Deciphering Streptococcal Biofilms
Abstract
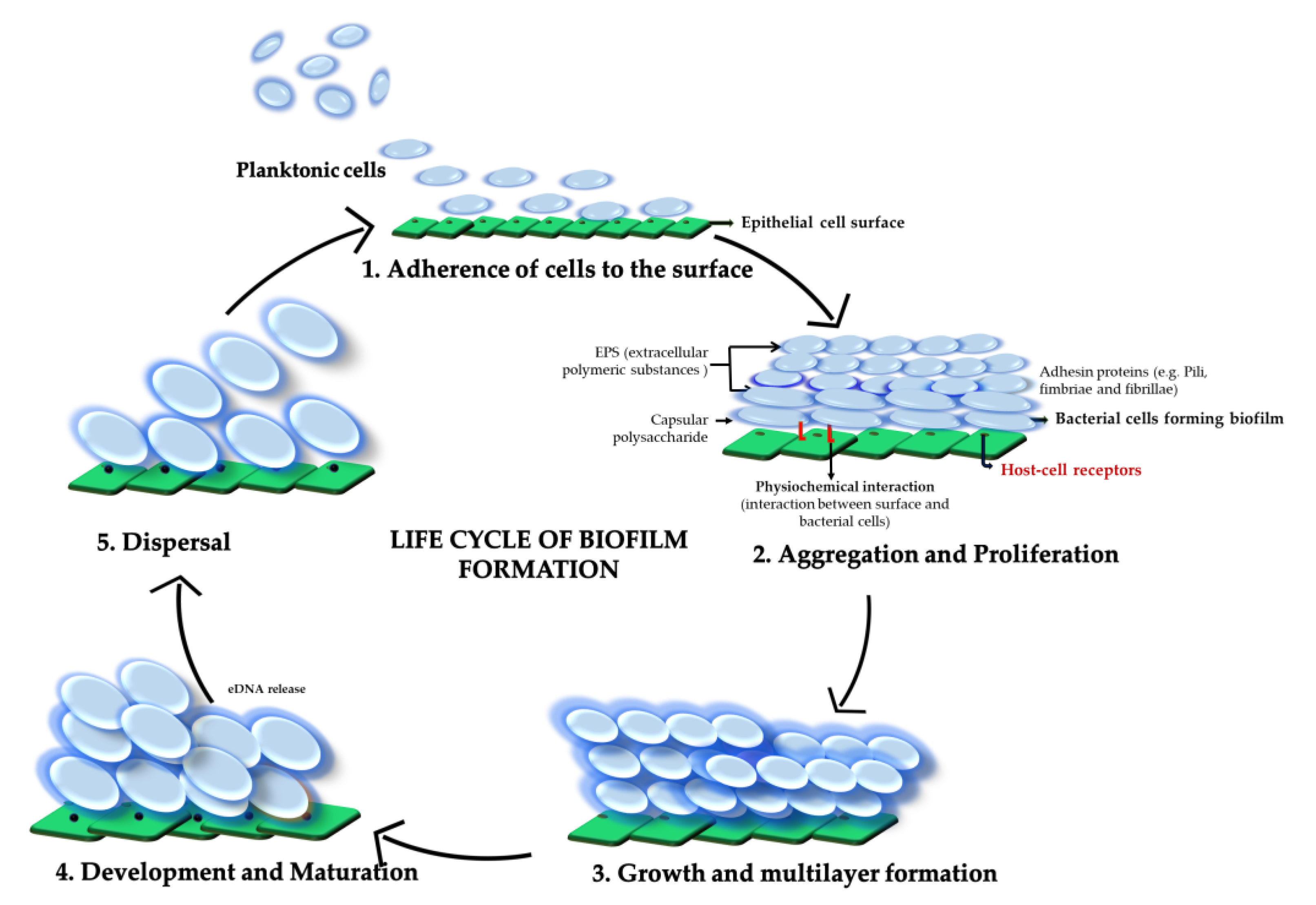
:1. Introduction
2. Biofilm Composition
2.1. Extracellular Polysaccharide
2.2. Nucleic Acids
2.3. Extracellular Proteins
3. Biofilm Formation in Different Streptococci
3.1. Betahemolytic Group A Streptococci: Streptococcus pyogenes (GAS)
3.2. Betahemolytic Group B Streptococci: Streptococcus agalactiae (GBS)
3.3. Betahemolytic Group C and G Streptococci: Streptococcus dysgalactiae subsp. equisimilis (SDSE)
3.4. Biofilm in Non-Beta-Hemolytic Streptococci: Streptococcus pneumoniae
3.5. Biofilm in Viridans Streptococci: Streptococcus mitis Group
3.6. Biofilm in Viridans Streptococci: Streptococcus anginosus Group
3.7. Biofilm in Viridans Streptococci: Streptococcus mutans
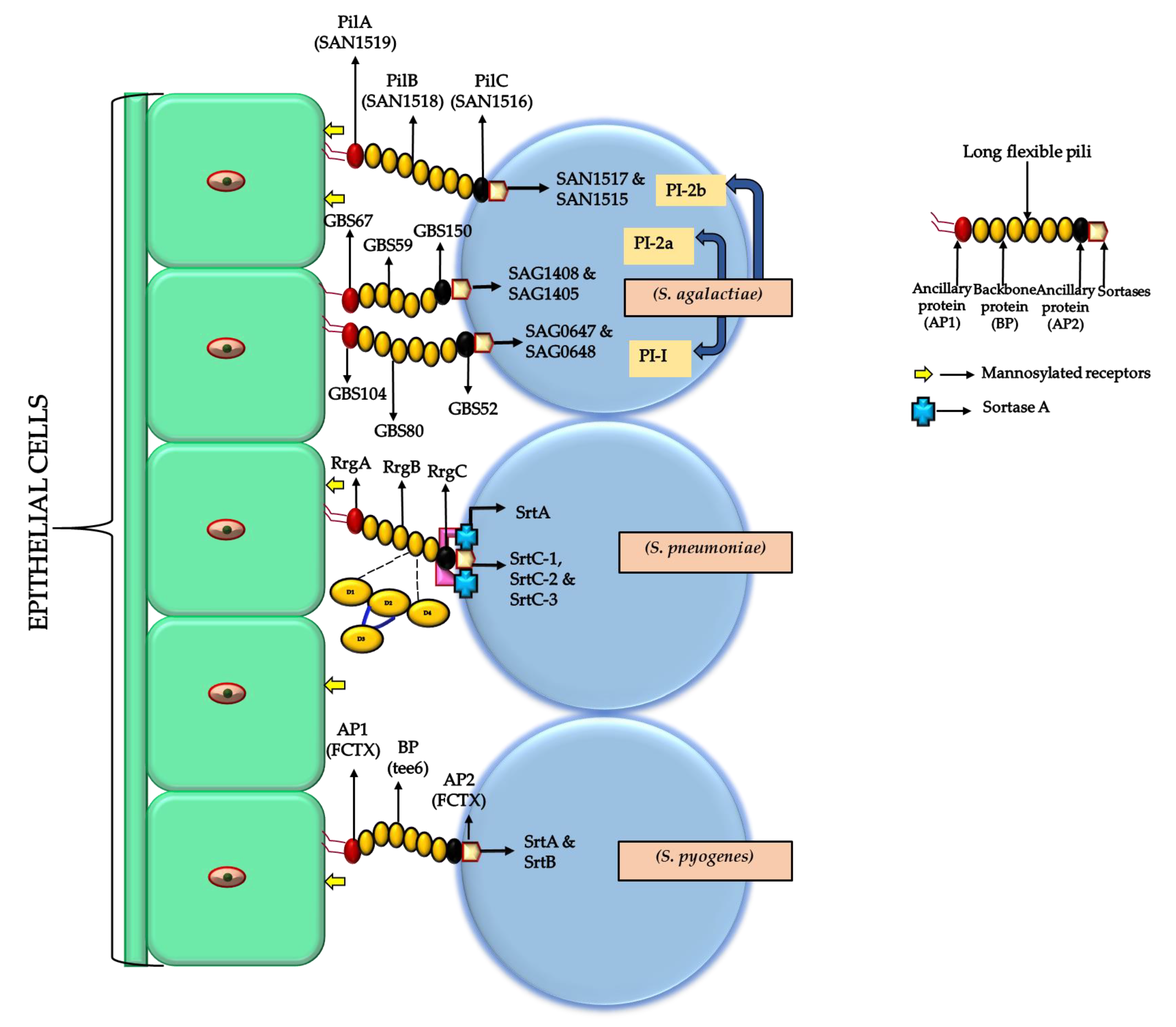
4. The Role of Pili and Streptococcal Cell Surface Proteins in Biofilm Formation (Pathogenesis and Virulence Factors)
4.1. Pili
4.2. Surface Proteins and Their Role in Biofilm Formation
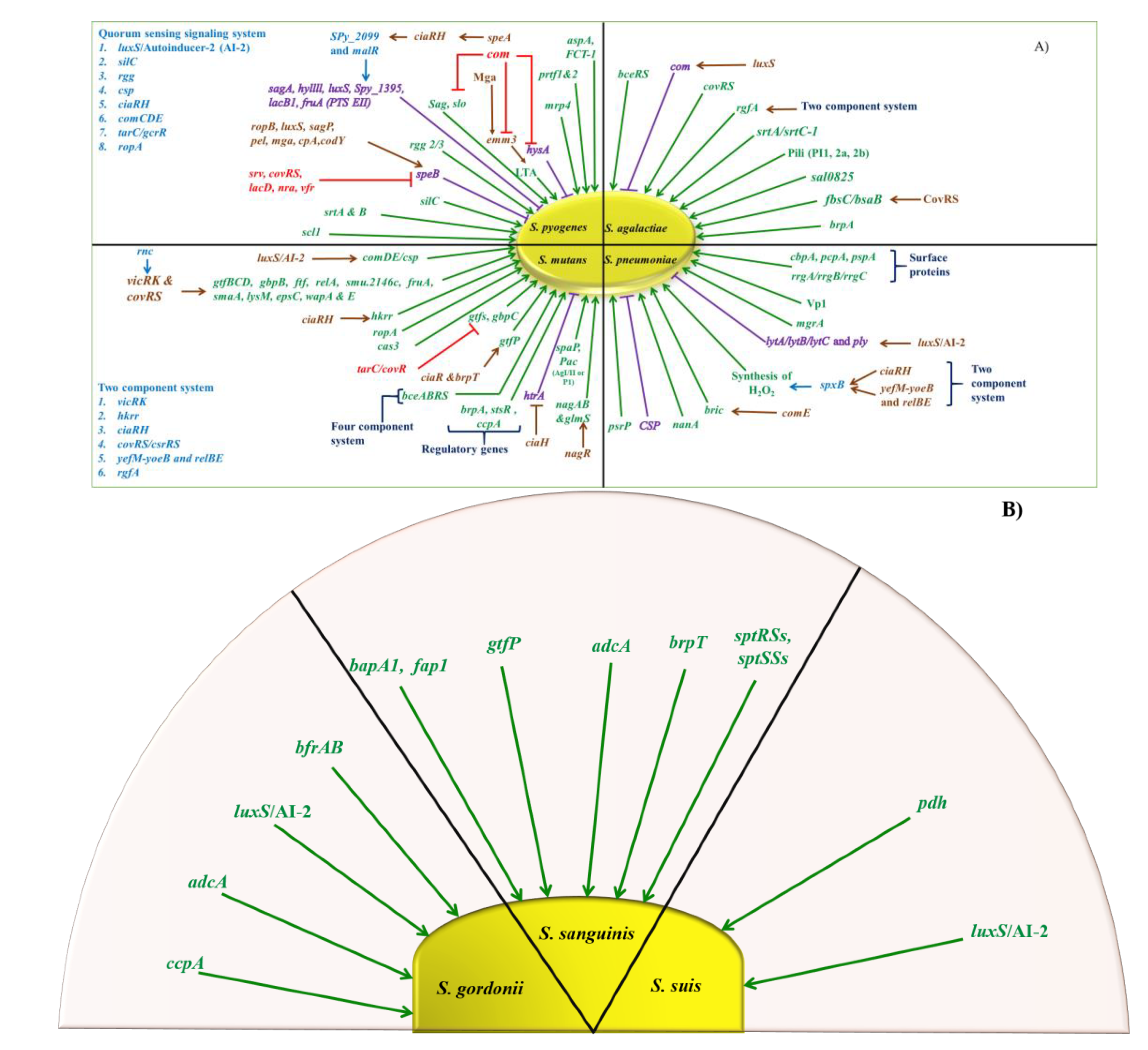
5. Regulation
5.1. GAS
5.2. GBS
5.3. S. pneumoniae
5.4. Viridans Streptococcus
6. Therapeutic Antibiofilm Approaches
6.1. Quorum Sensing Systems
6.2. Target Extracellular Polysaccharide (EPS) Matrix
6.3. Antimicrobial Peptides (AMPs)
6.4. Bacteriocins
6.5. Nanodrug Delivery System
6.6. Surfactants, Amino-Acids, Metal Chelators, and Various Enzymes
6.7. Phages Therapy
7. Conclusions and Future Prospective
Author Contributions
Funding
Conflicts of Interest
References
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Marrie, T.J.; Nelligan, J.; Costerton, J.W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation 1982, 66, 1339–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18. [Google Scholar] [CrossRef]
- Conley, J.; Olson, M.E.; Cook, L.S.; Ceri, H.; Phan, V.; Davies, H.D. Biofilm Formation by Group A Streptococci: Is There a Relationship with Treatment Failure? J. Clin. Microbiol. 2003, 41, 4043–4048. [Google Scholar] [CrossRef] [Green Version]
- Cox, G.; Wright, G.D. Intrinsic antibiotic resistance: Mechanisms, origins, challenges and solutions. Int. J. Med. Microbiol. 2013, 303, 287–292. [Google Scholar] [CrossRef]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Baldassarri, L.; Creti, R.; Recchia, S.; Imperi, M.; Facinelli, B.; Giovanetti, E.; Pataracchia, M.; Alfarone, G.; Orefici, G. Therapeutic Failures of Antibiotics Used To Treat Macrolide-Susceptible Streptococcus pyogenes Infections May Be Due to Biofilm Formation. J. Clin. Microbiol. 2006, 44, 2721–2727. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Terao, Y.; Okuni, H.; Ninomiya, K.; Sakata, H.; Ikebe, K.; Maeda, Y.; Kawabata, S. Biofilm formation or internalization into epithelial cells enable Streptococcus pyogenes to evade antibiotic eradication in patients with pharyngitis. Microb. Pathog. 2011, 51, 58–68. [Google Scholar] [CrossRef]
- Davey, M.E.; O’Toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef] [Green Version]
- Berlanga, M.; Guerrero, R. Living together in biofilms: The microbial cell factory and its biotechnological implications. Microb. Cell Factories 2016, 15, 165. [Google Scholar] [CrossRef] [Green Version]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stewart, P.S.; Franklin, M.J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 2008, 6, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Boles, B.R.; Singh, P.K. Endogenous oxidative stress produces diversity and adaptability in biofilm communities. Proc. Natl. Acad. Sci. USA 2008, 105, 12503–12508. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Ates, O. Systems Biology of Microbial Exopolysaccharides Production. Front. Bioeng. Biotechnol. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Nwodo, U.U.; Green, E.; Okoh, A.I. Bacterial exopolysaccharides: Functionality and prospects. Int. J. Mol. Sci. 2012, 13, 14002–14015. [Google Scholar] [CrossRef] [Green Version]
- Koo, H.; Xiao, J.; Klein, M.; Jeon, J. Exopolysaccharides Produced by Streptococcus mutans Glucosyltransferases Modulate the Establishment of Microcolonies within Multispecies Biofilms. J. Bacteriol. 2010, 192, 3024–3032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moscoso, M.; Claverys, J.-P. Release of DNA into the medium by competent Streptococcus pneumoniae: Kinetics, mechanism and stability of the liberated DNA. Mol. Microbiol. 2004, 54, 783–794. [Google Scholar] [CrossRef] [PubMed]
- Oggioni, M.R.; Trappetti, C.; Kadioglu, A.; Cassone, M.; Iannelli, F.; Ricci, S.; Andrew, P.W.; Pozzi, G. Switch from planktonic to sessile life: A major event in pneumococcal pathogenesis. Mol. Microbiol. 2006, 61, 1196–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allegrucci, M.; Sauer, K. Characterization of colony morphology variants isolated from Streptococcus pneumoniae biofilms. J. Bacteriol. 2007, 189, 2030–2038. [Google Scholar] [CrossRef] [Green Version]
- Camilli, R.; Pantosti, A.; Baldassarri, L. Contribution of serotype and genetic background to biofilm formation by Streptococcus pneumoniae. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2011, 30, 97–102. [Google Scholar] [CrossRef]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef]
- Moscoso, M.; García, E.; López, R. Biofilm Formation by Streptococcus pneumoniae: Role of Choline, Extracellular DNA, and Capsular Polysaccharide in Microbial Accretion. J. Bacteriol. 2006, 188, 7785–7795. [Google Scholar] [CrossRef] [Green Version]
- Carrolo, M.; Frias, M.J.; Pinto, F.R.; Melo-Cristino, J.; Ramirez, M. Prophage spontaneous activation promotes DNA release enhancing biofilm formation in Streptococcus pneumoniae. PLoS ONE 2010, 5, e15678. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Nistico, L.; Sambanthamoorthy, K.; Dice, B.; Nguyen, D.; Mershon, W.J.; Johnson, C.; Ze Hu, F.; Stoodley, P.; Ehrlich, G.D.; et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Fong, J.N.C.; Yildiz, F.H. Biofilm Matrix Proteins. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Lasa, I.; Penadés, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penadés, J.R.; Lasa, I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oli, M.W.; Otoo, H.N.; Crowley, P.J.; Heim, K.P.; Nascimento, M.M.; Ramsook, C.B.; Lipke, P.N.; Brady, L.J. Functional amyloid formation by Streptococcus mutans. Microbiology 2012, 158, 2903–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia, M.C.; Lee, J.T.; Ramsook, C.B.; Alsteens, D.; Dufrêne, Y.F.; Lipke, P.N. A Role for Amyloid in Cell Aggregation and Biofilm Formation. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Banas, J.A.; Vickerman, M.M. Glucan-binding Proteins of the Oral Streptococci. Crit. Rev. Oral Biol. Med. 2003, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Ramasubbu, N.; Fine, D.H. Detachment of Actinobacillus actinomycetemcomitans Biofilm Cells by an Endogenous β-Hexosaminidase Activity. J. Bacteriol. 2003, 185, 4693–4698. [Google Scholar] [CrossRef] [Green Version]
- Young, C.; Holder, R.C.; Dubois, L.; Reid, S.D. Streptococcus pyogenes biofilm. In Streptococcus pyogenes: Basic Biology to Clinical Manifestations; Ferretti, J.J., Stevens, D.L., Fischetti, V.A., Eds.; University of Oklahoma Health Sciences Center: Oklahoma City, OK, USA, 2016. [Google Scholar]
- Fiedler, T.; Köller, T.; Kreikemeyer, B. Streptococcus pyogenes biofilms—formation, biology, and clinical relevance. Front. Cell. Infect. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Shafreen, R.M.B.; Srinivasan, S.; Manisankar, P.; Pandian, S.K. Biofilm formation by Streptococcus pyogenes: Modulation of exopolysaccharide by fluoroquinolone derivatives. J. Biosci. Bioeng. 2011, 112, 345–350. [Google Scholar] [CrossRef]
- Lembke, C.; Podbielski, A.; Hidalgo-Grass, C.; Jonas, L.; Hanski, E.; Kreikemeyer, B. Characterization of Biofilm Formation by Clinically Relevant Serotypes of Group A Streptococci. Appl. Environ. Microbiol. 2006, 72, 12. [Google Scholar] [CrossRef] [Green Version]
- Thenmozhi, R.; Balaji, K.; Kumar, R.; Rao, T.S.; Pandian, S.K. Characterization of biofilms in different clinical M serotypes of Streptococcus pyogenes. J. Basic Microbiol. 2011, 51, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Rosini, R.; Margarit, I. Biofilm formation by Streptococcus agalactiae: Influence of environmental conditions and implicated virulence factors. Front. Cell. Infect. Microbiol. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Kaur, H.; Kumar, P.; Ray, P.; Kaur, J.; Chakraborti, A. Biofilm formation in clinical isolates of group B streptococci from north India. Microb. Pathog. 2009, 46, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Konto-Ghiorghi, Y.; Mairey, E.; Mallet, A.; Duménil, G.; Caliot, E.; Trieu-Cuot, P.; Dramsi, S. Dual role for pilus in adherence to epithelial cells and biofilm formation in Streptococcus agalactiae. PLoS Pathog. 2009, 5, e1000422. [Google Scholar] [CrossRef]
- Rinaudo, C.D.; Rosini, R.; Galeotti, C.L.; Berti, F.; Necchi, F.; Reguzzi, V.; Ghezzo, C.; Telford, J.L.; Grandi, G.; Maione, D. Specific Involvement of Pilus Type 2a in Biofilm Formation in Group B Streptococcus. PLoS ONE 2010, 5, e9216. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Urzo, N.; Martinelli, M.; Pezzicoli, A.; De Cesare, V.; Pinto, V.; Margarit, I.; Telford, J.L.; Maione, D. Acidic pH Strongly Enhances In Vitro Biofilm Formation by a Subset of Hypervirulent ST-17 Streptococcus agalactiae Strains. Appl. Environ. Microbiol. 2014, 80, 2176–2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, C.M.; Spellerberg, B. Human infections due to Streptococcus dysgalactiae subspecies equisimilis. Clin. Infect. Dis. 2009, 49, 766–772. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.-S.; Chen, S.-Y.; Lo, H.-H. Biofilm formation of beta-hemolytic group G Streptococcus dysgalactiae subspecies equisimilis isolates and its association with emm polymorphism. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2017, 125, 1027–1032. [Google Scholar] [CrossRef]
- Genteluci, G.L.; Silva, L.G.; Souza, M.C.; Glatthardt, T.; de Mattos, M.C.; Ejzemberg, R.; Alviano, C.S.; Figueiredo, A.M.S.; Ferreira-Carvalho, B.T. Assessment and characterization of biofilm formation among human isolates of Streptococcus dysgalactiae subsp. equisimilis. Int. J. Med. Microbiol. 2015, 305, 937–947. [Google Scholar] [CrossRef]
- Chao, Y.; Marks, L.R.; Pettigrew, M.M.; Hakansson, A.P. Streptococcus pneumoniae biofilm formation and dispersion during colonization and disease. Front. Cell. Infect. Microbiol. 2015, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenech, M.; García, E.; Moscoso, M. Biofilm formation in Streptococcus pneumoniae. Microb. Biotechnol. 2012, 5, 455–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.J.; Kumar, N.; Lizcano, A.; Shivshankar, P.; Dunning Hotopp, J.C.; Jorgensen, J.H.; Tettelin, H.; Orihuela, C.J. Streptococcus pneumoniae in Biofilms Are Unable to Cause Invasive Disease Due to Altered Virulence Determinant Production. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Domenech, M.; García, E.; Prieto, A.; Moscoso, M. Insight into the composition of the intercellular matrix of Streptococcus pneumoniae biofilms. Environ. Microbiol. 2012, 15. [Google Scholar] [CrossRef]
- Marks, L.R.; Davidson, B.A.; Knight, P.R.; Hakansson, A.P. Interkingdom signaling induces Streptococcus pneumoniae biofilm dispersion and transition from asymptomatic colonization to disease. mBio 2013, 4. [Google Scholar] [CrossRef] [Green Version]
- Rickard, A.H.; Gilbert, P.; High, N.J.; Kolenbrander, P.E.; Handley, P.S. Bacterial coaggregation: An integral process in the development of multi-species biofilms. Trends Microbiol. 2003, 11, 94–100. [Google Scholar] [CrossRef]
- Diaz, P.I.; Chalmers, N.I.; Rickard, A.H.; Kong, C.; Milburn, C.L.; Palmer, R., Jr.; Kolenbrander, P.E. Molecular characterization of subject-specific oral microflora during initial colonization of enamel. Appl. Environ. Microbiol. 2006, 72, 2837–2848. [Google Scholar] [CrossRef] [Green Version]
- Ammann, T.W.; Belibasakis, G.N.; Thurnheer, T. Impact of Early Colonizers on In Vitro Subgingival Biofilm Formation. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Thompson, A.; Mansfield, J.M.; Grassmann, A.A.; Maas, K.; Caimano, M.J.; Barao, V.A.R.; Vickerman, M.M.; Dongari-Bagtzoglou, A. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020, 14, 1207–1222. [Google Scholar] [CrossRef] [Green Version]
- McNab, R.; Ford, S.K.; El-Sabaeny, A.; Barbieri, B.; Cook, G.S.; Lamont, R.J. LuxS-Based Signaling in Streptococcus gordonii: Autoinducer 2 Controls Carbohydrate Metabolism and Biofilm Formation with Porphyromonas gingivalis. J. Bacteriol. 2003, 185, 274–284. [Google Scholar] [CrossRef] [Green Version]
- Zhu, B.; Ge, X.; Stone, V.; Kong, X.; El-Rami, F.; Liu, Y.; Kitten, T.; Xu, P. ciaR impacts biofilm formation by regulating an arginine biosynthesis pathway in Streptococcus sanguinis SK36. Sci. Rep. 2017, 7, 17183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vickerman, M.M.; Clewell, D.B.; Jones, G.W. Sucrose-promoted accumulation of growing glucosyltransferase variants of Streptococcus gordonii on hydroxyapatite surfaces. Infect. Immun. 1991, 59, 3523–3530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavalcanti, I.M.G.; Del Bel Cury, A.A.; Jenkinson, H.F.; Nobbs, A.H. Interactions between Streptococcus oralis, Actinomyces oris, and Candida albicans in the development of multispecies oral microbial biofilms on salivary pellicle. Mol. Oral Microbiol. 2017, 32, 60–73. [Google Scholar] [CrossRef] [Green Version]
- Fahim, A.; Himratul-Aznita, W.H.; Abdul-Rahman, P.S. Polymicrobial interactions between Streptococcus mitis, Streptococcus sanguinis and oral associated Candida albicans on an in vitro salivary biofilm and differential expression of ALS1, ALS2 and ALS3 genes. Biotechnol. Biotechnol. Equip. 2019, 33, 338–346. [Google Scholar] [CrossRef]
- Presterl, E.; Grisold, A.J.; Reichmann, S.; Hirschl, A.M.; Georgopoulos, A.; Graninger, W. Viridans streptococci in endocarditis and neutropenic sepsis: Biofilm formation and effects of antibiotics. J. Antimicrob. Chemother. 2005, 55, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Petersen, F.C.; Pecharki, D.; Scheie, A.A. Biofilm Mode of Growth of Streptococcus intermedius Favored by a Competence-Stimulating Signaling Peptide. J. Bacteriol. 2004, 186, 6327–6331. [Google Scholar] [CrossRef] [Green Version]
- Petersen, F.C.; Ahmed, N.A.A.M.; Naemi, A.; Scheie, A.A. LuxS-mediated signalling in Streptococcus anginosus and its role in biofilm formation. Antonie Van Leeuwenhoek 2006, 90, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Perez-Tanoira, R.; Aarnisalo, A.; Haapaniemi, A.; Saarinen, R.; Kuusela, P.; Kinnari, T.J. Bacterial biofilm in salivary stones. Eur. Arch. Otorhinolaryngol 2019, 276, 1815–1822. [Google Scholar] [CrossRef] [Green Version]
- Tavernier, S.; Sass, A.; De Bruyne, M.; Baeke, F.; De Rycke, R.; Crabbé, A.; Vandecandelaere, I.; Van Nieuwerburgh, F.; Coenye, T. Decreased susceptibility of Streptococcus anginosus to vancomycin in a multispecies biofilm is due to increased thickness of the cell wall. J. Antimicrob. Chemother. 2018, 73, 2323–2330. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; McLean, J.S.; Lux, R.; He, X.; Shi, W. The well-coordinated linkage between acidogenicity and aciduricity via insoluble glucans on the surface of Streptococcus mutans. Sci. Rep. 2016, 5, 18015. [Google Scholar] [CrossRef] [Green Version]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus Adherence and Colonization. Microbiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demuth, D.R.; Lammey, M.S.; Huck, M.; Lally, E.T.; Malamud, D. Comparison of Streptococcus mutans and Streptococcus sanguis receptors for human salivary agglutinin. Microb. Pathog. 1990, 9, 199–211. [Google Scholar] [CrossRef]
- Telford, J.L.; Barocchi, M.A.; Margarit, I.; Rappuoli, R.; Grandi, G. Pili in gram-positive pathogens. Nat. Rev. Microbiol. 2006, 4, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Bensi, G.; Capo, S.; Falugi, F.; Zingaretti, C.; Manetti, A.G.O.; Maggi, T.; Taddei, A.R.; Grandi, G.; Telford, J.L. Group A Streptococcus produce pilus-like structures containing protective antigens and Lancefield T antigens. Proc. Natl. Acad. Sci. USA 2005, 102, 15641–15646. [Google Scholar] [CrossRef] [Green Version]
- Lauer, P.; Rinaudo, C.D.; Soriani, M.; Margarit, I.; Maione, D.; Rosini, R.; Taddei, A.R.; Mora, M.; Rappuoli, R.; Grandi, G.; et al. Genome analysis reveals pili in Group B Streptococcus. Science 2005, 309, 105. [Google Scholar] [CrossRef] [Green Version]
- Rosini, R.; Rinaudo, C.D.; Soriani, M.; Lauer, P.; Mora, M.; Maione, D.; Taddei, A.; Santi, I.; Ghezzo, C.; Brettoni, C.; et al. Identification of novel genomic islands coding for antigenic pilus-like structures in Streptococcus agalactiae. Mol. Microbiol. 2006, 61, 126–141. [Google Scholar] [CrossRef]
- Barocchi, M.A.; Ries, J.; Zogaj, X.; Hemsley, C.; Albiger, B.; Kanth, A.; Dahlberg, S.; Fernebro, J.; Moschioni, M.; Masignani, V.; et al. A pneumococcal pilus influences virulence and host inflammatory responses. Proc. Natl. Acad. Sci. USA 2006, 103, 2857–2862. [Google Scholar] [CrossRef] [Green Version]
- Dramsi, S.; Trieu-Cuot, P.; Bierne, H. Sorting sortases: A nomenclature proposal for the various sortases of Gram-positive bacteria. Res. Microbiol. 2005, 156, 289–297. [Google Scholar] [CrossRef]
- Hilleringmann, M.; Ringler, P.; Müller, S.A.; De Angelis, G.; Rappuoli, R.; Ferlenghi, I.; Engel, A. Molecular architecture of Streptococcus pneumoniae TIGR4 pili. EMBO J. 2009, 28, 3921–3930. [Google Scholar] [CrossRef] [Green Version]
- Becherelli, M.; Manetti, A.G.O.; Buccato, S.; Viciani, E.; Ciucchi, L.; Mollica, G.; Grandi, G.; Margarit, I. The ancillary protein 1 of Streptococcus pyogenes FCT-1 pili mediates cell adhesion and biofilm formation through heterophilic as well as homophilic interactions. Mol. Microbiol. 2012, 83, 1035–1047. [Google Scholar] [CrossRef] [Green Version]
- Manetti, A.G.O.; Zingaretti, C.; Falugi, F.; Capo, S.; Bombaci, M.; Bagnoli, F.; Gambellini, G.; Bensi, G.; Mora, M.; Edwards, A.M.; et al. Streptococcus pyogenes pili promote pharyngeal cell adhesion and biofilm formation. Mol. Microbiol. 2007, 64, 968–983. [Google Scholar] [CrossRef]
- Manetti, A.G.O.; Köller, T.; Becherelli, M.; Buccato, S.; Kreikemeyer, B.; Podbielski, A.; Grandi, G.; Margarit, I. Environmental Acidification Drives S. pyogenes Pilus Expression and Microcolony Formation on Epithelial Cells in a FCT-Dependent Manner. PLoS ONE 2010, 5, e13864. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.R.; Nakata, M.; Sumitomo, T.; Kreikemeyer, B.; Podbielski, A.; Terao, Y.; Kawabata, S. Involvement of T6 Pili in Biofilm Formation by Serotype M6 Streptococcus pyogenes. J. Bacteriol. 2012, 194, 804–812. [Google Scholar] [CrossRef] [Green Version]
- Nakata, M.; Kimura, K.R.; Sumitomo, T.; Wada, S.; Sugauchi, A.; Oiki, E.; Higashino, M.; Kreikemeyer, B.; Podbielski, A.; Okahashi, N.; et al. Assembly Mechanism of FCT Region Type 1 Pili in Serotype M6 Streptococcus pyogenes. J. Biol. Chem. 2011, 286, 37566–37577. [Google Scholar] [CrossRef] [Green Version]
- Koller, T.; Manetti, A.G.O.; Kreikemeyer, B.; Lembke, C.; Margarit, I.; Grandi, G.; Podbielski, A. Typing of the pilus-protein-encoding FCT region and biofilm formation as novel parameters in epidemiological investigations of Streptococcus pyogenes isolates from various infection sites. J. Med. Microbiol. 2010, 59, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.M.; Manetti, A.G.O.; Falugi, F.; Zingaretti, C.; Capo, S.; Buccato, S.; Bensi, G.; Telford, J.L.; Margarit, I.; Grandi, G. Scavenger receptor gp340 aggregates group A streptococci by binding pili. Mol. Microbiol. 2008, 68, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- Falugi, F.; Zingaretti, C.; Pinto, V.; Mariani, M.; Amodeo, L.; Manetti, A.G.O.; Capo, S.; Musser, J.M.; Orefici, G.; Margarit, I.; et al. Sequence variation in group A Streptococcus pili and association of pilus backbone types with lancefield T serotypes. J. Infect. Dis. 2008, 198, 1834–1841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kratovac, Z.; Manoharan, A.; Luo, F.; Lizano, S.; Bessen, D.E. Population genetics and linkage analysis of loci within the FCT region of Streptococcus pyogenes. J. Bacteriol. 2007, 189, 1299–1310. [Google Scholar] [CrossRef] [Green Version]
- Kreikemeyer, B.; Gámez, G.; Margarit, I.; Giard, J.-C.; Hammerschmidt, S.; Hartke, A.; Podbielski, A. Genomic organization, structure, regulation and pathogenic role of pilus constituents in major pathogenic Streptococci and Enterococci. Int. J. Med. Microbiol. 2011, 301, 240–251. [Google Scholar] [CrossRef]
- Bessen, D.E.; Kalia, A. Genomic localization of a T serotype locus to a recombinatorial zone encoding extracellular matrix-binding proteins in Streptococcus pyogenes. Infect. Immun. 2002, 70, 1159–1167. [Google Scholar] [CrossRef] [Green Version]
- Kreikemeyer, B.; Nakata, M.; Oehmcke, S.; Gschwendtner, C.; Normann, J.; Podbielski, A. Streptococcus pyogenes collagen type I-binding Cpa surface protein. Expression profile, binding characteristics, biological functions, and potential clinical impact. J. Biol. Chem. 2005, 280, 33228–33239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisey, H.C.; Hensler, M.; Nizet, V.; Doran, K.S. Group B Streptococcal Pilus Proteins Contribute to Adherence to and Invasion of Brain Microvascular Endothelial Cells. J. Bacteriol. 2007, 189, 1464–1467. [Google Scholar] [CrossRef] [Green Version]
- Lazzarin, M.; Mu, R.; Fabbrini, M.; Ghezzo, C.; Rinaudo, C.D.; Doran, K.S.; Margarit, I. Contribution of pilus type 2b to invasive disease caused by a Streptococcus agalactiae ST-17 strain. BMC Microbiol. 2017, 17, 148. [Google Scholar] [CrossRef] [PubMed]
- Maisey, H.C.; Quach, D.; Hensler, M.E.; Liu, G.Y.; Gallo, R.L.; Nizet, V.; Doran, K.S. A group B streptococcal pilus protein promotes phagocyte resistance and systemic virulence. FASEB J. 2008, 22, 1715–1724. [Google Scholar] [CrossRef]
- Armistead, B.; Oler, E.; Adams Waldorf, K.; Rajagopal, L. The Double Life of Group B Streptococcus: Asymptomatic Colonizer and Potent Pathogen. J. Mol. Biol. 2019, 431, 2914–2931. [Google Scholar] [CrossRef]
- Martins, E.R.; Andreu, A.; Melo-Cristino, J.; Ramirez, M. Distribution of Pilus Islands in Streptococcus agalactiae That Cause Human Infections: Insights into Evolution and Implication for Vaccine Development. Clin. Vaccine Immunol. 2013, 20, 313–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, R.E.; Laut, C.; Gaddy, J.A.; Zadoks, R.N.; Davies, H.D.; Manning, S.D. Association between genotypic diversity and biofilm production in group B Streptococcus. BMC Microbiol. 2016, 16, 86. [Google Scholar] [CrossRef] [Green Version]
- Pang, M.; Sun, L.; He, T.; Bao, H.; Zhang, L.; Zhou, Y.; Zhang, H.; Wei, R.; Liu, Y.; Wang, R. Molecular and virulence characterization of highly prevalent Streptococcus agalactiae circulated in bovine dairy herds. Vet. Res. 2017, 48, 65. [Google Scholar] [CrossRef] [Green Version]
- Alvim, D.C.S.S.; Ferreira, A.F.M.; Leal, M.A.; Oliveira, L.M.A.; Botelho, A.M.N.; Botelho, A.C.N.; Figueiredo, A.M.S.; Fracalanzza, S.E.L.; Teixeira, L.M.; Pinto, T.C.A. Biofilm production and distribution of pilus variants among Streptococcus agalactiae isolated from human and animal sources. Biofouling 2019, 35, 938–944. [Google Scholar] [CrossRef]
- Soriani, M.; Telford, J.L. Relevance of pili in pathogenic streptococci pathogenesis and vaccine development. Future Microbiol. 2010, 5, 735–747. [Google Scholar] [CrossRef]
- Springman, A.; Lacher, D.W.; Waymire, E.A.; Wengert, S.L.; Singh, P.; Zadoks, R.N.; Davies, H.; Manning, S.D. Pilus distribution among lineages of group b streptococcus: An evolutionary and clinical perspective. BMC Microbiol. 2014, 14, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, T.D.; Ness, S.; Kaufmann, B.K.; Hartmann, B.; Schilling, A.F.; Sudhop, S.; Hilleringmann, M.; Clausen-Schaumann, H. Pilus-1 Backbone Protein RrgB of Streptococcus pneumoniae Binds Collagen I in a Force-Dependent Way. ACS Nano 2019, 13, 7155–7165. [Google Scholar] [CrossRef] [PubMed]
- Paterson, N.; Baker, E. Structure of the Full-Length Major Pilin from Streptococcus pneumoniae: Implications for Isopeptide Bond Formation in Gram-Positive Bacterial Pili. PLoS ONE 2011, 6, e22095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaik, M.M.; Maccagni, A.; Tourcier, G.; Di Guilmi, A.M.; Dessen, A. Structural Basis of Pilus Anchoring by the Ancillary Pilin RrgC of Streptococcus pneumoniae. J. Biol. Chem. 2014, 289, 16988–16997. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Elías, E.J.; Marcano, J.; Camilli, A. Isolation of Streptococcus pneumoniae Biofilm Mutants and Their Characterization during Nasopharyngeal Colonization. Infect. Immun. 2008, 76, 5049–5061. [Google Scholar] [CrossRef] [Green Version]
- Okahashi, N.; Okinaga, T.; Sakurai, A.; Terao, Y.; Nakata, M.; Nakashima, K.; Shintani, S.; Kawabata, S.; Ooshima, T.; Nishihara, T. Streptococcus sanguinis induces foam cell formation and cell death of macrophages in association with production of reactive oxygen species. FEMS Microbiol. Lett. 2011, 323, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Zähner, D.; Gandhi, A.R.; Yi, H.; Stephens, D.S. Mitis Group Streptococci Express Variable Pilus Islet 2 Pili. PLoS ONE 2011, 6, e25124. [Google Scholar] [CrossRef] [Green Version]
- Foster, T.J. The MSCRAMM Family of Cell-Wall-Anchored Surface Proteins of Gram-Positive Cocci. Trends Microbiol. 2019, 27, 927–941. [Google Scholar] [CrossRef]
- Brady, L.J.; Maddocks, S.E.; Larson, M.R.; Forsgren, N.; Persson, K.; Deivanayagam, C.C.; Jenkinson, H.F. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol. Microbiol. 2010, 77, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Lukomski, S.; Bachert, B.A.; Squeglia, F.; Berisio, R. Collagen-like proteins of pathogenic streptococci. Mol. Microbiol. 2017, 103, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Maestro, B.; Sanz, J.M. Choline Binding Proteins from Streptococcus pneumoniae: A Dual Role as Enzybiotics and Targets for the Design of New Antimicrobials. Antibiotics 2016, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caswell, C.C.; Oliver-Kozup, H.; Han, R.; Lukomska, E.; Lukomski, S. Scl1, the multifunctional adhesin of group A Streptococcus, selectively binds cellular fibronectin and laminin, and mediates pathogen internalization by human cells. FEMS Microbiol. Lett. 2010, 303, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver-Kozup, H.; Martin, K.H.; Schwegler-Berry, D.; Green, B.J.; Betts, C.; Shinde, A.V.; Van De Water, L.; Lukomski, S. The group A streptococcal collagen-like protein-1, Scl1, mediates biofilm formation by targeting the extra domain A-containing variant of cellular fibronectin expressed in wounded tissue. Mol. Microbiol. 2013, 87, 672–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wozniak, A.; Scioscia, N.; Geoffroy, E.; Ponce, I.; García, P. Importance of adhesins in the recurrence of pharyngeal infections caused by Streptococcus pyogenes. J. Med. Microbiol. 2017, 66, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Lukomski, S.; Nakashima, K.; Abdi, I.; Cipriano, V.J.; Ireland, R.M.; Reid, S.D.; Adams, G.G.; Musser, J.M. Identification and characterization of the scl gene encoding a group A Streptococcus extracellular protein virulence factor with similarity to human collagen. Infect. Immun. 2000, 68, 6542–6553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmussen, M.; Edén, A.; Björck, L. SclA, a novel collagen-like surface protein of Streptococcus pyogenes. Infect. Immun. 2000, 68, 6370–6377. [Google Scholar] [CrossRef] [PubMed]
- Oliver-Kozup, H.A.; Elliott, M.; Bachert, B.A.; Martin, K.H.; Reid, S.D.; Schwegler-Berry, D.E.; Green, B.J.; Lukomski, S. The streptococcal collagen-like protein-1 (Scl1) is a significant determinant for biofilm formation by group A Streptococcus. BMC Microbiol. 2011, 11, 262. [Google Scholar] [CrossRef] [Green Version]
- Courtney, H.S.; Ofek, I.; Penfound, T.; Nizet, V.; Pence, M.A.; Podbielbski, A.; Hasty, D.L.; Dale, J.B. Relationship between Expression of the Family of M Proteins and Lipoteichoic Acid to Hydrophobicity and Biofilm Formation in Streptococcus pyogenes. PLoS ONE 2009, 4, 10. [Google Scholar] [CrossRef]
- Hall, M.; Nylander, S.; Jenkinson, H.F.; Persson, K. Structure of the C-terminal domain of AspA (antigen I/II-family) protein from Streptococcus pyogenes. FEBS Open Bio 2014, 4, 283–289. [Google Scholar] [CrossRef] [Green Version]
- Maddocks, S.E.; Wright, C.J.; Nobbs, A.H.; Brittan, J.L.; Franklin, L.; Strömberg, N.; Kadioglu, A.; Jepson, M.A.; Jenkinson, H.F. Streptococcus pyogenes antigen I/II-family polypeptide AspA shows differential ligand-binding properties and mediates biofilm formation. Mol. Microbiol. 2011, 81, 1034–1049. [Google Scholar] [CrossRef] [Green Version]
- Connolly, K.; Braden, A.; Holder, R.; Reid, S. Srv Mediated Dispersal of Streptococcal Biofilms Through SpeB Is Observed in CovRS+ Strains. PLoS ONE 2011, 6, e28640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buscetta, M.; Papasergi, S.; Firon, A.; Pietrocola, G.; Biondo, C.; Mancuso, G.; Midiri, A.; Romeo, L.; Teti, G.; Speziale, P.; et al. FbsC, a Novel Fibrinogen-binding Protein, Promotes Streptococcus agalactiae-Host Cell Interactions. J. Biol. Chem. 2014, 289, 21003–21015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, S.; Wessels, M.R. BsaB, a Novel Adherence Factor of Group B Streptococcus. Infect. Immun. 2014, 82, 1007–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuzeville, S.; Dramsi, S.; Madec, J.-Y.; Haenni, M.; Payot, S. Antigen I/II encoded by integrative and conjugative elements of Streptococcus agalactiae and role in biofilm formation. Microb. Pathog. 2015, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gosink, K.K.; Mann, E.R.; Guglielmo, C.; Tuomanen, E.I.; Masure, H.R. Role of Novel Choline Binding Proteins in Virulence of Streptococcus pneumoniae. Infect. Immun. 2000, 68, 5690–5695. [Google Scholar] [CrossRef] [Green Version]
- Hammerschmidt, S. Adherence molecules of pathogenic pneumococci. Curr. Opin. Microbiol. 2006, 9, 12–20. [Google Scholar] [CrossRef]
- López, R.; García, E. Recent trends on the molecular biology of pneumococcal capsules, lytic enzymes, and bacteriophage. FEMS Microbiol. Rev. 2004, 28, 553–580. [Google Scholar] [CrossRef] [Green Version]
- López, R.; García, E.; García, P.; García, J.L. Cell Wall Hydrolases. Pneumococcus 2004, 75–88. [Google Scholar] [CrossRef]
- Tokuda, M.; Okahashi, N.; Takahashi, I.; Nakai, M.; Nagaoka, S.; Kawagoe, M.; Koga, T. Complete nucleotide sequence of the gene for a surface protein antigen of Streptococcus sobrinus. Infect. Immun. 1991, 59, 3309–3312. [Google Scholar] [CrossRef] [Green Version]
- LaPolla, R.J.; Haron, J.A.; Kelly, C.G.; Taylor, W.R.; Bohart, C.; Hendricks, M.; Pyati, J.P.; Graff, R.T.; Ma, J.K.; Lehner, T. Sequence and structural analysis of surface protein antigen I/II (SpaA) of Streptococcus sobrinus. Infect. Immun. 1991, 59, 2677–2685. [Google Scholar] [CrossRef] [Green Version]
- Tamura, H.; Kikuchi, T.; Shirato, R.; Kato, H. Cloning and DNA sequencing of the surface protein antigen I/II (PAa) of Streptococcus cricetus. FEMS Microbiol. Lett. 2001, 196, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Schilling, K.; Giertsen, E.; Pearson, S.; Lee, S.F.; Bleiweis, A.; Beeman, D. Role of a cell surface-associated protein in adherence and dental caries. Infect. Immun. 1991, 59, 4606–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beg, A.M.; Jones, M.N.; Miller-Torbert, T.; Holt, R.G. Binding of Streptococcus mutans to extracellular matrix molecules and fibrinogen. Biochem. Biophys. Res. Commun. 2002, 298, 75–79. [Google Scholar] [CrossRef]
- Pecharki, D.; Petersen, F.C.; Assev, S.; Scheie, A.A. Involvement of antigen I/II surface proteins in Streptococcus mutans and Streptococcus intermedius biofilm formation. Oral Microbiol. Immunol. 2005, 20, 366–371. [Google Scholar] [CrossRef]
- Besingi, R.N.; Wenderska, I.B.; Senadheera, D.B.; Cvitkovitch, D.G.; Long, J.R.; Wen, Z.T.; Brady, L.J. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology 2017, 163, 488–501. [Google Scholar] [CrossRef]
- Lynch, D.; Fountain, T.; Mazurkiewicz, J.; Banas, J. Glucan-Binding Proteins are Essential for Shaping Streptococcus mutans Biofilm Architecture. FEMS Microbiol. Lett. 2007, 268, 158–165. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.; Chen, Y.-Y.M.; Ruiz, T.; Wu, H. New Cell Surface Protein Involved in Biofilm Formation by Streptococcus parasanguinis. Infect. Immun. 2011, 79, 3239–3248. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H.; Caparon, M.G. Patterns of virulence gene expression differ between biofilm and tissue communities of Streptococcus pyogenes. Mol. Microbiol. 2005, 57, 1545–1556. [Google Scholar] [CrossRef]
- Marks, L.R.; Mashburn-Warren, L.; Federle, M.J.; Hakansson, A.P. Streptococcus pyogenes Biofilm Growth In Vitro and In Vivo and Its Role in Colonization, Virulence, and Genetic Exchange. J. Infect. Dis. 2014, 210, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Doern, C.D.; Roberts, A.L.; Hong, W.; Nelson, J.; Lukomski, S.; Swords, W.E.; Reid, S.D. Biofilm formation by group A Streptococcus: A role for the streptococcal regulator of virulence (Srv) and streptococcal cysteine protease (SpeB). Microbiology. 2009, 155, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Vyas, H.K.N.; Proctor, E.-J.; McArthur, J.; Gorman, J.; Sanderson-Smith, M. Current Understanding of Group A Streptococcal Biofilms. Curr. Drug Targets 2019, 20, 982–993. [Google Scholar] [CrossRef]
- Carroll, R.K.; Musser, J.M. From transcription to activation: How group A streptococcus, the flesh-eating pathogen, regulates SpeB cysteine protease production. Mol. Microbiol. 2011, 81, 588–601. [Google Scholar] [CrossRef]
- Federle, M.J.; McIver, K.S.; Scott, J.R. A response regulator that represses transcription of several virulence operons in the group A streptococcus. J. Bacteriol. 1999, 181, 3649–3657. [Google Scholar] [CrossRef] [Green Version]
- Graham, M.R.; Smoot, L.M.; Migliaccio, C.A.L.; Virtaneva, K.; Sturdevant, D.E.; Porcella, S.F.; Federle, M.J.; Adams, G.J.; Scott, J.R.; Musser, J.M. Virulence control in group A Streptococcus by a two-component gene regulatory system: Global expression profiling and in vivo infection modeling. Proc. Natl. Acad. Sci. USA 2002, 99, 13855–13860. [Google Scholar] [CrossRef] [Green Version]
- Sugareva, V.; Arlt, R.; Fiedler, T.; Riani, C.; Podbielski, A.; Kreikemeyer, B. Serotype- and strain- dependent contribution of the sensor kinase CovS of the CovRS two-component system to Streptococcus pyogenes pathogenesis. BMC Microbiol. 2010, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Podbielski, A.; Woischnik, M.; Pohl, B.; Schmidt, K.H. What is the size of the group A streptococcal vir regulon? The Mga regulator affects expression of secreted and surface virulence factors. Med. Microbiol. Immunol. 1996, 185, 171–181. [Google Scholar] [CrossRef]
- Ribardo, D.A.; McIver, K.S. Defining the Mga regulon: Comparative transcriptome analysis reveals both direct and indirect regulation by Mga in the group A streptococcus. Mol. Microbiol. 2006, 62, 491–508. [Google Scholar] [CrossRef]
- Hondorp, E.R.; McIver, K.S. The Mga virulence regulon: Infection where the grass is greener. Mol. Microbiol. 2007, 66, 1056–1065. [Google Scholar] [CrossRef]
- Luo, F.; Lizano, S.; Bessen, D.E. Heterogeneity in the Polarity of Nra Regulatory Effects on Streptococcal Pilus Gene Transcription and Virulence. Infect. Immun. 2008, 76, 2490–2497. [Google Scholar] [CrossRef] [Green Version]
- Lyon, W.R.; Madden, J.C.; Levin, J.C.; Stein, J.L.; Caparon, M.G. Mutation of luxS affects growth and virulence factor expression in Streptococcus pyogenes. Mol. Microbiol. 2001, 42, 145–157. [Google Scholar] [CrossRef]
- Marouni, M.J.; Sela, S. The luxS Gene of Streptococcus pyogenes Regulates Expression of Genes That Affect Internalization by Epithelial Cells. Infect. Immun. 2003, 71, 5633–5639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siller, M.; Janapatla, R.P.; Pirzada, Z.A.; Hassler, C.; Zinkl, D.; Charpentier, E. Functional analysis of the group A streptococcal luxS/AI-2 system in metabolism, adaptation to stress and interaction with host cells. BMC Microbiol. 2008, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, J.C.; LaSarre, B.; Jimenez, J.C.; Aggarwal, C.; Federle, M.J. Two group A streptococcal peptide pheromones act through opposing Rgg regulators to control biofilm development. PLoS Pathog. 2011, 7, e1002190. [Google Scholar] [CrossRef]
- Pérez-Pascual, D.; Gaudu, P.; Fleuchot, B.; Besset, C.; Rosinski-Chupin, I.; Guillot, A.; Monnet, V.; Gardan, R. RovS and its associated signaling peptide form a cell-to-cell communication system required for Streptococcus agalactiae pathogenesis. mBio 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Parker, R.E.; Knupp, D.; Al Safadi, R.; Rosenau, A.; Manning, S.D. Contribution of the RgfD Quorum Sensing Peptide to rgf Regulation and Host Cell Association in Group B Streptococcus. Genes 2017, 8, 23. [Google Scholar] [CrossRef] [Green Version]
- Park, S.E.; Jiang, S.; Wessels, M.R. CsrRS and Environmental pH Regulate Group B Streptococcus Adherence to Human Epithelial Cells and Extracellular Matrix. Infect. Immun. 2012, 80, 3975–3984. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Luo, M.; Zhou, H.; Li, C.; Luk, A.; Zhao, G.; Fung, K.; Ip, M. Role of Two-Component System Response Regulator bceR in the Antimicrobial Resistance, Virulence, Biofilm Formation, and Stress Response of Group B Streptococcus. Front. Microbiol. 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Safadi, R.A.; Mereghetti, L.; Salloum, M.; Lartigue, M.-F.; Virlogeux-Payant, I.; Quentin, R.; Rosenau, A. Two-Component System RgfA/C Activates the fbsB Gene Encoding Major Fibrinogen-Binding Protein in Highly Virulent CC17 Clone Group B Streptococcus. PLoS ONE 2011, 6, e14658. [Google Scholar] [CrossRef] [Green Version]
- Spellerberg, B.; Rozdzinski, E.; Martin, S.; Weber-Heynemann, J.; Lütticken, R. rgf Encodes a Novel Two-Component Signal Transduction System of Streptococcus agalactiae. Infect. Immun. 2002, 70, 2434–2440. [Google Scholar] [CrossRef] [Green Version]
- Patras, K.A.; Derieux, J.; Al-Bassam, M.M.; Adiletta, N.; Vrbanac, A.; Lapek, J.D.; Zengler, K.; Gonzalez, D.J.; Nizet, V. Group B Streptococcus Biofilm Regulatory Protein A Contributes to Bacterial Physiology and Innate Immune Resistance. J. Infect. Dis. 2018, 218, 1641–1652. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.E.; Ludewick, H.P.; Kunkel, R.M.; Zähner, D.; Klugman, K.P. The LuxS-Dependent Quorum-Sensing System Regulates Early Biofilm Formation by Streptococcus pneumoniae Strain D39. Infect. Immun. 2011, 79, 4050–4060. [Google Scholar] [CrossRef] [Green Version]
- Trappetti, C.; Gualdi, L.; Meola, L.; Jain, P.; Korir, C.; Edmonds, P.; Iannelli, F.; Ricci, S.; Pozzi, G.; Oggioni, M.R. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 2011, 11, 75. [Google Scholar] [CrossRef] [Green Version]
- Vidal, J.E.; Howery, K.E.; Ludewick, H.P.; Nava, P.; Klugman, K.P. Quorum-Sensing Systems LuxS/Autoinducer 2 and Com Regulate Streptococcus pneumoniae Biofilms in a Bioreactor with Living Cultures of Human Respiratory Cells. Infect. Immun. 2013, 81, 1341–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.D.; Eutsey, R.; West-Roberts, J.; Domenech, A.; Xu, W.; Abdullah, I.T.; Mitchell, A.P.; Veening, J.-W.; Yesilkaya, H.; Hiller, N.L. Function of BriC peptide in the pneumococcal competence and virulence portfolio. PLoS Pathog. 2018, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuevas, R.A.; Eutsey, R.; Kadam, A.; West-Roberts, J.A.; Woolford, C.A.; Mitchell, A.P.; Mason, K.M.; Hiller, N.L. A novel streptococcal cell–cell communication peptide promotes pneumococcal virulence and biofilm formation. Mol. Microbiol. 2017, 105, 554–571. [Google Scholar] [CrossRef]
- Hemsley, C.; Joyce, E.; Hava, D.L.; Kawale, A.; Camilli, A. MgrA, an Orthologue of Mga, Acts as a Transcriptional Repressor of the Genes within the rlrA Pathogenicity Islet in Streptococcus pneumoniae. J. Bacteriol. 2003, 185, 6640–6647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senadheera, D.B.; Cordova, M.; Ayala, E.A.; Chávez de Paz, L.E.; Singh, K.; Downey, J.S.; Svensäter, G.; Goodman, S.D.; Cvitkovitch, D.G. Regulation of Bacteriocin Production and Cell Death by the VicRK Signaling System in Streptococcus mutans. J. Bacteriol. 2012, 194, 1307–1316. [Google Scholar] [CrossRef] [Green Version]
- Senadheera, M.D.; Guggenheim, B.; Spatafora, G.A.; Huang, Y.-C.C.; Choi, J.; Hung, D.C.I.; Treglown, J.S.; Goodman, S.D.; Ellen, R.P.; Cvitkovitch, D.G. A VicRK Signal Transduction System in Streptococcus mutans Affects gtfBCD, gbpB, and ftf Expression, Biofilm Formation, and Genetic Competence Development. J. Bacteriol. 2005, 187, 4064–4076. [Google Scholar] [CrossRef] [Green Version]
- Duque, C.; Stipp, R.N.; Wang, B.; Smith, D.J.; Höfling, J.F.; Kuramitsu, H.K.; Duncan, M.J.; Mattos-Graner, R.O. Downregulation of GbpB, a Component of the VicRK Regulon, Affects Biofilm Formation and Cell Surface Characteristics of Streptococcus mutans. Infect. Immun. 2011, 79, 786–796. [Google Scholar] [CrossRef] [Green Version]
- Ayala, E.; Downey, J.S.; Mashburn-Warren, L.; Senadheera, D.B.; Cvitkovitch, D.G.; Goodman, S.D. A Biochemical Characterization of the DNA Binding Activity of the Response Regulator VicR from Streptococcus mutans. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Qi, F.; Merritt, J.; Lux, R.; Shi, W. Inactivation of the ciaH Gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect. Immun. 2004, 72, 4895–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-J.; Lemos, J.A.C.; Burne, R.A. Role of HtrA in Growth and Competence of Streptococcus mutans UA159. J. Bacteriol. 2005, 187, 3028–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biswas, S.; Biswas, I. Role of HtrA in Surface Protein Expression and Biofilm Formation by Streptococcus mutans. Infect. Immun. 2005, 73, 6923–6934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.-J.; Wen, Z.; Burne, R. Multilevel Control of Competence Development and Stress Tolerance in Streptococcus mutans UA159. Infect. Immun. 2006, 74, 1631–1642. [Google Scholar] [CrossRef] [Green Version]
- Idone, V.; Brendtro, S.; Gillespie, R.; Kocaj, S.; Peterson, E.; Rendi, M.; Warren, W.; Michalek, S.; Krastel, K.; Cvitkovitch, D.; et al. Effect of an orphan response regulator on Streptococcus mutans sucrose-dependent adherence and cariogenesis. Infect. Immun. 2003, 71, 4351–4360. [Google Scholar] [CrossRef] [Green Version]
- Biswas, S.; Biswas, I. Regulation of the glucosyltransferase (gtfBC) operon by CovR in Streptococcus mutans. J. Bacteriol. 2006, 188, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.T.; Burne, R.A. LuxS-Mediated Signaling in Streptococcus mutans Is Involved in Regulation of Acid and Oxidative Stress Tolerance and Biofilm Formation. J. Bacteriol. 2004, 186, 2682–2691. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Liang, J.; Tang, Z.; Ma, R.; Peng, H.; Huang, Z. Role of the luxS gene in initial biofilm formation by Streptococcus mutans. J. Mol. Microbiol. Biotechnol. 2015, 25, 60–68. [Google Scholar] [CrossRef]
- Leung, V.; Dufour, D.; Lévesque, C.M. Death and survival in Streptococcus mutans: Differing outcomes of a quorum-sensing signaling peptide. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.-H.; Tang, N.; Aspiras, M.B.; Lau, P.C.Y.; Lee, J.H.; Ellen, R.P.; Cvitkovitch, D.G. A Quorum-Sensing Signaling System Essential for Genetic Competence in Streptococcus mutans Is Involved in Biofilm Formation. J. Bacteriol. 2002, 184, 2699–2708. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.T.; Baker, H.V.; Burne, R.A. Influence of BrpA on Critical Virulence Attributes of Streptococcus mutans. J. Bacteriol. 2006, 188, 2983–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitoun, J.P.; Liao, S.; Yao, X.; Ahn, S.-J.; Isoda, R.; Nguyen, A.H.; Brady, L.J.; Burne, R.A.; Abranches, J.; Wen, Z.T. BrpA Is Involved in Regulation of Cell Envelope Stress Responses in Streptococcus mutans. Appl. Environ. Microbiol. 2012, 78, 2914–2922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Hou, J.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Emergent Properties in Streptococcus mutans Biofilms Are Controlled through Adhesion Force Sensing by Initial Colonizers. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, Z.T.; Suntharaligham, P.; Cvitkovitch, D.G.; Burne, R.A. Trigger Factor in Streptococcus mutans Is Involved in Stress Tolerance, Competence Development, and Biofilm Formation. Infect. Immun. 2005, 73, 219–225. [Google Scholar] [CrossRef] [Green Version]
- Mao, M.-Y.; Li, M.; Lei, L.; Yin, J.-X.; Yang, Y.-M.; Hu, T. The Regulator Gene rnc Is Closely Involved in Biofilm Formation in Streptococcus mutans. Caries Res. 2018, 52, 347–358. [Google Scholar] [CrossRef]
- Mao, M.-Y.; Yang, Y.-M.; Li, K.-Z.; Lei, L.; Li, M.; Yang, Y.; Tao, X.; Yin, J.-X.; Zhang, R.; Ma, X.-R.; et al. The rnc Gene Promotes Exopolysaccharide Synthesis and Represses the vicRKX Gene Expressions via MicroRNA-Size Small RNAs in Streptococcus mutans. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Li, Z.; Xiang, Z.; Zeng, J.; Li, Y.; Li, J. A GntR Family Transcription Factor in Streptococcus mutans Regulates Biofilm Formation and Expression of Multiple Sugar Transporter Genes. Front. Microbiol. 2019, 9, 3224. [Google Scholar] [CrossRef]
- Kawada-Matsuo, M.; Mazda, Y.; Oogai, Y.; Kajiya, M.; Kawai, T.; Yamada, S.; Miyawaki, S.; Oho, T.; Komatsuzawa, H. GlmS and NagB Regulate Amino Sugar Metabolism in Opposing Directions and Affect Streptococcus mutans Virulence. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [Green Version]
- Zeng, L.; Burne, R.A. NagR Differentially Regulates the Expression of the glmS and nagAB Genes Required for Amino Sugar Metabolism by Streptococcus mutans. J. Bacteriol. 2015, 197, 3533–3544. [Google Scholar] [CrossRef] [Green Version]
- Blehert, D.S.; Palmer, R.J.; Xavier, J.B.; Almeida, J.S.; Kolenbrander, P.E. Autoinducer 2 Production by Streptococcus gordonii DL1 and the Biofilm Phenotype of a luxS Mutant Are Influenced by Nutritional Conditions. J. Bacteriol. 2003, 185, 4851–4860. [Google Scholar] [CrossRef] [Green Version]
- Zheng, L.; Chen, Z.; Itzek, A.; Herzberg, M.C.; Kreth, J. CcpA Regulates Biofilm Formation and Competence in Streptococcus gordonii. Mol. Oral Microbiol. 2012, 27, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Stone, V.N.; Ge, X.; Tang, M.; Elrami, F.; Xu, P. TetR Family Regulator brpT Modulates Biofilm Formation in Streptococcus sanguinis. PLoS ONE 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, B.; Wang, S.; Li, J.; Gong, S.; Sun, L.; Yi, L. pdh modulate virulence through reducing stress tolerance and biofilm formation of Streptococcus suis serotype 2. Virulence 2019, 10, 588–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, T.M.; Stipp, R.N.; Alves, L.A.; Harth-Chu, E.N.; Höfling, J.F.; Mattos-Graner, R.O. Novel Two-Component System of Streptococcus sanguinis Affecting Functions Associated with Viability in Saliva and Biofilm Formation. Infect. Immun. 2018, 86, e00942-17. [Google Scholar] [CrossRef] [Green Version]
- Ge, X.; Kitten, T.; Chen, Z.; Lee, S.P.; Munro, C.L.; Xu, P. Identification of Streptococcus sanguinis Genes Required for Biofilm Formation and Examination of Their Role in Endocarditis Virulence. Infect. Immun. 2008, 76, 2551–2559. [Google Scholar] [CrossRef] [Green Version]
- Yadav, M.K.; Vidal, J.E.; Go, Y.Y.; Kim, S.H.; Chae, S.-W.; Song, J.-J. The LuxS/AI-2 Quorum-Sensing System of Streptococcus pneumoniae Is Required to Cause Disease, and to Regulate Virulence- and Metabolism-Related Genes in a Rat Model of Middle Ear Infection. Front. Cell. Infect. Microbiol. 2018, 8, 138. [Google Scholar] [CrossRef]
- Liu, B.; Yi, L.; Li, J.; Wang, Y.; Mao, C.; Wang, Y. Autoinducer-2 influences tetracycline resistance in Streptococcus suis by regulating the tet(M) gene via transposon Tn916. Res. Vet. Sci. 2020, 128, 269–274. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Sun, L.; Grenier, D.; Yi, L. The LuxS/AI-2 system of Streptococcus suis. Appl. Microbiol. Biotechnol. 2018, 102, 7231–7238. [Google Scholar] [CrossRef]
- Suntharalingam, P.; Cvitkovitch, D.G. Quorum sensing in streptococcal biofilm formation. Trends Microbiol. 2005, 13, 3–6. [Google Scholar] [CrossRef]
- Stipp, R.N.; Boisvert, H.; Smith, D.J.; Höfling, J.F.; Duncan, M.J.; Mattos-Graner, R.O. CovR and VicRK Regulate Cell Surface Biogenesis Genes Required for Biofilm Formation in Streptococcus mutans. PLoS ONE 2013, 8, e58271. [Google Scholar] [CrossRef]
- Tang, B.; Gong, T.; Zhou, X.; Lu, M.; Zeng, J.; Peng, X.; Wang, S.; Li, Y. Deletion of cas3 gene in Streptococcus mutans affects biofilm formation and increases fluoride sensitivity. Arch. Oral Biol. 2019, 99, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Mascher, T.; Zähner, D.; Merai, M.; Balmelle, N.; de Saizieu, A.; Hakenbeck, R. The Streptococcus pneumoniae cia Regulon: CiaR Target Sites and Transcription Profile Analysis. J. Bacteriol. 2003, 185, 60–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.T.; Domenech, M.; Moreno-Córdoba, I.; Navarro-Martínez, V.; Nieto, C.; Moscoso, M.; García, E.; Espinosa, M. The Streptococcus pneumoniae yefM-yoeB and relBE Toxin-Antitoxin Operons Participate in Oxidative Stress and Biofilm Formation. Toxins 2018, 10, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-H.; Lau, P.C.Y.; Tang, N.; Svensäter, G.; Ellen, R.P.; Cvitkovitch, D.G. Novel Two-Component Regulatory System Involved in Biofilm Formation and Acid Resistance in Streptococcus mutans. J. Bacteriol. 2002, 184, 6333–6342. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.-L.; Salim, H.; Dong, G.; Parcells, M.; Li, Y.-H. The BceABRS four-component system that is essential for cell envelope stress response is involved in sensing and response to host defence peptides and is required for the biofilm formation and fitness of Streptococcus mutans. J. Med. Microbiol. 2018, 67, 874–883. [Google Scholar] [CrossRef]
- Li, Y.; Burne, R.A. Regulation of the gtfBC and ftf genes of Streptococcus mutans in biofilms in response to pH and carbohydrate. Microbiol. Read. Engl. 2001, 147, 2841–2848. [Google Scholar] [CrossRef] [Green Version]
- Banas, J.A. Virulence properties of Streptococcus mutans. Front. Biosci. 2004, 9, 1267. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.T.; Burne, R.A. Functional Genomics Approach to Identifying Genes Required for Biofilm Development by Streptococcus mutans. Appl. Environ. Microbiol. 2002, 68, 9. [Google Scholar] [CrossRef] [Green Version]
- Shadbad, M.A.; Kafil, H.S.; Rezaee, M.A.; Farzami, M.R.; Dehkharghani, A.D.; Sadeghi, J.; Gholizadeh, P.; Aghazadeh, M. Filament genes and biofilm formation in Streptococcus agalactiae: Rev. Med. Microbiol. 2020, 31, 17–25. [Google Scholar] [CrossRef]
- Froeliger, E.H.; Fives-Taylor, P. Streptococcus parasanguis fimbria-associated adhesin fap1 is required for biofilm formation. Infect. Immun. 2001, 69, 2512–2519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, C.; Shivshankar, P.; Stol, K.; Trakhtenbroit, S.; Sullam, P.; Sauer, K.; Hermans, P.; Orihuela, J. The Pneumococcal Serine-Rich Repeat Protein Is an Intra-Species Bacterial Adhesin That Promotes Bacterial Aggregation In Vivo and in Biofilms. PLoS Pathog. 2010, 6, e1001044. [Google Scholar] [CrossRef]
- Spellerberg, B.; Cundell, D.R.; Sandros, J.; Pearce, B.J.; Idänpään-Heikkilä, I.; Rosenow, C.; Masure, H.R. Pyruvate oxidase, as a determinant of virulence in Streptococcus pneumoniae. Mol. Microbiol. 1996, 19, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Soong, G.; Planet, P.; Brower, J.; Ratner, A.J.; Prince, A. The NanA Neuraminidase of Streptococcus pneumoniae Is Involved in Biofilm Formation. Infect. Immun. 2009, 77, 3722–3730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domenech, M.; García, E. The N -Acetylglucosaminidase LytB of Streptococcus pneumoniae Is Involved in the Structure and Formation of Biofilms. Appl. Environ. Microbiol. 2020, 86, e00280-20. [Google Scholar] [CrossRef]
- Babbar, A.; Barrantes, I.; Pieper, D.H.; Itzek, A. Superantigen SpeA attenuates the biofilm forming capacity of Streptococcus pyogenes. J. Microbiol. 2019, 57, 626–636. [Google Scholar] [CrossRef]
- Wu, H.; Moser, C.; Wang, H.-Z.; Høiby, N.; Song, Z.-J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Lovetri, K.; Madhyastha, S. Antimicrobial and Antibiofilm Activity of Quorum Sensing Peptides and Peptide Analogues Against Oral Biofilm Bacteria. Methods Mol. Biol. 2010, 618, 383–392. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Ramanathan, T. Antiquorum sensing and biofilm potential of 5- Hydroxymethylfurfural against Gram positive pathogens. Microb. Pathog. 2018, 125, 48–50. [Google Scholar] [CrossRef]
- Cook, L.C.; Federle, M.J. Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol. Rev. 2014, 38, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Mah, T.F.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Mulcahy, H.; Charron-Mazenod, L.; Lewenza, S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008, 4, e1000213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craigen, B.; Dashiff, A.; Kadouri, D.E. The Use of Commercially Available Alpha-Amylase Compounds to Inhibit and Remove Staphylococcus aureus Biofilms. Open Microbiol. J. 2011, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Ramasubbu, N.; Ragunath, C.; Sundar, K.; Mishra, P.; Gyémánt, G.; Kandra, L. Structure-function relationships in human salivary α-amylase: Role of aromatic residues. Biologia 2005, 60, 47–56. [Google Scholar]
- Darouiche, R.O.; Mansouri, M.D.; Gawande, P.V.; Madhyastha, S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB combination. J. Antimicrob. Chemother. 2009, 64, 88–93. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2006, 114, 131–138. [Google Scholar] [CrossRef]
- Kaplan, J.B. Therapeutic Potential of Biofilm-Dispersing Enzymes: Int. J. Artif. Organs 2018. [Google Scholar] [CrossRef]
- Leroy, C.; Delbarre, C.; Ghillebaert, F.; Compere, C.; Combes, D. Effects of commercial enzymes on the adhesion of a marine biofilm-forming bacterium. Biofouling 2008, 24, 11–22. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Hall-Stoodley, L.; Stoodley, P. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 2015, 60, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Pletzer, D.; Hancock, R.E.W. Antibiofilm Peptides: Potential as Broad-Spectrum Agents. J. Bacteriol. 2016, 198, 2572–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente-Núñez, C.; Korolik, V.; Bains, M.; Nguyen, U.; Breidenstein, E.B.M.; Horsman, S.; Lewenza, S.; Burrows, L.; Hancock, R.E.W. Inhibition of Bacterial Biofilm Formation and Swarming Motility by a Small Synthetic Cationic Peptide. Antimicrob. Agents Chemother. 2012, 56, 2696–2704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Fuente-Núñez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernández, D.; Brackman, G.; Coenye, T.; Hancock, R.E.W. D-Enantiomeric Peptides that Eradicate Wild-Type and Multidrug-Resistant Biofilms and Protect against Lethal Pseudomonas aeruginosa Infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tao, R.; Tong, Z.; Ding, Y.; Kuang, R.; Zhai, S.; Liu, J.; Ni, L. Effect of a novel antimicrobial peptide chrysophsin-1 on oral pathogens and Streptococcus mutans biofilms. Peptides 2012, 33, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Tao, R.; Tong, Z.; Lin, Y.; Xue, Y.; Wang, W.; Kuang, R.; Wang, P.; Tian, Y.; Ni, L. Antimicrobial and antibiofilm activity of pleurocidin against cariogenic microorganisms. Peptides 2011, 32, 1748–1754. [Google Scholar] [CrossRef]
- Cao, Y.; Yin, H.; Wang, W.; Pei, P.; Wang, Y.; Wang, X.; Jiang, J.; Luo, S.-Z.; Chen, L. Killing Streptococcus mutans in mature biofilm with a combination of antimicrobial and antibiofilm peptides. Amino Acids 2020, 52, 1–14. [Google Scholar] [CrossRef]
- Guo, L.; McLean, J.S.; Yang, Y.; Eckert, R.; Kaplan, C.W.; Kyme, P.; Sheikh, O.; Varnum, B.; Lux, R.; Shi, W.; et al. Precision-guided antimicrobial peptide as a targeted modulator of human microbial ecology. Proc. Natl. Acad. Sci. USA 2015, 112, 7569–7574. [Google Scholar] [CrossRef] [Green Version]
- Shahrour, H.; Ferrer-Espada, R.; Dandache, I.; Bárcena-Varela, S.; Sánchez-Gómez, S.; Chokr, A.; Martinez-de-Tejada, G. AMPs as Anti-biofilm agents for human therapy and prophylaxis. In Antimicrobial Peptides; Matsuzaki, K., Ed.; Advances in Experimental Medicine and Biology; Springer Singapore: Singapore, 2019; Volume 1117, pp. 257–279. ISBN 9789811335877. [Google Scholar]
- Field, D.; Seisling, N.; Cotter, P.; Ross, R.; Hill, C. Synergistic Nisin-Polymyxin Combinations for the Control of Pseudomonas Biofilm Formation. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Kajwadkar, R.; Shin, J.M.; Lin, G.-H.; Fenno, J.C.; Rickard, A.H.; Kapila, Y.L. High-purity Nisin Alone or in Combination with Sodium Hypochlorite Is Effective against Planktonic and Biofilm Populations of Enterococcus faecalis. J. Endod. 2017, 43, 989–994. [Google Scholar] [CrossRef] [PubMed]
- Yamakami, K.; Tsumori, H.; Sakurai, Y.; Shimizu, Y.; Nagatoshi, K.; Sonomoto, K. Sustainable inhibition efficacy of liposome-encapsulated nisin on insoluble glucan-biofilm synthesis by Streptococcus mutans. Pharm. Biol. 2013, 51, 267–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Z.; Zhang, L.; Ling, J.; Jian, Y.; Huang, L.; Deng, D. An In Vitro Study on the Effect of Free Amino Acids Alone or in Combination with Nisin on Biofilms as well as on Planktonic Bacteria of Streptococcus mutans. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rukavina, Z.; Vanić, Ž. Current Trends in Development of Liposomes for Targeting Bacterial Biofilms. Pharmaceutics 2016, 8, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote Streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Allaker, R.P. The use of nanoparticles to control oral biofilm formation. J. Dent. Res. 2010, 89, 1175–1186. [Google Scholar] [CrossRef]
- Fernandes, T.; Bhavsar, C.; Sawarkar, S.; D’souza, A. Current and novel approaches for control of dental biofilm. Int. J. Pharm. 2018, 536, 199–210. [Google Scholar] [CrossRef]
- Ramazanzadeh, B.; Jahanbin, A.; Yaghoubi, M.; Shahtahmassbi, N.; Ghazvini, K.; Shakeri, M.; Shafaee, H. Comparison of Antibacterial Effects of ZnO and CuO Nanoparticles Coated Brackets against Streptococcus Mutans. J. Dent. Shiraz Iran 2015, 16, 200–205. [Google Scholar]
- Al-Adham, I.S.I.; Dinning, A.J.; Eastwood, I.M.; Austin, P.; Collier, P.J. Cell membrane effects of some common biocides. J. Ind. Microbiol. Biotechnol. 1998, 21, 6–10. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Singh, A.; Ward, O.P. Physiological aspects. Part 1 in a series of papers devoted to surfactants in microbiology and biotechnology. Biotechnol. Adv. 2006, 24, 604–620. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef]
- Chen, X.; Stewart, P.S. Biofilm removal caused by chemical treatments. Water Res. 2000, 34, 4229–4233. [Google Scholar] [CrossRef]
- Simões, M.; Pereira, M.O.; Vieira, M.J. Effect of mechanical stress on biofilms challenged by different chemicals. Water Res. 2005, 39, 5142–5152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, T.; Englert, D.; Lee, J.; Hegde, M.; Wood, T.K.; Jayaraman, A. Differential Effects of Epinephrine, Norepinephrine, and Indole on Escherichia coli O157:H7 Chemotaxis, Colonization, and Gene Expression. Infect. Immun. 2007, 75, 4597–4607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Z.; Lin, H.; Ji, X.; Yan, G.; Lei, L.; Han, W.; Gu, J.; Huang, J. Therapeutic applications of lytic phages in human medicine. Microb. Pathog. 2020, 142, 104048. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Köller, T.; Kreikemeyer, B.; Nelson, D.C. Rapid degradation of Streptococcus pyogenes biofilms by PlyC, a bacteriophage-encoded endolysin. J. Antimicrob. Chemother. 2013, 68, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Shi, Y.; Ji, W.; Meng, X.; Zhang, J.; Wang, H.; Lu, C.; Sun, J.; Yan, Y. Application of a Bacteriophage Lysin To Disrupt Biofilms Formed by the Animal Pathogen Streptococcus suis. Appl. Environ. Microbiol. 2011, 77, 8272–8279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Xiong, W.; Liu, P.; Xie, X.; Zeng, J.; Sun, Y.; Zeng, Z. Metagenomic Insights Into the Contribution of Phages to Antibiotic Resistance in Water Samples Related to Swine Feedlot Wastewater Treatment. Front. Microbiol. 2018, 9, 2474. [Google Scholar] [CrossRef] [Green Version]
- Dalmasso, M.; de Haas, E.; Neve, H.; Strain, R.; Cousin, F.J.; Stockdale, S.R.; Ross, R.P.; Hill, C. Isolation of a Novel Phage with Activity against Streptococcus mutans Biofilms. PLoS ONE 2015, 10. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Bi, Y.; Shang, X.; Wang, M.; Linden, S.B.; Li, Y.; Li, Y.; Nelson, D.C.; Wei, H. Antibiofilm Activities of a Novel Chimeolysin against Streptococcus mutans under Physiological and Cariogenic Conditions. Antimicrob. Agents Chemother. 2016, 60, 7436–7443. [Google Scholar] [CrossRef] [Green Version]
| FCT (Fibronectin-Binding, Collagen-Binding, T-Antigen) Type Encoding pili | Biofilm Phenotype |
|---|---|
| FCT type 1 | Strong biofilm, independent of media or pH |
| FCT type 9 | Poor biofilm, under all tested condition |
| FCT-2, FCT-3, FCT-5, and FCT-6 | Biofilm phenotype dependent upon on culture conditions and is triggered by low pH |
| FCT type 4 | Inhomogeneous response to environmental conditions with respect to biofilm formation. |
| Streptococci Species | Pilus Operon | Backbone Protein | Ancillary Protein-1 (Adhesin) | Ancillary Protein-2 | Gene Encoding for Sortase Enzyme |
|---|---|---|---|---|---|
| S. agalactiae | PI-1 | * GBS80 (* mandatory) | GBS 104 | GBS 52 | SAG 0647 and SAG 0648 |
| S. agalactiae | PI-2a | GBS 59 | GBS 67 | GBS 150 | SAG 1408 and SAG 1405 |
| S. agalactiae | PI-2b | SAN 1518 | SAN 1519 | SAN 1516 | SAN1517 and SAN 1515 |
| S. pyogenes | fctX operon | Tee6 | fctX | fctX | SrtB and SrtA |
| S. pneumoniae | RrgB | RrgA | RrgC | SrtC-1, SrtC-2, and SrtC-3 |
| Virulence Factors | Genes | Function | Streptococci Species | Reference |
|---|---|---|---|---|
| Quorum sensing system | luxS | Key regulator of early biofilm formation | S. gordonii | [61] |
| Involvement in virulence, competence, biofilm formation, acid and oxidative stress tolerance, and carbohydrate metabolism | S. agalactiae | [72] | ||
| Regulation of lytA and early biofilm formation | S. pneumoniae | [162,197] | ||
| Regulatory role in biofilm formation | S. mutans | [178] | ||
| Key regulator of early biofilm formation | S. suis | [198,199] | ||
| Streptococcal invasion locus (silC) | Regulator affecting biofilm architecture and density | S. pyogenes | [41] | |
| Regulatory gene of glucosyltransferase (rgg) | Universal streptococcal regulator involved in intraspecies communication, increased biogenesis of biofilms by rgg2 rgg3 | S. pyogenes | [142] | |
| Competence-stimulating peptide (CSP) | Competence development, involved in later stages of biofilm production | S. pneumoniae | [23,200] | |
| comCDE | Regulation of competence through production of competence-stimulating peptide (CSP) | S. mutans | [178,181] | |
| Two-component signaling system | bfrAB | Regulator involved in the maturation of multispecies biofilms | S. gordonii | [72] |
| bceRS | Control of oxidative stress response and biofilm production | S. agalactiae | [158] | |
| Histidine kinase (ciaH) | Regulatory role in biofilm formation, acid tolerance, and genetic competence | S. mutans | [172] | |
| vicR/K system (vicK) | Modulates the expression of several genes such as gtfBCD, gbpB, ftf, wapE, smaA, SMU.2146c, lysM, and epsC that affect the synthesis of EPS and biofilm formation | S. mutans | [169,201,202] | |
| ciaR/H | Control of the competence operon | S. pneumoniae | [203] | |
| yefM-yoeB and relBE | Control of resistance towards oxidative stress and involvement in biofilm formation | S. pneumoniae | [204] | |
| rgfA | Control of adherence to fibrinogen | S. agalactiae | [159,160] | |
| covR/S | Major virulence and adherence regulator | [123,124,157] | ||
| Histidine kinase (hk11) and response regulator (rr11) | Control of biofilm formation and acid resistance | S. mutans | [205] | |
| sptRSs and sptSSs | Coordination of cell wall homeostasis, involved in H2O2 production, and competence | S. sanguinis | [195] | |
| Four-component system | bceA, bceB, bceR, or bceS | Regulation of sensitivity towards antimicrobial peptides and requirement for biofilm formation | S. mutans | [206] |
| CRISPR/Cas systems | cas3 gene | Bacterial immunity, effect on biofilm formation, and fluoride sensitivity | S. mutans | [202] |
| Extracellular enzyme | Glucosyltransferases (gtfB, gtfC, and gtfD) and fructosyltransferases (ftfs) | Carbohydrate metabolism for the generation of exopolysaccharide | S. mutans | [207] |
| Sugar metabolism enzyme | Pyruvate dehydrogenase (pdh) | Control of environmental stress and promotion of biofilm formation | S. suis | [194] |
| Glucan binding | Glucan-binding protein (gbpA, gbpB, and gbpC) | Adhesion and promotion of biofilm formation | S. mutans | [208,209] |
| Amyloid proteins | Wall-associated protein (wapA and wapE) | Production of extracellular matrix | S. mutans | [136] |
| Regulatory proteins | Biofilm regulatory protein (brpA) | Regulation of acid and oxidative stress tolerance and biofilm formation | S. mutans | [182,205,210] |
| Virulence regulator stress tolerance | S. agalactiae | [211] | ||
| Sugar Transporter Systems Regulator (stsR) | Formation of biofilm and production of extracellular polysaccharides (EPS) at early stage | S. mutans | [188] | |
| Catabolite control protein (ccpA) | Global transcriptional regulator of carbon catabolite repression, involvement in biofilm formation | S. mutans | [210] | |
| Surface protease | Serine protease (htrA) | Processing and maturation of extracellular proteins including surface associated glycolytic enzymes (GbpB, GtfB, and FTF) contributing to biofilm formation | S. mutans | [174] |
| Surface-associated proteins | Fimbria-associated serine-rich repeat adhesin (fap1) and (bapA1) | Adhesins with important role in biofilm initiation | S. sanguinis | [212] |
| Choline-binding protein adhesin (cbpA), putative adhesin (pcpA), and pneumococcal surface protein A (pspA) | Adhesins binding to the teichoic acids of the cell wall, involvement in immune evasion, and promotion of biofilm formation | S. pneumoniae | [27,53] | |
| Pneumococcal serine-rich repeat protein (psrP) | Adhesion to host cells and mature biofilm formation | S. pneumoniae | [213] | |
| Pyruvate oxidase (spxB) | Responsible for the synthesis of H2O2 | [53,214] | ||
| Pili/fimbriae | Genomic island (PI-1, -2a, -2b). All islands contain 3 genes encoding pilus component | Pilus assembly and creation of biofilms | S. agalactiae | [93,94,95] |
| rrgA, rrgB, and rrgC | Pilus subunits and involvement in biofilm formation | S. pneumoniae | [106] | |
| FCT-1 region (fctX, srtB, and tee6) | Pili and biofilm formation | S. pyogenes | [84] | |
| Adhesin | Bacterial surface adhesin of GBS (BsaB) (sal0825) | Attachment of GBS to epithelial cells, extracellular matrix and promotion of biofilm production | S. agalactiae | [124] |
| Fibrinogen-binding protein (fbsC) | Fibrinogen binding, promotion of invasion of epithelial and endothelial barriers, biofilm formation | S. agalactiae | [123] | |
| Antigen | Neuraminidase (nanA) | Release of sialic acid residues, modification of immune defense proteins, promotion of colonization and biofilm formation | S. pneumoniae | [215] |
| Autolysin | lytA (amidase), lytB (glucosaminidase), and lytC (lysozyme) | Cell separation, autolysis and promotion of biofilm dispersion | S. pneumoniae | [27,216] |
| M-protein | emm | Key virulence factor, antiphagocytic, immune evasion, adhesin, and contribution to biofilm formation | S. pyogenes | [38,119,142] |
| Hyaluronic acid capsule | Hyaluronate synthase (hasA) | Escape of phagocytosis involvement in biofilm maturation | S. pyogenes | [38,139] |
| Sorting signal | Sortase A (srtA), sortase C (srtC-1) | Pilus polymerization and cell wall attachment | S. agalactiae | [43,46] |
| Sortase (srt A and srtA) | Pili assemblance and biofilm production | S. pyogenes | [39] | |
| Transcriptional regulator | Streptococcal regulator of virulence (srv) | Transcriptional regulator of virulence and contribution to biofilm dispersal by degrading SpeB | S. pyogenes | [122,142] |
| Streptococcal antigen I/II (AglI/II) family polypeptides | Group A Streptococcus protein A (aspA) | Adhesion to human salivary glycoproteins and facilitation of colonization to develop biofilm | S. pyogenes | [38,39] |
| Collagen-like protein | Streptococcal collagen-like gene-1 (scl-1) | Cell surface adhesin | S. pyogenes | [142] |
| MSCRAMM family proteins | Fibronectin-binding protein F (prtF1 and prtF2) and mrp4 | Adherence to host epithelial cells | S. pyogenes | [38,39,111] |
| Exotoxin | (speA) | Superantigen involved in the dispersal of biofilm | S. pyogenes | [122,217] |
| Cysteine protease (speB) | Cleavage of streptococcal cell surface virulence factors such as M protein, protein F, and C5a peptidase. Dispersal of biofilm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.; Verma, S.; Bauer, R.; Kumari, M.; Dua, M.; Johri, A.K.; Yadav, V.; Spellerberg, B. Deciphering Streptococcal Biofilms. Microorganisms 2020, 8, 1835. https://doi.org/10.3390/microorganisms8111835
Yadav P, Verma S, Bauer R, Kumari M, Dua M, Johri AK, Yadav V, Spellerberg B. Deciphering Streptococcal Biofilms. Microorganisms. 2020; 8(11):1835. https://doi.org/10.3390/microorganisms8111835
Chicago/Turabian StyleYadav, Puja, Shalini Verma, Richard Bauer, Monika Kumari, Meenakshi Dua, Atul Kumar Johri, Vikas Yadav, and Barbara Spellerberg. 2020. "Deciphering Streptococcal Biofilms" Microorganisms 8, no. 11: 1835. https://doi.org/10.3390/microorganisms8111835
APA StyleYadav, P., Verma, S., Bauer, R., Kumari, M., Dua, M., Johri, A. K., Yadav, V., & Spellerberg, B. (2020). Deciphering Streptococcal Biofilms. Microorganisms, 8(11), 1835. https://doi.org/10.3390/microorganisms8111835






