Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Isolates
2.2. Culture Media
2.3. Batch Culture Conditions
2.4. Analytical Methods
2.4.1. Biomass and Lipids Quantification
2.4.2. Volatile Fatty Acids Quantification
2.4.3. Total Organic Carbon and Nitrogen Quantification
2.4.4. Lipid Profile Analysis (Fatty Acid Methyl Esters, FAME)
2.4.5. Statistical Analysis
3. Results and Discussion
3.1. Time Course Growth and SCOs Production in Acetic Acid
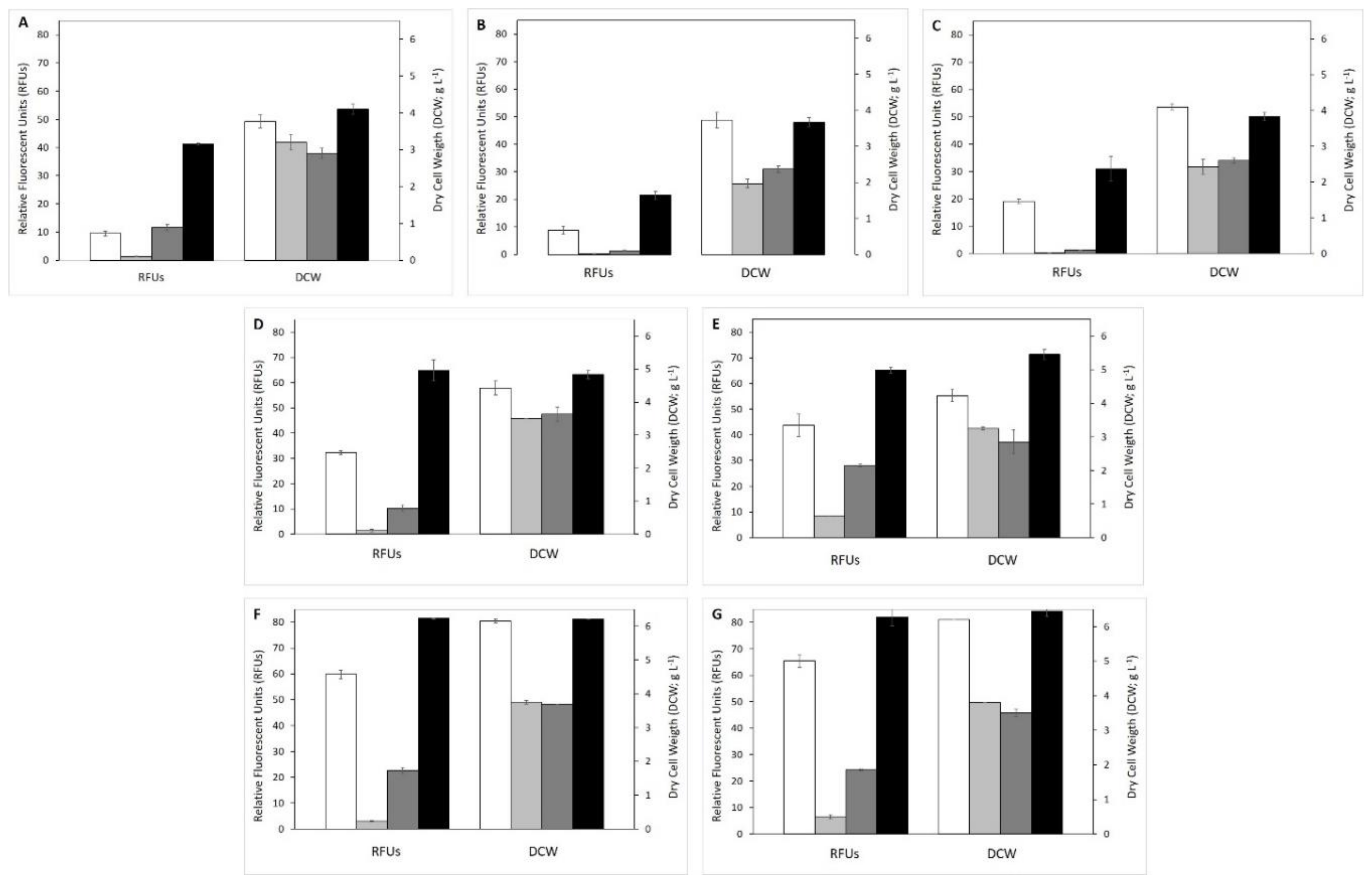
3.2. Effect of Different VFAs on Biomass Growth and SCOs Production
3.2.1. Individual VFAs Effect in Biomass and Lipid Production
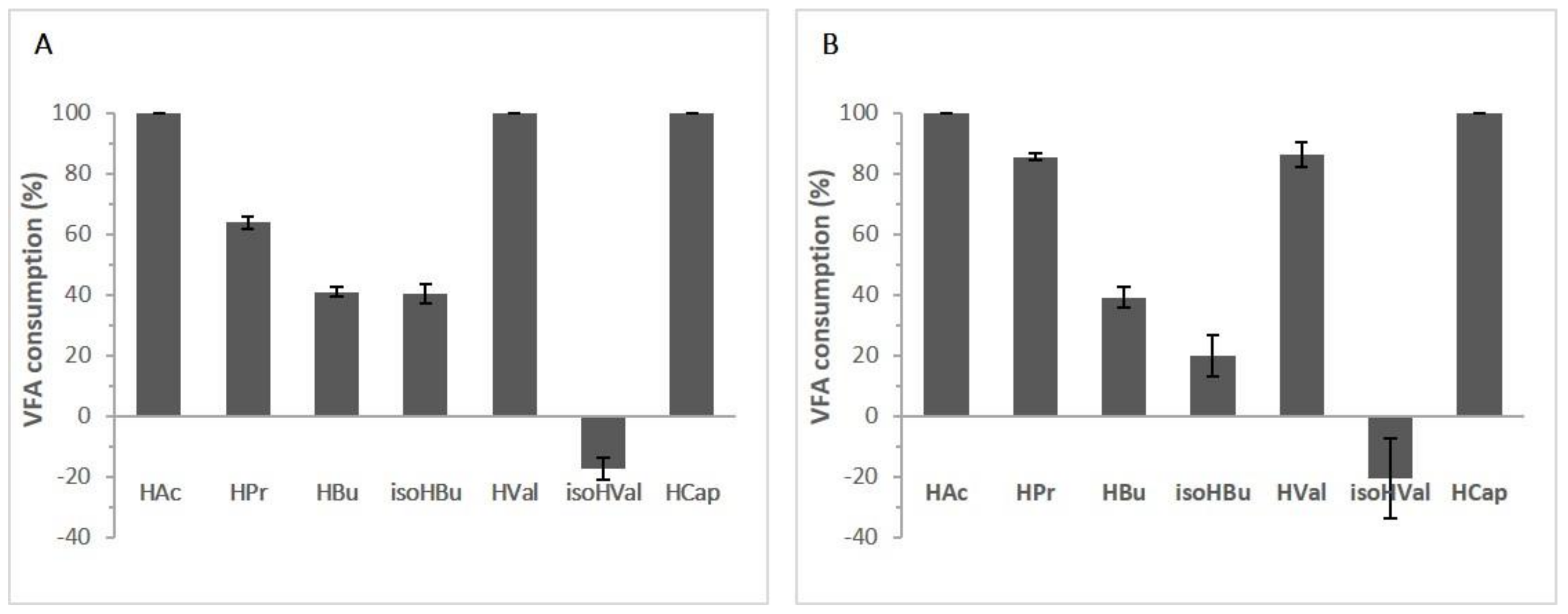
3.2.2. Effect of VFAs Mixtures in Biomass and Lipid Production
3.3. Effluent Filtrate as Fermentation Medium
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Jong, E.; Jungmeier, G. Biorefinery Concepts in Comparison to Petrochemical Refineries; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9780444634535. [Google Scholar]
- Adrio, J.L. Oleaginous yeasts: Promising platforms for the production of oleochemicals and biofuels. Biotechnol. Bioeng. 2017, 114, 1915–1920. [Google Scholar] [CrossRef]
- Metzger, J.O.; Bornscheuer, U. Lipids as renewable resources: Current state of chemical and biotechnological conversion and diversification. Appl. Microbiol. Biotechnol. 2006, 71, 13–22. [Google Scholar] [CrossRef]
- Xue, S.J.; Chi, Z.; Zhang, Y.; Li, Y.F.; Liu, G.L.; Jiang, H.; Hu, Z.; Chi, Z.M. Fatty acids from oleaginous yeasts and yeast-like fungi and their potential applications. Crit. Rev. Biotechnol. 2018, 38, 1049–1060. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Garay, L.A.; Sestric, R.; Levin, D.; Block, D.E.; German, J.B.; Boundy-Mills, K.L. Oleaginous yeasts for biodiesel: Current and future trends in biology and production. Biotechnol. Adv. 2014, 32, 1336–1360. [Google Scholar] [CrossRef]
- Thorpe, R.F.; Ratledge, C. Acid Distribution in Triglycerides. J. Gen. Microbiol. 1972, 72, 151–163. [Google Scholar] [CrossRef]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Miranda, C.; Bettencourt, S.; Pozdniakova, T.; Pereira, J.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Modified high-throughput Nile red fluorescence assay for the rapid screening of oleaginous yeasts using acetic acid as carbon source. BMC Microbiol. 2020, 20, 60. [Google Scholar] [CrossRef]
- Garay, L.A.; Sitepu, I.R.; Cajka, T.; Chandra, I.; Shi, S.; Lin, T.; German, J.B.; Fiehn, O.; Boundy-Mills, K.L. Eighteen new oleaginous yeast species. J. Ind. Microbiol. Biotechnol. 2016, 43, 887–900. [Google Scholar] [CrossRef]
- Ayadi, I.; Kamoun, O.; Trigui-Lahiani, H.; Hdiji, A.; Gargouri, A.; Belghith, H.; Guerfali, M. Single cell oil production from a newly isolated Candida viswanathii Y-E4 and agro-industrial by-products valorization. J. Ind. Microbiol. Biotechnol. 2016, 43, 901–914. [Google Scholar] [CrossRef]
- Arous, F.; Frikha, F.; Triantaphyllidou, I.E.; Aggelis, G.; Nasri, M.; Mechichi, T. Potential utilization of agro-industrial wastewaters for lipid production by the oleaginous yeast Debaryomyces etchellsii. J. Clean. Prod. 2016, 133, 899–909. [Google Scholar] [CrossRef]
- Dourou, M.; Kancelista, A.; Juszczyk, P.; Sarris, D.; Bellou, S.; Triantaphyllidou, I.E.; Rywinska, A.; Papanikolaou, S.; Aggelis, G. Bioconversion of olive mill wastewater into high-added value products. J. Clean. Prod. 2016, 139, 957–969. [Google Scholar] [CrossRef]
- Park, Y.K.; Dulermo, T.; Ledesma-Amaro, R.; Nicaud, J.M. Optimization of odd chain fatty acid production by Yarrowia lipolytica. Biotechnol. Biofuels 2018, 11, 1–12. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol. 2013, 143, 525–530. [Google Scholar] [CrossRef]
- Hoornweg, D.; Bhada-Tata, P. What a Waste: A Global Review of Solid Waste Management—Review, Global Management, Solid Waste; The World Bank: Washington, DC, USA, 2012; pp. 1–116. [Google Scholar]
- Gao, R.; Li, Z.; Zhou, X.; Cheng, S.; Zheng, L. Oleaginous yeast Yarrowia lipolytica culture with synthetic and food waste-derived volatile fatty acids for lipid production. Biotechnol. Biofuels 2017, 10, 247. [Google Scholar] [CrossRef]
- Fontanille, P.; Kumar, V.; Christophe, G.; Nouaille, R.; Larroche, C. Bioconversion of volatile fatty acids into lipids by the oleaginous yeast Yarrowia lipolytica. Bioresour. Technol. 2012, 114, 443–449. [Google Scholar] [CrossRef]
- Kolouchová, I.; Schreiberová, O.; Sigler, K.; Masák, J.; Řezanka, T. Biotransformation of volatile fatty acids by oleaginous and non-oleaginous yeast species. FEMS Yeast Res. 2015, 15, fov076. [Google Scholar] [CrossRef]
- Llamas, M.; Dourou, M.; González-Fernández, C.; Aggelis, G.; Tomás-Pejó, E. Screening of oleaginous yeasts for lipid production using volatile fatty acids as substrate. Biomass Bioenergy 2020, 138, 105553. [Google Scholar] [CrossRef]
- Gientka, I.; Gadaszewska, M.; Błażejak, S.; Kieliszek, M.; Bzducha-Wróbel, A.; Stasiak-Różańska, L.; Kot, A.M. Evaluation of lipid biosynthesis ability by Rhodotorula and Sporobolomyces strains in medium with glycerol. Eur. Food Res. Technol. 2017, 243, 275–286. [Google Scholar] [CrossRef]
- Ratledge, C. Fatty acid biosynthesis in microorganisms being used for Single Cell Oil production. Biochimie 2004, 86, 807–815. [Google Scholar] [CrossRef]
- Niehus, X.; Casas-Godoy, L.; Vargas-Sánchez, M.; Sandoval, G. A Fast and Simple Qualitative Method for Screening Oleaginous Yeasts on Agar. J. Lipids 2018, 2018, 5325804. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Sources of microbial oils with emphasis to Mortierella (Umbelopsis) isabellina fungus. World J. Microbiol. Biotechnol. 2019, 35, 63. [Google Scholar] [CrossRef]
- Bellou, S.; Triantaphyllidou, I.E.; Aggeli, D.; Elazzazy, A.M.; Baeshen, M.N.; Aggelis, G. Microbial oils as food additives: Recent approaches for improving microbial oil production and its polyunsaturated fatty acid content. Curr. Opin. Biotechnol. 2016, 37, 24–35. [Google Scholar] [CrossRef]
- Ratledge, C. Microbial oils: An introductory overview of current status and future prospects. OCL—Oilseeds fats. Crop. Lipids 2013, 20. [Google Scholar] [CrossRef]
- Fillet, S.; Ronchel, C.; Callejo, C.; Fajardo, M.J.; Moralejo, H.; Adrio, J.L. Engineering Rhodosporidium toruloides for the production of very long-chain monounsaturated fatty acid-rich oils. Appl. Microbiol. Biotechnol. 2017, 101, 7271–7280. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, X.; Xia, X.; Dong, M. A systematic optimization of medium chain fatty acid biosynthesis via the reverse beta-oxidation cycle in Escherichia coli. Metab. Eng. 2017, 41, 115–124. [Google Scholar] [CrossRef]
- Franklin, S.; Decker, S.M.; Wee, J. United States Patent: Fuel and Chemical Production from Oleaginous Yeast 2011. U.S. Patent Application No. 13/087311, 14 April 2011. [Google Scholar]
- Hassan, M.; Blanc, P.J.; Granger, L.M.; Pareilleux, A.; Goma, G. Lipid production by an unsaturated fatty acid auxotroph of the oleaginous yeast Apiotrichum curvatum grown in single-stage continuous culture. Appl. Microbiol. Biotechnol. 1993, 40, 483–488. [Google Scholar] [CrossRef]
- Ykema, A.; Kater, M.M.; Smit, H. Lipid production in wheypermeate by an unsaturated fatty acid mutant of the oleaginous yeast Apiotrichum curvatum. Biotechnol. Lett. 1989, 11, 477–482. [Google Scholar] [CrossRef]
- Heredia, L.; Ratledge, C. Simultaneous utilization of glucose and xylose by Candida curvata D in continuous culture. Biotechnol. Lett. 1988, 10, 25–30. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Nuñez, A.; Foglia, T.A. Production of sophorolipids by Candida bombicola grown on soy molasses as substrate. Biotechnol. Lett. 2004, 26, 1241–1245. [Google Scholar] [CrossRef]
- Dey, P.; Maiti, M.K. Molecular characterization of a novel isolate of Candida tropicalis for enhanced lipid production. J. Appl. Microbiol. 2013, 114, 1357–1368. [Google Scholar] [CrossRef]
- Louhasakul, Y.; Cheirsilp, B.; Maneerat, S.; Prasertsan, P. Potential use of flocculating oleaginous yeasts for bioconversion of industrial wastes into biodiesel feedstocks. Renew. Energy 2019, 136, 1311–1319. [Google Scholar] [CrossRef]
- Polburee, P.; Yongmanitchai, W.; Lertwattanasakul, N.; Ohashi, T.; Fujiyama, K.; Limtong, S. Characterization of oleaginous yeasts accumulating high levels of lipid when cultivated in glycerol and their potential for lipid production from biodiesel-derived crude glycerol. Fungal Biol. 2015, 119, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y.; Valéro, J.R. Wastewater sludge as raw material for microbial oils production. Appl. Energy 2014, 135, 192–201. [Google Scholar] [CrossRef]
- Rangaswamy, V.; Saran, S.; Kannabiran, M.; Thiru, M.; Sankh, S. Bioprocess for Biodiesel Production from a Yeast Strain 2017. U.S. Patent 9,725,745 B2, 8 August 2017. [Google Scholar]
- Sankh, S.; Thiru, M.; Saran, S.; Rangaswamy, V. Biodiesel production from a newly isolated Pichia kudriavzevii strain. Fuel 2013, 106, 690–696. [Google Scholar] [CrossRef]
- Woodbine, M. Microbial fat: Microorganisms as potential fat producers. Prog. Ind. Microbiol. 1959, 1, 181–245. [Google Scholar]
- Sitepu, I.R.; Ignatia, L.; Franz, A.K.; Wong, D.M.; Faulina, S.A.; Tsui, M.; Kanti, A.; Boundy-Mills, K. An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J. Microbiol. Methods 2012, 91, 321–328. [Google Scholar] [CrossRef]
- Dourou, M.; Mizerakis, P.; Papanikolaou, S.; Aggelis, G. Storage lipid and polysaccharide metabolism in Yarrowia lipolytica and Umbelopsis isabellina. Appl. Microbiol. Biotechnol. 2017, 101, 7213–7226. [Google Scholar] [CrossRef]
- Beopoulos, A.; Nicaud, J.M. Yeast: A new oil producer? OCL—Ol. Corps Gras Lipides 2012, 19, 22–28. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Chevalot, I.; Komaitis, M.; Aggelis, G.; Marc, I. Kinetic profile of the cellular lipid composition in an oleaginous Yarrowia lipolytica capable of producing a cocoa-butter substitute from industrial fats. Antonie Van Leeuwenhoek 2001, 80, 215–224. [Google Scholar] [CrossRef]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Erten, H. Lipids by yarrowia lipolytica strains cultivated on glucose in batch cultures. Microorganisms 2020, 8, 1054. [Google Scholar] [CrossRef]
- Xenopoulos, E.; Giannikakis, I.; Chatzifragkou, A.; Koutinas, A.; Papanikolaou, S. Lipid production by yeasts growing on commercial xylose in submerged cultures with process water being partially replaced by olive millwastewaters. Processes 2020, 8, 819. [Google Scholar] [CrossRef]
- Serrano-Carreon, L.; Hathout, Y.; Bensoussan, M.; Belin, J. Lipid accumulation in Trichoderma species. FEMS Microbiol. Lett. 1992, 93, 181–187. [Google Scholar] [CrossRef]
- Kim, N.-J.; Lim, S.-J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application. In Emerging Areas in Bioengineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 173–190. [Google Scholar]
- Fei, Q.; Chang, H.N.; Shang, L.; dal rae Choi, J.; Kim, N.J.; Kang, J.W. The effect of volatile fatty acids as a sole carbon source on lipid accumulation by Cryptococcus albidus for biodiesel production. Bioresour. Technol. 2011, 102, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Fradinho, J.C.; Oehmen, A.; Reis, M.A.M. Photosynthetic mixed culture polyhydroxyalkanoate (PHA) production from individual and mixed volatile fatty acids (VFAs): Substrate preferences and co-substrate uptake. J. Biotechnol. 2014, 185, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Arous, F.; Azabou, S.; Triantaphyllidou, I.E.; Aggelis, G.; Jaouani, A.; Nasri, M.; Mechichi, T. Newly isolated yeasts from Tunisian microhabitats: Lipid accumulation and fatty acid composition. Eng. Life Sci. 2017, 17, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Bhattacharya, A.; Khare, S.K. Trends in Oil Production from Oleaginous Yeast Using Biomass: Biotechnological Potential and Constraints. Appl. Biochem. Microbiol. 2018, 54, 361–369. [Google Scholar] [CrossRef]
- Katre, G.; Joshi, C.; Khot, M.; Zinjarde, S.; Ravikumar, A. Evaluation of single cell oil (SCO) from a tropical marine yeast yarrowia lipolytica NCIM 3589 as a potential feedstock for biodiesel. AMB Express 2012, 2, 36. [Google Scholar] [CrossRef]
- Chalima, A.; Hatzidaki, A.; Karnaouri, A.; Topakas, E. Integration of a dark fermentation effluent in a microalgal-based biorefinery for the production of high-added value omega-3 fatty acids. Appl. Energy 2019, 241, 130–138. [Google Scholar] [CrossRef]
- Zhang, S.; Skerker, J.M.; Rutter, C.D.; Maurer, M.J.; Arkin, A.P.; Rao, C.V. Engineering Rhodosporidium toruloides for Increased Lipid Production. Biotechnol. Bioeng. 2015, 113, 1056–1066. [Google Scholar] [CrossRef]
- Qiao, K.; Wasylenko, T.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 2017, 35, 173–177. [Google Scholar] [CrossRef]
- Islam, M.A.; Yousuf, A.; Karim, A.; Pirozzi, D.; Khan, M.R.; Wahid, Z.A. Bioremediation of palm oil mill effluent and lipid production by Lipomyces starkeyi: A combined approach. J. Clean. Prod. 2018, 172, 1779–1787. [Google Scholar] [CrossRef]
- Viñarta, S.C.; Angelicola, M.V.; Barros, J.M.; Fernández, P.M.; Mac Cormak, W.; Aybar, M.J.; de Figueroa, L.I.C. Oleaginous yeasts from Antarctica: Screening and preliminary approach on lipid accumulation. J. Basic Microbiol. 2016, 56, 1360–1368. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mohan, S.V. Microbial lipid production by Cryptococcus curvatus from vegetable waste hydrolysate. Bioresour. Technol. 2018, 254, 284–289. [Google Scholar] [CrossRef] [PubMed]

| YP:HAc | YP:HPr | YP:HBut | YP:HAc:HPr | YP:HAc:HBut | YP:HAc:HPr:HBut | YP:HAc:HPr:HBut:isoHBut:HVal | Effluent Filtrate | |
|---|---|---|---|---|---|---|---|---|
| Yeast extract | 5 | 5 | 5 | 5 | 5 | 5 | 5 | - |
| Peptone | 10 | 10 | 10 | 10 | 10 | 10 | 10 | - |
| HAc | 15/5.1 | - | - | 5.1 | 5.1 | 5.1 | 5.1 | 5.1 |
| HPr | - | 2.7 | - | 2.7 | - | 2.7 | 2.7 | 2.7 |
| HBut | - | - | 3.5 | - | 3.5 | 3.5 | 3.5 | 3.5 |
| isoHBut | - | - | - | - | - | - | 0.2 | 0.2 |
| HVal | - | - | - | - | - | - | 0.9 | 0.9 |
| isoHVal | - | - | - | - | - | - | - | 0.3 |
| HCap | - | - | - | - | - | - | - | 1.0 |
| Total dissolved organic carbon | 13.6/9.7 | 9.0 | 9.5 | 11.0 | 11.6 | 12.9 | 13.5 | 6.9 |
| Total dissolved nitrogen | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 3.2 | 0.9 |
| Carbon/Nitrogen | 4.3/3.0 | 2.8 | 3.0 | 3.4 | 3.6 | 4.0 | 4.2 | 7.6 |
| pH | 6.9 | 6.9 | 6.9 | 6.9 | 6.9 | 6.9 | 6.9 | 7.0 |
| Medium | Yeasts | Dry Cell Weight (g L−1) | Lipid Content (%, w/w) | Lipid Output (g L−1) | YX/S (g g−1 Cconsumed) | YL/S (g g−1 Cconsumed) | YL/S (g g−1 VFAconsumed) | Carbon Source Reduction (%) |
|---|---|---|---|---|---|---|---|---|
| YP:HAc 15 g L−1 | A. brassicae V134 | 2.5 ± 0.2 | 55 ± 2 | 1.4 ± 0.2 | 0.41 ± 0.04 | 0.23 ± 0.02 | 0.09 ± 0.01 | 100 |
| C. tropicalis V139 | 3.56 ± 0.03 | 27 ± 1 | 0.92 ± 0.05 | 0.60 ± 0.01 | 0.15 ± 0.01 | 0.062 ± 0.004 | 100 | |
| M. pulcherrima V213 | 3.5 ± 0.3 | 29 ± 1 | 1.03 ± 0.03 | 0.59 ± 0.01 | 0.17 ± 0.01 | 0.070 ± 0.002 | 100 | |
| P. kudriavzevii V194 | 3.86 ± 0.04 | 64 ± 4 | 2.5 ± 0.1 | 0.65 ± 0.01 | 0.41 ± 0.02 | 0.17 ± 0.01 | 100 | |
| Effluent filtrate | A. brassicae V134 | 8.3 ± 0.1 | 43.2 ± 3 | 3.40 ± 0.1 | 1.24 ± 0.02 | 0.51 ± 0.02 | 0.25 ± 0.01 | 100 |
| C. tropicalis V139 | 6.6 ± 0.2 | 19.6 ± 2 | 1.38 ± 0.2 | 1.36 ± 0.03 | 0.29 ± 0.04 | 0.14 ± 0.02 | 72 | |
| M. pulcherrima V213 | 6.5 ± 0.4 | 23.6 ± 2 | 1.65 ± 0.1 | 1.31 ± 0.08 | 0.33 ± 0.02 | 0.16 ± 0.01 | 74 | |
| P. kudriavzevii V194 | 7.8 ± 0.4 | 46.7 ± 2 | 3.33 ± 0.07 | 1.17 ± 0.06 | 0.50 ± 0.01 | 0.25 ± 0.01 | 99 |
| C14:0 | C15:0 | C16:0 | C16:1 | C17:1 | C18:0 | C18:1n9 | C18:2n6 | C18:3n6 | C20:1n9 | Sum | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Myristic Acid | Pentadecanoic Acid | Palmitic Acid | Palmitoleic Acid | Heptadecenoic Acid | Stearic Acid | Oleic Acid | Linoleic Acid | γ-Linolenic Acid | Eicosenoic Acid | |||
| YP:HAc 15 g L−1 | A. brassicae V134 | 1.05 ± 0.01 | 26.8 ± 0.3 | 19.5 ± 0.1 | 47.5 ± 0.4 | 2.4 ± 0.1 | 97 | |||||
| C. tropicalis V139 | 13.9 ± 0.4 | 9.7 ± 0.4 | 8.7 ± 0.5 | 61.8 ± 0.3 | 4.2 ± 0.4 | 98 | ||||||
| M. pulcherrima V213 | 15.4 ± 0.4 | 9.2 ± 0.2 | 10.0 ± 0.6 | 60.0 ± 0.8 | 3.4 ± 0.1 | 98 | ||||||
| P. kudriavzevii V194 | 25.6 ± 0.4 | 18.5 ± 0.6 | 50.3 ± 0.1 | 2.8 ± 0.1 | 97 | |||||||
| Effluent filtrate | A. brassicae V134 | 1.5 ± 0.1 | 19.5 ± 0.1 | 5.2 ± 0.5 | 19.9 ± 0.1 | 44 ± 0.4 | 4 ± 2 | 1.2 ± 0.2 | 3.1 ± 0.3 | 98 | ||
| C. tropicalis V139 | 2.1 ± 0.1 | 10.2 ± 0.3 | 2.9 ± 0.1 | 15.5 ± 0.6 | 11.8 ± 0.3 | 41.5 ± 0.2 | 9.5 ± 0.4 | 2.6 ± 0.1 | 96 | |||
| M. pulcherrima V213 | 2.0 ± 0.5 | 7.7 ± 0.1 | 2.4 ± 0.1 | 22 ± 6 | 12.0 ± 0.3 | 42 ± 8 | 3.1 ± 0.4 | 1.0 ± 0.2 | 3.4 ± 0.8 | 95 | ||
| P. kudriavzevii V194 | 1.6 ± 0.1 | 20.7 ± 0.2 | 4.5 ± 0.1 | 19.9 ± 0.2 | 42.3 ± 0.3 | 4.9 ± 0.3 | 2.5 ± 0.1 | 96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettencourt, S.; Miranda, C.; Pozdniakova, T.A.; Sampaio, P.; Franco-Duarte, R.; Pais, C. Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids. Microorganisms 2020, 8, 1809. https://doi.org/10.3390/microorganisms8111809
Bettencourt S, Miranda C, Pozdniakova TA, Sampaio P, Franco-Duarte R, Pais C. Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids. Microorganisms. 2020; 8(11):1809. https://doi.org/10.3390/microorganisms8111809
Chicago/Turabian StyleBettencourt, Sara, Catarina Miranda, Tatiana A. Pozdniakova, Paula Sampaio, Ricardo Franco-Duarte, and Célia Pais. 2020. "Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids" Microorganisms 8, no. 11: 1809. https://doi.org/10.3390/microorganisms8111809
APA StyleBettencourt, S., Miranda, C., Pozdniakova, T. A., Sampaio, P., Franco-Duarte, R., & Pais, C. (2020). Single Cell Oil Production by Oleaginous Yeasts Grown in Synthetic and Waste-Derived Volatile Fatty Acids. Microorganisms, 8(11), 1809. https://doi.org/10.3390/microorganisms8111809







