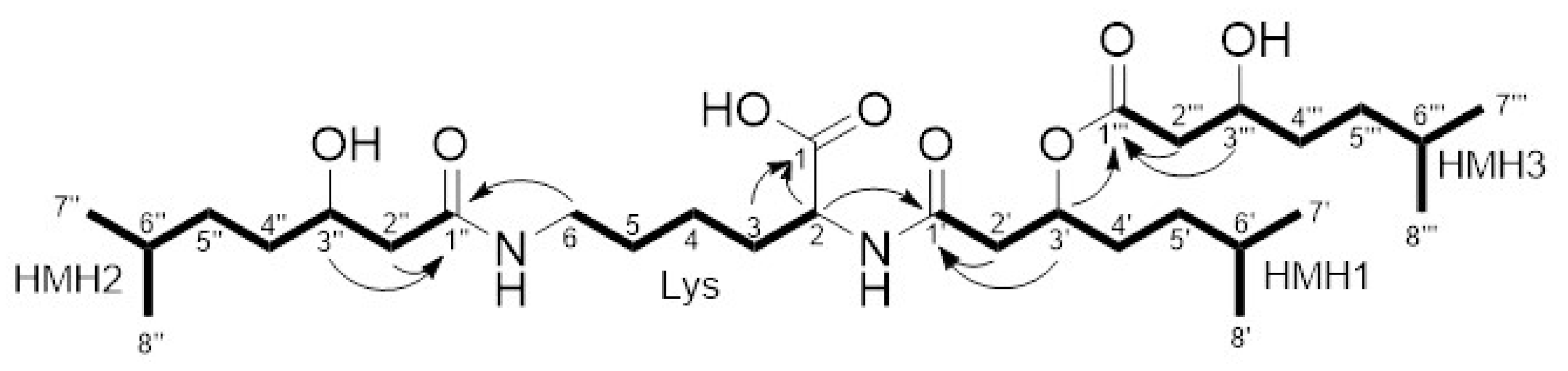
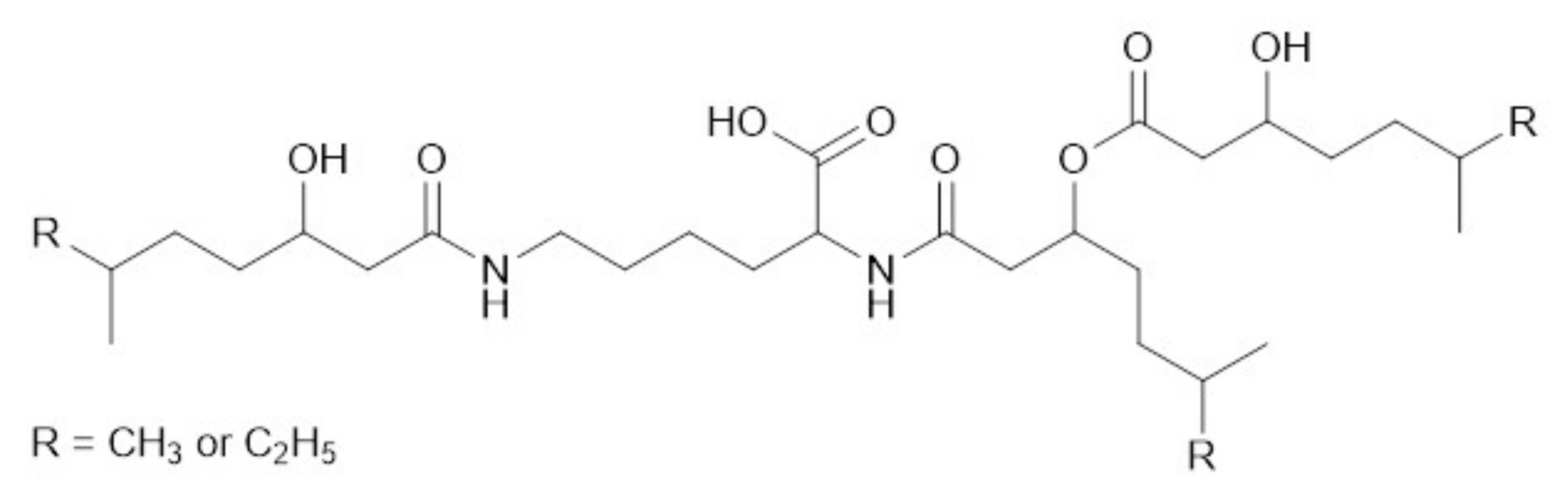
3.2. Purification and Structure Elucidation
3.3. Determination of the Minimal Dudomycin Gene Cluster
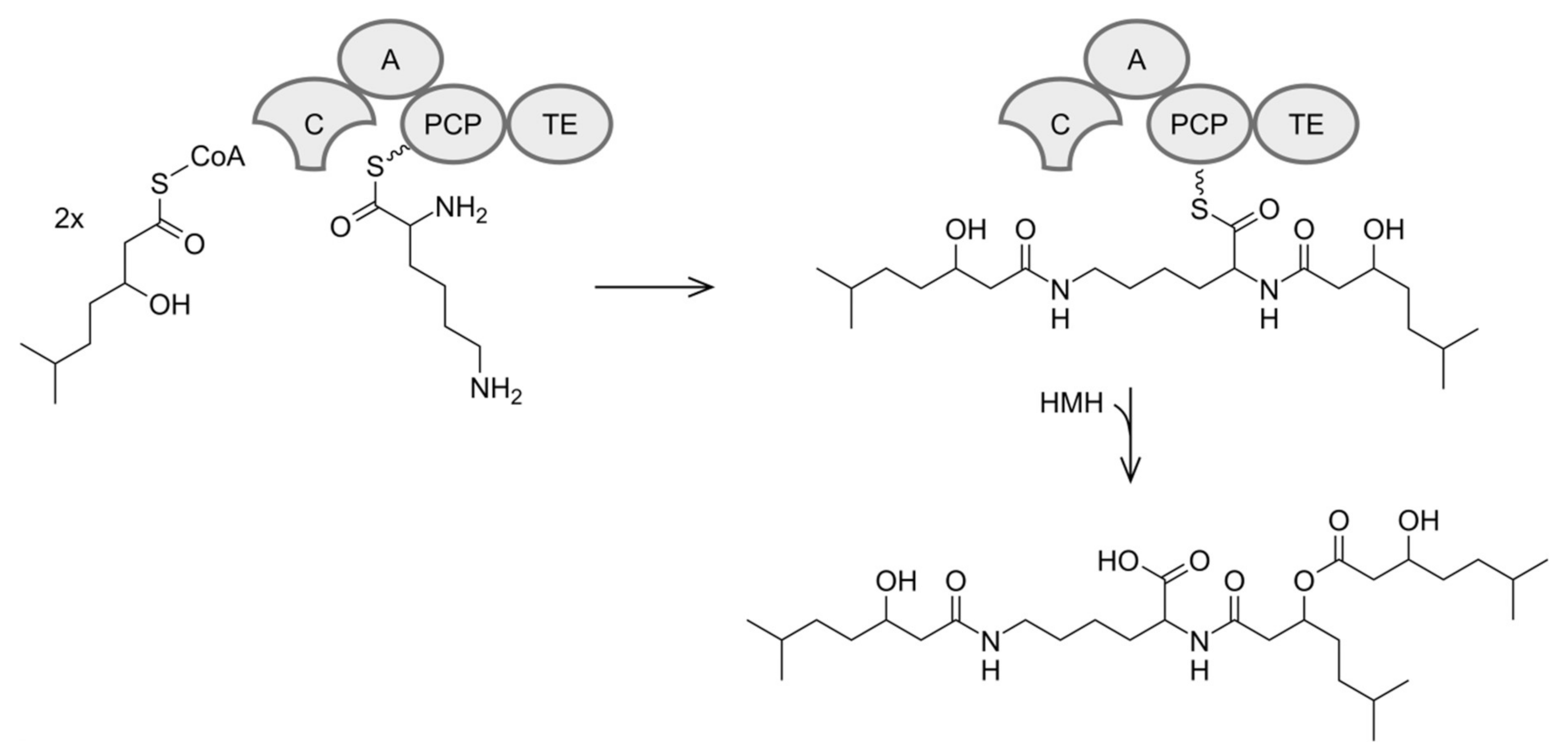
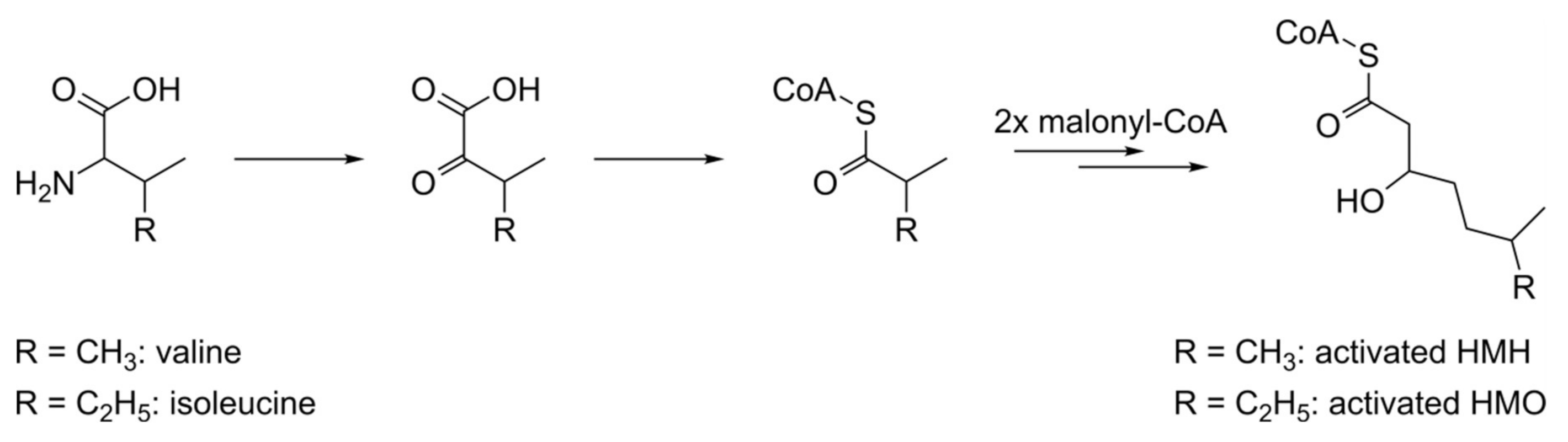
3.4. Biosynthesis of Dudomycins
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beutler, J.A. Natural Products as a Foundation for Drug Discovery. Curr. Protoc. Pharmacol. 2009, 46. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A New Golden Age of Natural Products Drug Discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.S. Vancomycin use—An historical review. J. Antimicrob. Chemother. 1984, 14, 1–5. [Google Scholar] [CrossRef]
- Hotson, I.K. The avermectins: A new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 1982, 53, 87–90. [Google Scholar]
- Ottesen, E.A.; Campbell, W. Ivermectin in human medicine. J. Antimicrob. Chemother. 1994, 34, 195–203. [Google Scholar] [CrossRef]
- D’Angio, G.J.; Evans, A.; Breslow, N.; Beckwith, B.; Bishop, H.; Farewell, V.; Goodwin, W.; Leape, L.; Palmer, N.; Sinks, L.; et al. The treatment of Wilms’ Tumor: Results of the second national Wilms’ Tumor study. Cancer 1981, 47, 2302–2311. [Google Scholar] [CrossRef]
- Turan, T.; Karacay, O.; Tulunay, G.; Boran, N.; Koc, S.; Bozok, S.; Kose, M.F. Results with EMA/CO (etoposide, methotrexate, actinomycin D, cyclophosphamide, vincristine) chemotherapy in gestational trophoblastic neoplasia. Int. J. Gynecol. Cancer. 2006, 16, 1432–1438. [Google Scholar] [CrossRef]
- Myronovskyi, M.; Luzhetskyy, A. Heterologous production of small molecules in the optimized Streptomyces hosts. Nat. Prod. Rep. 2019, 36, 1281–1294. [Google Scholar] [CrossRef]
- Baltz, R. Renaissance in antibacterial discovery from actinomycetes. Curr. Opin. Pharmacol. 2008, 8, 557–563. [Google Scholar] [CrossRef]
- Estévez, M.R.; Myronovskyi, M.; Gummerlich, N.; Nadmid, S.; Luzhetskyy, A. Heterologous Expression of the Nybomycin Gene Cluster from the Marine Strain Streptomyces albus subsp. chlorinus NRRL B-24108. Mar. Drugs 2018, 16, 435. [Google Scholar] [CrossRef] [PubMed]
- Myronovskyi, M.; Rosenkränzer, B.; Stierhof, M.; Petzke, L.; Seiser, T.; Luzhetskyy, A. Identification and Heterologous Expression of the Albucidin Gene Cluster from the Marine Strain Streptomyces albus Subsp. chlorinus NRRL B-24108. Microorganisms 2020, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M.R.; Gummerlich, N.; Myronovskyi, M.; Zapp, J.; Luzhetskyy, A. Benzanthric Acid, a Novel Metabolite From Streptomyces albus Del14 Expressing the Nybomycin Gene Cluster. Front. Chem. 2020, 7. [Google Scholar] [CrossRef]
- Estévez, M.R.; Myronovskyi, M.; Rosenkränzer, B.; Paululat, T.; Petzke, L.; Ristau, J.; Luzhetskyy, A. Novel fredericamycin variant overproduced by a streptomycin-resistant Streptomyces albus subsp. chlorinus Strain. Mar. Drugs 2020, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Myronovskyi, M.; Rosenkränzer, B.; Nadmid, S.; Pujic, P.; Normand, P.; Luzhetskyy, A. Generation of a cluster-free Streptomyces albus chassis strains for improved heterologous expression of secondary metabolite clusters. Metab. Eng. 2018, 49, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Y.; Rebets, Y.; Estévez, M.R.; Zapp, J.; Myronovskyi, M.; Luzhetskyy, A. Engineering of Streptomyces lividans for heterologous expression of secondary metabolite gene clusters. Microb. Cell Fact. 2020, 19, 5. [Google Scholar] [CrossRef]
- Green, J.M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2012. [Google Scholar]
- Kieser, T.; Bibb, M.J.; Buttner, M.J.; Chater, K.F.; Hopwood, D.A. Practical Streptomyces Genetics. A Laboratory Manual; John Innes Foundation: Norwich, UK, 2000. [Google Scholar]
- Rebets, Y.; Kormanec, J.; Lutzhetskyy, A.; Bernaerts, K.; Anné, J. Cloning and expression of metagenomic dna in Streptomyces lividans and subsequent fermentation for optimized production. Methods Mol. Biol. 2017, 1539, 99–144. [Google Scholar] [CrossRef]
- Court, D.L.; Sawitzke, J.A.; Thomason, L.C. Genetic Engineering Using Homologous Recombination. Annu. Rev. Genet. 2002, 36, 361–388. [Google Scholar] [CrossRef]
- Medema, M.H.; Blin, K.; Cimermancic, P.; de Jager, V.; Zakrzewski, P.; Fischbach, M.A.; Weber, T.; Takano, E.; Breitling, R. antiSMASH: Rapid identification, annotation and analysis of secondary metabolite biosynthesis gene clusters in bacterial and fungal genome sequences. Nucleic Acids Res. 2011, 39, W339–W346. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Gaitatzis, N.; Kunze, B.; Muller, R. In vitro reconstitution of the myxochelin biosynthetic machinery of Stigmatella aurantiaca Sg a15: Biochemical characterization of a reductive release mechanism from nonribosomal peptide synthetases. Proc. Natl. Acad. Sci. USA 2001, 98, 11136–11141. [Google Scholar] [CrossRef] [PubMed]
- Keating, T.A.; Marshall, C.G.; Walsh, C.T. Reconstitution and Characterization of the Vibrio cholerae Vibriobactin Synthetase from VibB, VibE, VibF, and VibH. Biochemistry 2000, 39, 15522–15530. [Google Scholar] [CrossRef] [PubMed]
- Gago, G.; Diacovich, L.; Arabolaza, A.; Tsai, S.-C.; Gramajo, H. Fatty acid biosynthesis in actinomycetes. FEMS Microbiol. Rev. 2011, 35, 475–497. [Google Scholar] [CrossRef] [PubMed]
- Kaneda, T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991, 55, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hutchinson, C.R. Regulation of expression of the valine (branched-chain amino acid) dehydrogenase-encoding gene from Streptomyces coelicolor. Gene 1995, 162, 69–74. [Google Scholar] [CrossRef]
- Manderscheid, N. Strain Development for Heterologous Expression of Secondary Metabolite Clusters in Actinobacteria. Ph.D. Thesis, Universität des Saarlandes, Saarbrücken, Germany, 2015. [Google Scholar]
- Vancurová, I.; Vancura, A.; Volc, J.; Neuzil, J.; Flieger, M.; Basarová, G.; Běhal, V. Isolation and characterization of valine dehydrogenase from Streptomyces aureofaciens. J. Bacteriol. 1988, 170, 5192–5196. [Google Scholar] [CrossRef]
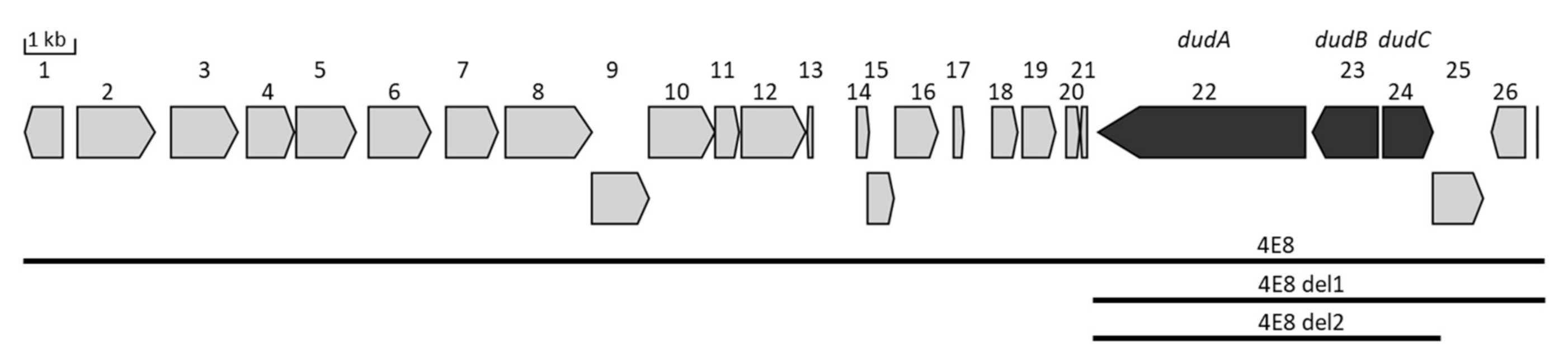
| Gene # | Locus Tag | Putative Function |
|---|---|---|
| 1 | SACHL_42600 | glycosyltransferase |
| 2 | SACHL_42610 | hypothetical protein |
| 3 | SACHL_42620 | phosphatase |
| 4 | SACHL_42630 | N,N’-diacetyllegionaminic acid synthase |
| 5 | SACHL_42640 | hypothetical protein |
| 6 | SACHL_42650 | hydrolase |
| 7 | SACHL_42660 | membrane lipoprotein precursor |
| 8 | SACHL_42670 | galactose/methyl galactoside import ATP-binding protein |
| 9 | SACHL_42680 | ribose transport system permease protein |
| 10 | SACHL_42690 | branched-chain amino acid transport system/permease component |
| 11 | SACHL_42700 | cytidine deaminase |
| 12 | SACHL_42710 | pyrimidine-nucleoside phosphorylase |
| 13 | SACHL_42720 | hypothetical protein |
| 14 | SACHL_42730 | hypothetical protein |
| 15 | SACHL_42740 | hypothetical protein |
| 16 | SACHL_42750 | hypothetical protein |
| 17 | SACHL_42760 | hypothetical protein |
| 18 | SACHL_42770 | hypothetical protein |
| 19 | SACHL_42780 | ubiquinone biosynthesis O-methyltransferase |
| 20 | SACHL_42790 | zinc metallo-peptidase |
| 21 | SACHL_42800 | hypothetical protein |
| 22 [dudA] | SACHL_42810 | dimodular nonribosomal peptide synthase |
| 23 [dudB] | SACHL_42820 | inner membrane transport protein |
| 24 [dudC] | SACHL_42830 | transcriptional regulator |
| 25 | SACHL_42840 | demethylrebeccamycin-d-glucose O-methyltransferase |
| 26 | SACHL_42850 | hypothetical protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lasch, C.; Stierhof, M.; Estévez, M.R.; Myronovskyi, M.; Zapp, J.; Luzhetskyy, A. Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108. Microorganisms 2020, 8, 1800. https://doi.org/10.3390/microorganisms8111800
Lasch C, Stierhof M, Estévez MR, Myronovskyi M, Zapp J, Luzhetskyy A. Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108. Microorganisms. 2020; 8(11):1800. https://doi.org/10.3390/microorganisms8111800
Chicago/Turabian StyleLasch, Constanze, Marc Stierhof, Marta Rodríguez Estévez, Maksym Myronovskyi, Josef Zapp, and Andriy Luzhetskyy. 2020. "Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108" Microorganisms 8, no. 11: 1800. https://doi.org/10.3390/microorganisms8111800
APA StyleLasch, C., Stierhof, M., Estévez, M. R., Myronovskyi, M., Zapp, J., & Luzhetskyy, A. (2020). Dudomycins: New Secondary Metabolites Produced after Heterologous Expression of an Nrps Cluster from Streptomyces albus ssp. Chlorinus Nrrl B-24108. Microorganisms, 8(11), 1800. https://doi.org/10.3390/microorganisms8111800





