The Fungicidal Action of Micafungin is Independent on Both Oxidative Stress Generation and HOG Pathway Signaling in Candida albicans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Growth Conditions
2.2. Minimum Inhibitory Concentration (MIC)
2.3. Preparation of Cell-Free Extracts
2.4. Enzymatic Assays
2.5. ROS and Mitochondrial Membrane Potential Determination
2.6. Real-Time Quantitative RT-PCR (qRT PCR) Analysis
3. Results
3.1. Homozygous Disruption of HOG1 Gene does not Increase the Susceptibility to MF
3.2. Differential Action of MF vs. AMB on Intracellular ROS Production and Mitochondrial Membrane Potential
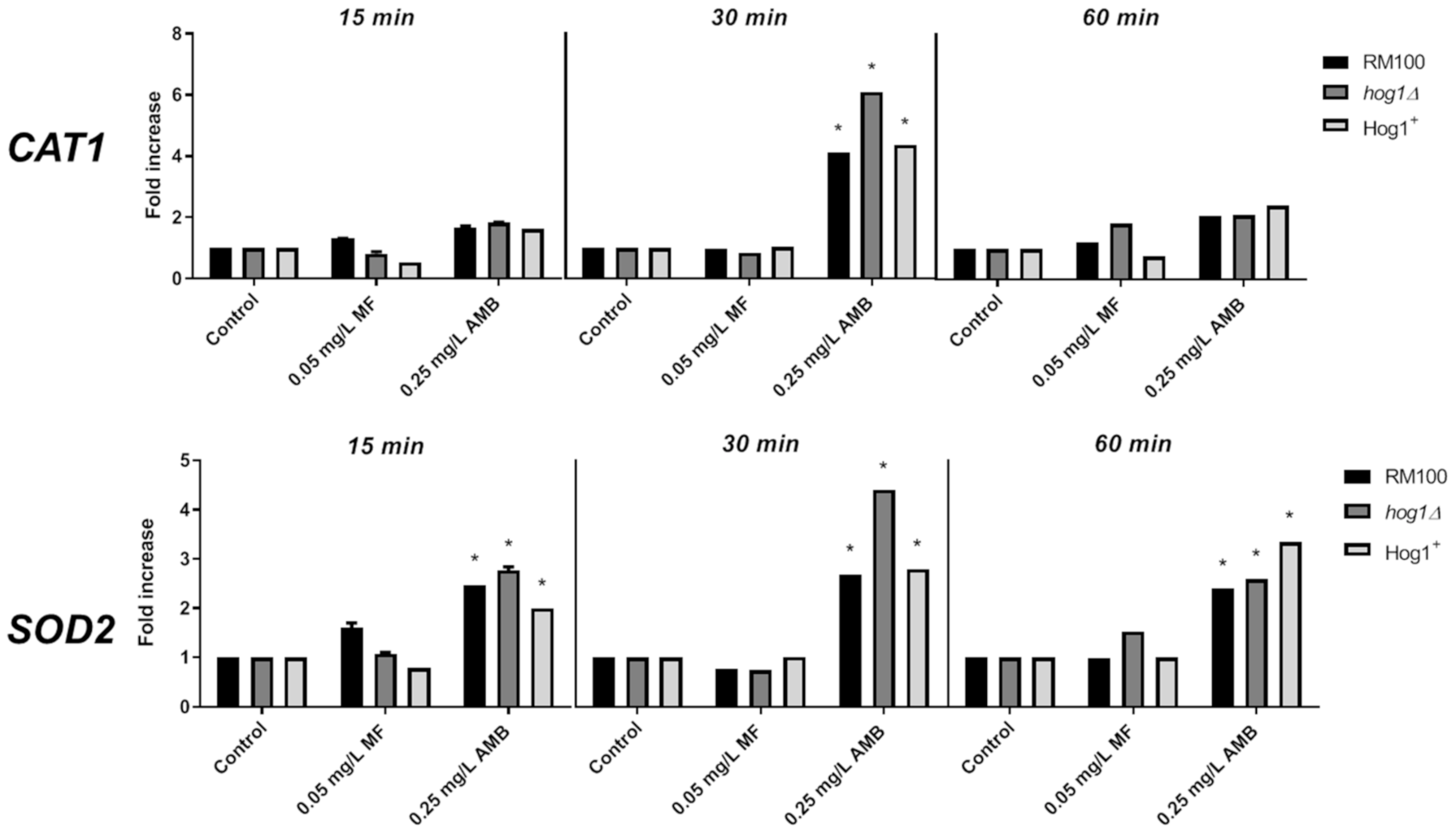
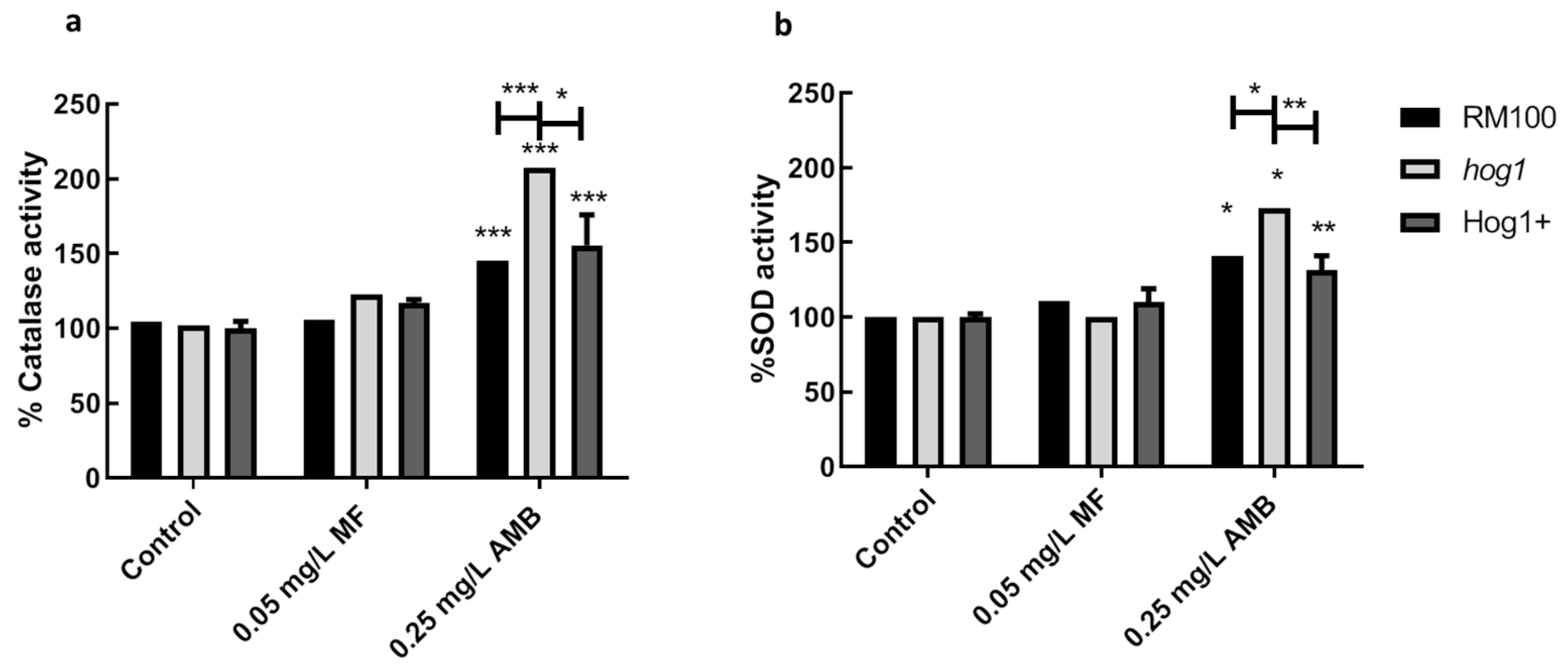
3.3. Role of AMB and MF on Antioxidant Gene Expression and Enzymatic Activities
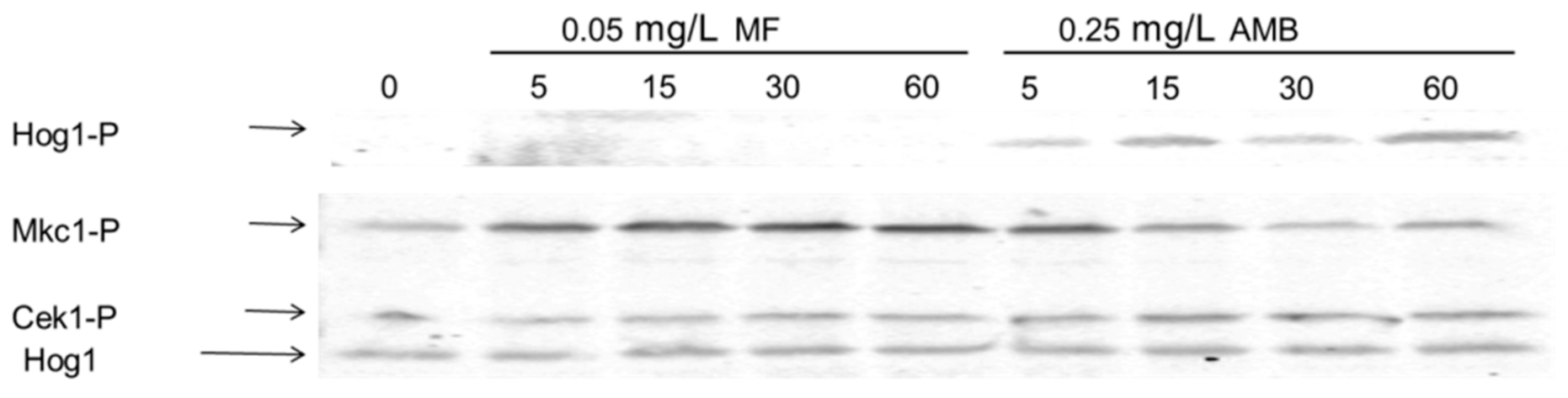
3.4. The Presence of AMB and MF is Sensed through Different Signaling Pathways in C. albicans
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gudlaugsson, O.; Gillespie, S.; Lee, K.; Berg, J.V.; Hu, J.; Messer, S.; Herwaldt, L.; Pfaller, M.; Diekema, D. Attributable mortality of nosocomial candidemia, revisited. Clin. Infect. Dis. 2003, 37, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, J.A. Invasive fungal infections in the intensive care unit. Semin. Respir. Crit. Care Med. 2010, 31, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Fortun, J.; Martin-Davila, P.; de la Pedrosa, E.G.-G.; Pintado, V.; Cobo, J.; Fresco, G.; Meije, Y.; Ros, L.; Alvarez, M.E.; Luengo, J.; et al. Emerging trends in candidemia: A higher incidence but a similar outcome. J. Infect. 2012, 65, 64–70. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive mycoses in North America. Crit. Rev. Microbiol. 2010, 36, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Castanheira, M. Nosocomial Candidiasis: Antifungal Stewardship and the Importance of Rapid Diagnosis. Med. Mycol. 2016, 54, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlroth, J.; Choi, B.; Spellberg, B. Nosocomial fungal infections: Epidemiology, diagnosis, and treatment. Med. Mycol. 2007, 45, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Quindos, G.; Marcos-Arias, C.; San-Millan, R.; Mateo, E.; Eraso, E. The continuous changes in the aetiology and epidemiology of invasive candidiasis: From familiar Candida albicans to multiresistant Candida auris. Int. Microbiol. 2018, 21, 107–119. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Smith, C.J.; Diekema, D.J.; Castanheira, M. Bacterial and fungal pathogens isolated from patients with bloodstream infection: Frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY Antimicrobial Surveillance Program (2012–2017). Diagn. Microbiol. Infect. Dis. 2020, 97, 115016. [Google Scholar] [CrossRef]
- Ermishkin, L.N.; Kasumov, K.M.; Potzeluyev, V.M. Single ionic channels induced in lipid bilayers by polyene antibiotics Amphotericin B and nystatine. Nature 1976, 262, 698–699. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Vincent, B.M.; Lancaster, A.K.; Scherz-Shouval, R.; Whitesell, L.; Lindquist, S. Fitness Trade-offs Restrict the Evolution of Resistance to Amphotericin B. PLoS Biol. 2013, 11, e1001692. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S.; Rautemaa-Richardson, R.; Alastruey-Izquierdo, A. The global problem of antifungal resistance: Prevalence, mechanisms, and management. Lancet Infect. Dis. 2017, 17, E383–E392. [Google Scholar] [CrossRef]
- Cornely, F.B.; Cornely, O.A.; Salmanton-Garcia, J.; Koehler, F.C.; Koehler, P.; Seifert, H.; Wingen-Heimann, S.; Mellinghoff, S.C. Attributable mortality of candidemia after introduction of echinocandins. Mycoses 2020, 63, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Perlin, D.S. Echinocandin Resistance in Candida. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S612–S617. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Roman, E.; Sanchez-Fresneda, R.; Casas, C.; Herrero, E.; Arguelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is an universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug: AMPHORES study. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Parraga, P.; Sanchez-Fresneda, R.; Zaragoza, O.; Arguelles, J.C. Amphotericin B induces trehalose synthesis and simultaneously activates an antioxidant enzymatic response in Candida albicans. Biochem. Biophys. Acta 2011, 1810, 777–783. [Google Scholar] [CrossRef]
- Kultz, D. Phylogenetic and functional classification of mitogen- and stress-activated protein kinases. J. Mol. Evol. 1998, 46, 571–588. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Román, E.; Nombela, C.; Pla, J. The MAP kinase signal transduction network in Candida albicans. Microbiology 2006, 152, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, B.; Nantel, A.; Whiteway, M. Stress-induced gene expression in Candida albicans: Absence of a general stress response. Mol. Biol. Cell 2003, 14, 1460–1467. [Google Scholar] [CrossRef] [Green Version]
- Enjalbert, B.; Smith, D.A.; Cornell, M.J.; Alam, I.; Nicholls, S.; Brown, A.J.; Quinn, J. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol. Biol. Cell 2006, 17, 1018–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero-de-Dios, C.; Day, A.M.; Tillmann, A.T.; Kastora, S.L.; Stead, D.; Salgado, P.S.; Quinn, J.; Brown, A.J.P. Redox Regulation, Rather than Stress-Induced Phosphorylation, of a Hog1 Mitogen-Activated Protein Kinase Modulates Its Nitrosative-Stress-Specific Outputs. MBio 2018, 9, e02229-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guirao-Abad, J.P.; Sanchez-Fresneda, R.; Roman, E.; Pla, J.; Arguelles, J.C.; Alonso-Monge, R. The MAPK Hog1 mediates the response to amphotericin B in Candida albicans. Fungal Genet. Biol. 2020, 136, 103302. [Google Scholar] [CrossRef] [PubMed]
- Belenky, P.; Camacho, D.; Collins, J.J. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 2013, 3, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Kelly, J.; Rowan, R.; McCann, M.; Kavanagh, K. Exposure to caspofungin activates Cap and Hog pathways in Candida albicans. Med. Mycol. 2009, 47, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Zhang, B.; Li, J.; Zhang, B.; Wang, H.; Li, M. Endoplasmic reticulum-derived reactive oxygen species (ROS) is involved in toxicity of cell wall stress to Candida albicans. Free Radic. Biol. Med. 2016, 99, 572–583. [Google Scholar] [CrossRef]
- Guirao-Abad, J.P.; Sanchez-Fresneda, R.; Alburquerque, B.; Hernandez, J.A.; Arguelles, J.C. ROS formation is a differential contributory factor to the fungicidal action of Amphotericin B and Micafungin in Candida albicans. Int. J. Med. Microbiol. 2017, 307, 241–248. [Google Scholar] [CrossRef]
- Pappas, P.G.; Rotstein, C.M.; Betts, R.F.; Nucci, M.; Talwar, D.; De Waele, J.J.; Vazquez, J.A.; Dupont, B.F.; Horn, D.L.; Ostrosky-Zeichner, L.; et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 2007, 45, 883–893. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Monge, R.; Navarro-García, F.; Román, E.; Negredo, A.I.; Eisman, B.; Nombela, C.; Pla, J. The Hog1 mitogen-activated protein kinase is essential in the oxidative stress response and chlamydospore formation in Candida albicans. Eukaryot. Cell 2003, 2, 351–361. [Google Scholar] [CrossRef] [Green Version]
- José, C.S.; Alonso-Monge, R.; Pérez-Díaz, R.M.; Pla, J.; Nombela, C. The mitogen-activated protein kinase homolog HOG1 gene controls glycerol accumulation in the pathogenic fungus Candida albicans. J. Bacteriol. 1996, 178, 5850–5852. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Clinical and Laboratory Standars Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Sangalli-Leite, F.; Scorzoni, L.; Mesa-Arango, A.C.; Casas, C.; Herrero, E.; Gianinni, M.J.; Rodriguez-Tudela, J.L.; Cuenca-Estrella, M.; Zaragoza, O. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect. 2011, 13, 457–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-García, F.; Alonso-Monge, R.; Rico, H.; Pla, J.; Sentandreu, R.; Nombela, C. A role for the MAP kinase gene MKC1 in cell wall construction and morphological transitions in Candida albicans. Microbiology 1998, 144, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-García, F.; Eisman, B.; Fiuza, S.M.; Nombela, C.; Pla, J. The MAP kinase Mkc1p is activated under different stress conditions in Candida albicans. Microbiology 2005, 151, 2737–2749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arana, D.M.; Nombela, C.; Alonso-Monge, R.; Pla, J. The Pbs2 MAP kinase kinase is essential for the oxidative-stress response in the fungal pathogen Candida albicans. Microbiology 2005, 151, 1033–1049. [Google Scholar] [CrossRef]
- Urrialde, V.; Prieto, D.; Pla, J.; Alonso-Monge, R. The Pho4 transcription factor mediates the response to arsenate and arsenite in Candida albicans. Front. Microbiol. 2015, 6, 118. [Google Scholar] [CrossRef] [Green Version]
- Román, E.; Nombela, C.; Pla, J. The Sho1 adaptor protein links oxidative stress to morphogenesis and cell wall biosynthesis in the fungal pathogen Candida albicans. Mol. Cell. Biol. 2005, 25, 10611–10627. [Google Scholar] [CrossRef] [Green Version]
- Suleyman, G.; Alangaden, G.J. Nosocomial Fungal Infections: Epidemiology, Infection Control, and Prevention. Infect. Dis. Clin. N. Am. 2016, 30, 1023–1052. [Google Scholar] [CrossRef] [PubMed]
- Sanglard, D.; Odds, F.C. Resistance of Candida species to antifungal agents: Molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2002, 2, 73–85. [Google Scholar] [CrossRef]
- Casadevall, A.; Kontoyiannis, D.P.; Robert, V. On the Emergence of Candida auris: Climate Change, Azoles, Swamps, and Birds. MBio 2019, 10, e01397-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Douglas, C.M. Fungal β(1,3)-D-glucan synthesis. Med. Mycol. 2001, 39 (Suppl. 1), 55–66. [Google Scholar] [CrossRef]
- Mora-Duarte, J.; Betts, R.; Rotstein, C.; Colombo, A.L.; Thompson-Moya, L.; Smietana, J.; Lupinacci, R.; Sable, C.; Kartsonis, N.; Perfect, J.; et al. Comparison of caspofungin and Amphotericin B for invasive candidiasis. N. Engl. J. Med. 2002, 347, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Echinocandin antifungal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Morrison, V.A. Echinocandin antifungals: Review and update. Expert Rev. Anti-Infect. Ther. 2006, 4, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Sokol-Anderson, M.L.; Sligh, J.E., Jr.; Elberg, S.; Brajtburg, J.; Kobayashi, G.S.; Medoff, G. Role of cell defense against oxidative damage in the resistance of Candida albicans to the killing effect of amphotericin B. Antimicrob. Agents Chemother. 1988, 32, 702–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, B.; Cheng, S.; Clancy, C.J.; Nguyen, M.H. Caspofungin kills Candida albicans by causing both cellular apoptosis and necrosis. Antimicrob. Agents Chemother. 2013, 57, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satish, S.; Jimenez-Ortigosa, C.; Zhao, Y.; Lee, M.H.; Dolgov, E.; Kruger, T.; Park, S.; Denning, D.W.; Kniemeyer, O.; Brakhage, A.A.; et al. Stress-Induced Changes in the Lipid Microenvironment of β-(1,3)-d-Glucan Synthase Cause Clinically Important Echinocandin Resistance in Aspergillus fumigatus. mBio 2019, 10, e00779-19. [Google Scholar] [CrossRef] [Green Version]
- Sumiyoshi, M.; Miyazaki, T.; Makau, J.N.; Mizuta, S.; Tanaka, Y.; Ishikawa, T.; Makimura, K.; Hirayama, T.; Takazono, T.; Saijo, T.; et al. Novel and potent antimicrobial effects of caspofungin on drug-resistant Candida and bacteria. Sci. Rep. 2020, 10, 17745. [Google Scholar] [CrossRef]
- Peter, T.; Bissinger, R.; Signoretto, E.; Mack, A.F.; Lang, F. Micafungin-Induced Suicidal Erythrocyte Death. Cell. Physiol. Biochem. 2016, 39, 584–595. [Google Scholar] [CrossRef]
- Alonso-Monge, R.; Navarro-García, F.; Molero, G.; Díez-Orejas, R.; Gustin, M.; Pla, J.; Sánchez, M.; Nombela, C. Role of the mitogen-activated protein kinase Hog1p in morphogenesis and virulence of Candida albicans. J. Bacteriol. 1999, 181, 3058–3068. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; De Micheli, M.; Coleman, S.T.; Sanglard, D.; Moye-Rowley, W.S. Analysis of the oxidative stress regulation of the Candida albicans transcription factor, Cap1p. Mol. Microbiol. 2000, 36, 618–629. [Google Scholar] [CrossRef]
- Urrialde, V.; Alburquerque, B.; Guirao-Abad, J.P.; Pla, J.; Arguelles, J.C.; Alonso-Monge, R. Arsenic inorganic compounds cause oxidative stress mediated by the transcription factor PHO4 in Candida albicans. Microbiol. Res. 2017, 203, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Morales-Menchen, A.; Navarro-Garcia, F.; Guirao-Abad, J.P.; Roman, E.; Prieto, D.; Coman, I.V.; Pla, J.; Alonso-Monge, R. Non-canonical Activities of Hog1 Control Sensitivity of Candida albicans to Killer Toxins From Debaryomyces hansenii. Front. Cell Infect. Microbiol. 2018, 8, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Primer’s Name | Sequence |
|---|---|---|
| ACT1 | o-ACTQTup | TGGTGGTTCTATCTTGGCTTCA |
| o-ACTQTlw | ATCCACATTTGTTGGAAAGTAGA | |
| CAT1 | o-CAT1up-QT | ATCCCAGTGAACTGTCCTGTCA |
| o-CAT1lw-QT | ACCATTAACAGTCATAGCACCATCTCT | |
| SOD2 | o-SOD2up-QT | TGCTTCCAAGACTTTCACTAGATCTAA |
| o-SOD2lw-QT | TGGTTCAGTAGCGGAGAATTCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Monge, R.; Guirao-Abad, J.P.; Sánchez-Fresneda, R.; Pla, J.; Yagüe, G.; Argüelles, J.C. The Fungicidal Action of Micafungin is Independent on Both Oxidative Stress Generation and HOG Pathway Signaling in Candida albicans. Microorganisms 2020, 8, 1867. https://doi.org/10.3390/microorganisms8121867
Alonso-Monge R, Guirao-Abad JP, Sánchez-Fresneda R, Pla J, Yagüe G, Argüelles JC. The Fungicidal Action of Micafungin is Independent on Both Oxidative Stress Generation and HOG Pathway Signaling in Candida albicans. Microorganisms. 2020; 8(12):1867. https://doi.org/10.3390/microorganisms8121867
Chicago/Turabian StyleAlonso-Monge, Rebeca, José P. Guirao-Abad, Ruth Sánchez-Fresneda, Jesús Pla, Genoveva Yagüe, and Juan Carlos Argüelles. 2020. "The Fungicidal Action of Micafungin is Independent on Both Oxidative Stress Generation and HOG Pathway Signaling in Candida albicans" Microorganisms 8, no. 12: 1867. https://doi.org/10.3390/microorganisms8121867





