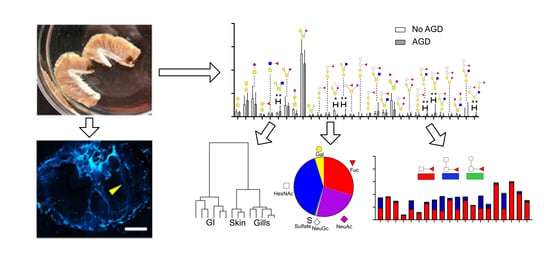
Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon
Abstract
1. Introduction
2. Materials and Methods
2.1. Swedish Freshwater Atlantic Salmon
2.2. Australian Marine Atlantic Salmon
2.3. Mucin Extraction
2.4. O-glycan Analysis by Liquid-Chromatography–Mass Spectrometry (LC-MS)
2.5. Fucosidase Treatment
2.6. Ex Vivo Imaging
2.7. Morphology and Histology
2.8. Statistics
3. Results
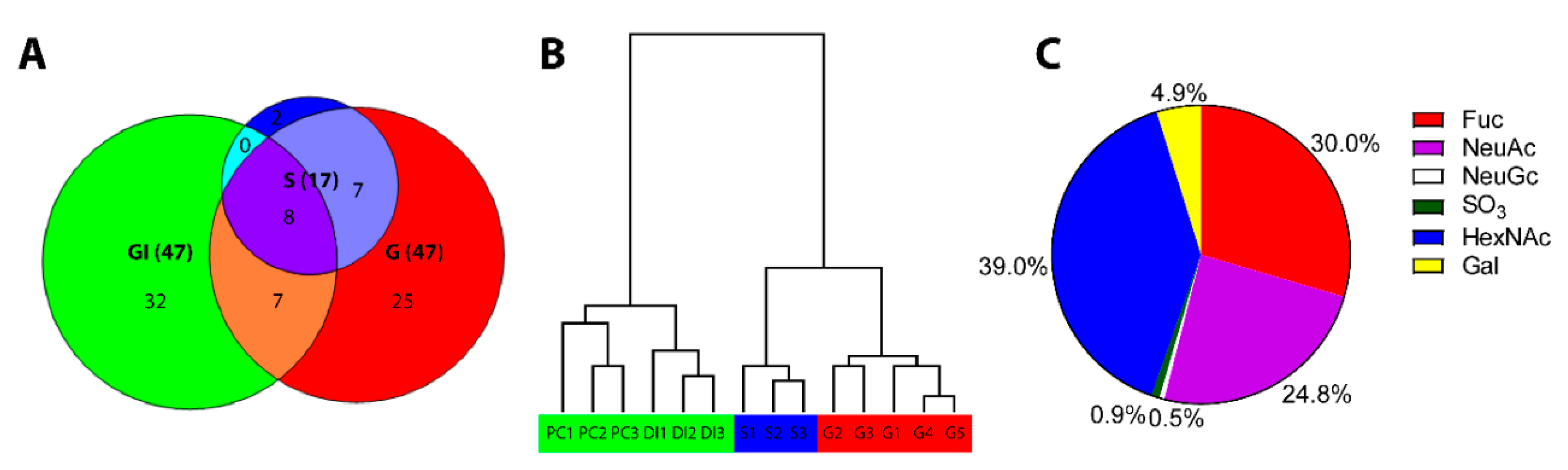
3.1. ATS-SE Gill Mucin Glycans Are More Fucosylated and Less Sialylated Compared to Skin and Intestinal Mucin Glycans
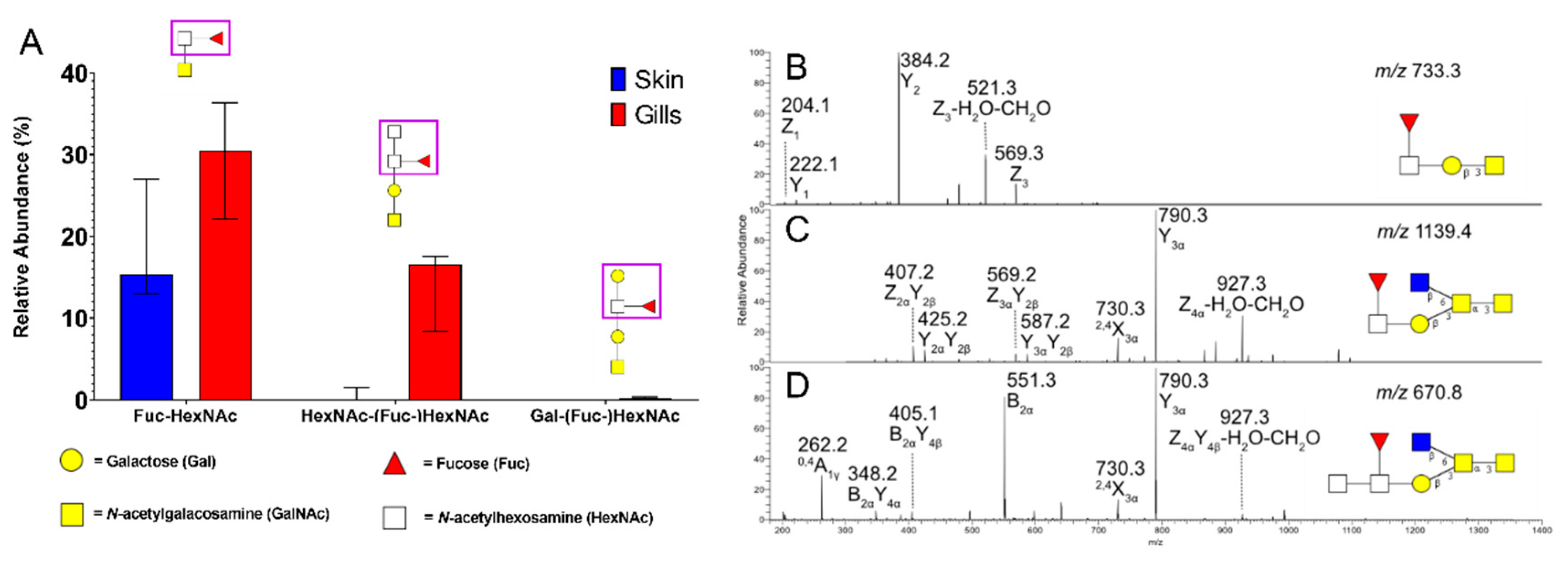
3.2. Gill Mucin O-glycans Carry Fucosylated Structures Similar to the Mammalian Histo-Blood-Group Antigens
3.3. Fucosylated Mucins Appear to Coat Sialylated Mucin Strands in Gill Mucus
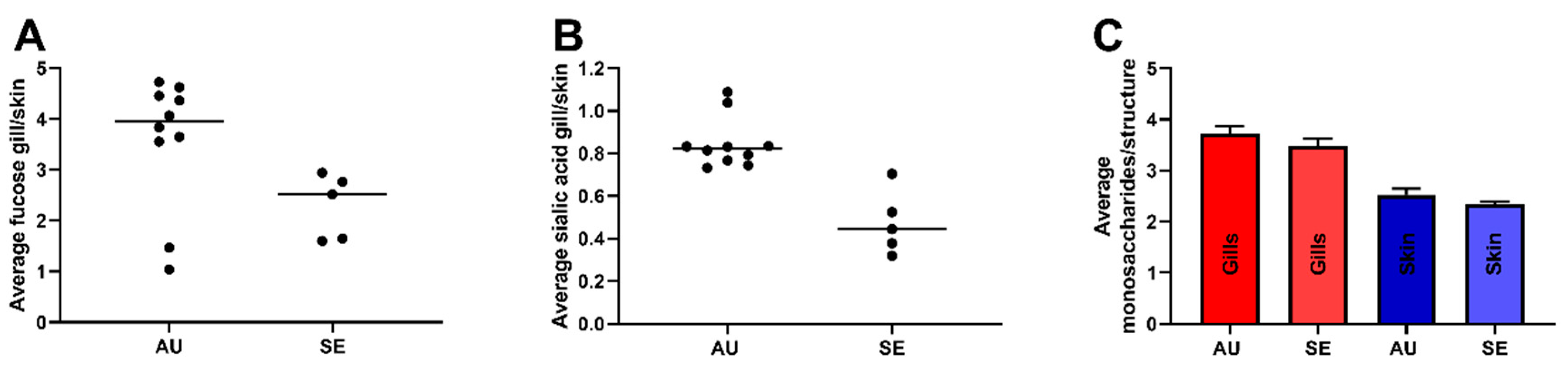
3.4. Gill O-Glycans were Larger, More Fucosylated and Less Sialylated Compared to Skin O-glycans both among ATS-SE and ATS-AU
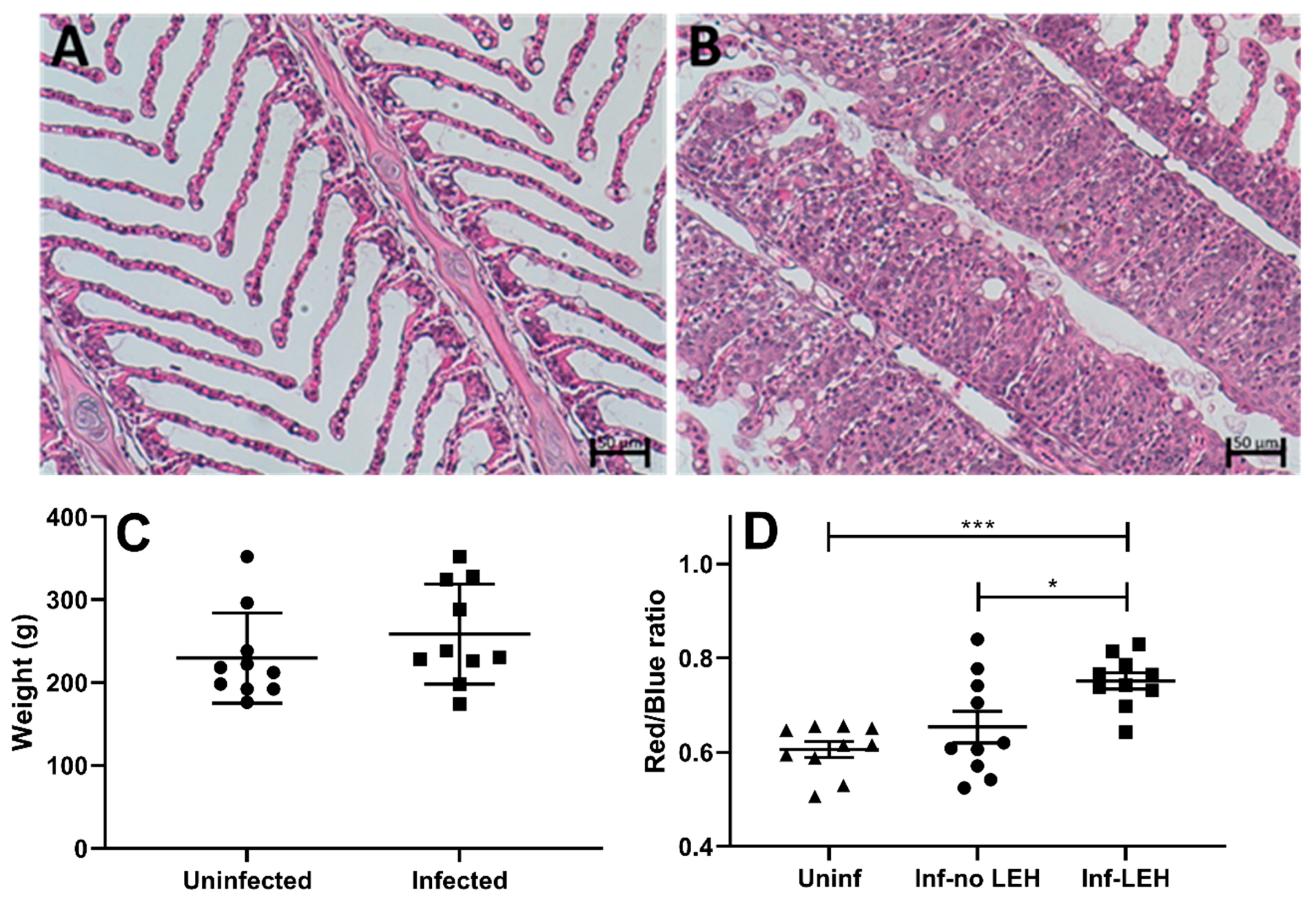
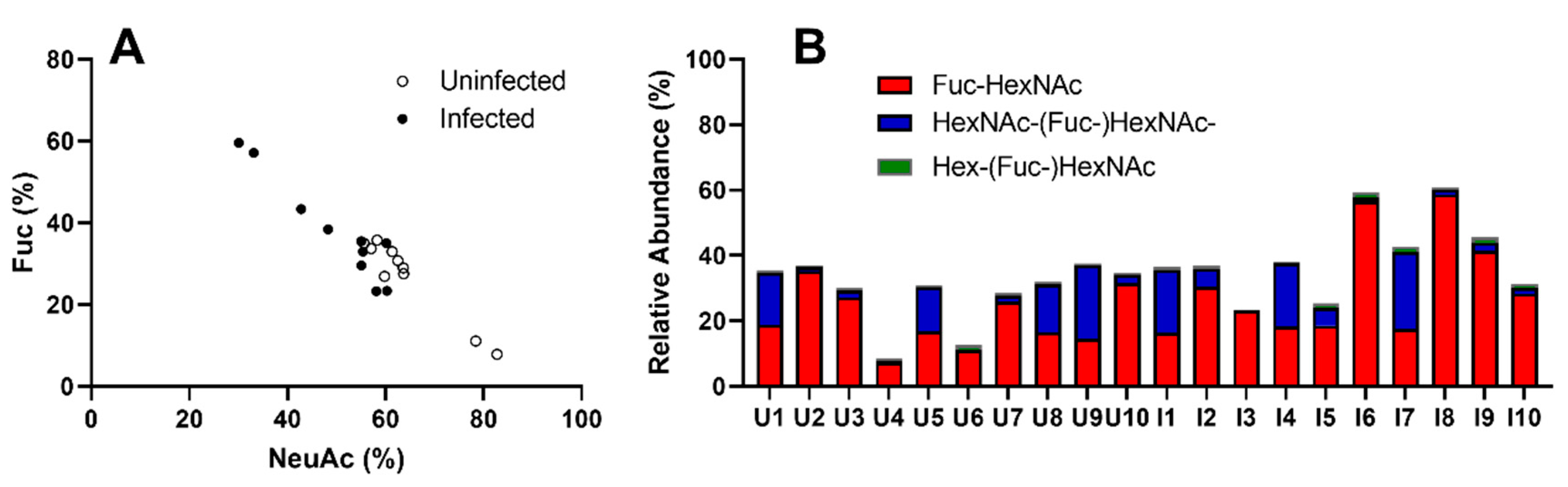
3.5. AGD Alters Gill Glycosylation in ATS-AU
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Quintana-Hayashi, M.P.; Padra, M.; Padra, J.T.; Benktander, J.; Linden, S.K. Mucus-Pathogen Interactions in the Gastrointestinal Tract of Farmed Animals. Microorganisms 2018, 6, 55. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.A.; Linden, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Benktander, J.; Venkatakrishnan, V.; Padra, J.T.; Sundh, H.; Sundell, K.; Murughan, A.V.M.; Maynard, B.; Linden, S.K. Effects of size and geographical origin on Atlantic salmon, Salmo salar, mucin O-glycan repertoire. Mol. Cell. Proteom. 2019, 18, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Padra, J.T.; Sundell, K.; Sundh, H.; Karlsson, N.G.; Linden, S.K. Atlantic Salmon Carries a Range of Novel O-Glycan Structures Differentially Localized on Skin and Intestinal Mucins. J. Proteome Res. 2015, 14, 3239–3251. [Google Scholar] [CrossRef] [PubMed]
- Padra, M.; Adamczyk, B.; Flahou, B.; Erhardsson, M.; Chahal, G.; Smet, A.; Jin, C.; Thorell, A.; Ducatelle, R.; Haesebrouck, F.; et al. Helicobacter suis infection alters glycosylation and decreases the pathogen growth inhibiting effect and binding avidity of gastric mucins. Mucosal Immunol. 2019, 12, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, V.; Quintana-Hayashi, M.P.; Mahu, M.; Haesebrouck, F.; Pasmans, F.; Linden, S.K. Brachyspira hyodysenteriae Infection Regulates Mucin Glycosylation Synthesis Inducing an Increased Expression of Core-2 O-Glycans in Porcine Colon. J. Proteome Res. 2017, 16, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Kenny, D.T.; Skoog, E.C.; Padra, M.; Adamczyk, B.; Vitizeva, V.; Thorell, A.; Venkatakrishnan, V.; Linden, S.K.; Karlsson, N.G. Structural diversity of human gastric mucin glycans. Mol. Cell. Proteom. 2017, 16, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Venkatakrishnan, V.; Padra, J.T.; Sundh, H.; Sundell, K.; Jin, C.; Langeland, M.; Carlberg, H.; Vidakovic, A.; Lundh, T.; Karlsson, N.G.; et al. Exploring the Arctic charr intestinal glycome: Evidence for increased N-glycolylneuraminic acid levels and changed host-pathogen interactions in response to inflammation. J. Proteome Res. 2019, 18, 1760–1773. [Google Scholar] [CrossRef] [PubMed]
- Padra, J.T.; Sundh, H.; Sundell, K.; Venkatakrishnan, V.; Jin, C.; Samuelsson, T.; Karlsson, N.G.; Linden, S.K. Aeromonas salmonicida growth in response to Atlantic salmon mucins differ between epithelial sites, is governed by sialylated and N-acetylhexosamine containing O-glycans and is affected by Ca2. Infect. Immun. 2017, 85. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, P.B.; Bridle, A.R.; Cadoret, K.; Nowak, B.F. In vitro cultured Neoparamoeba perurans causes amoebic gill disease in Atlantic salmon and fulfils Koch’s postulates. Int. J. Parasitol. 2012, 42, 511–515. [Google Scholar] [CrossRef]
- Oldham, T.; Rodger, H.; Nowak, B.F. Incidence and distribution of amoebic gill disease (AGD)–An epidemiological review. Aquaculture 2016, 457, 35–42. [Google Scholar] [CrossRef]
- Ruane, N.; Jones, S. Amoebic gill disease (AGD) of farmed Atlantic salmon (Salmo salar L.). In ICES Identification Leaflets for Diseases and Parasites of Fish and Shellfish; ICES: Copenhagen, Denmark, 2013. [Google Scholar]
- Munday, B.L.; Zilberg, D.; Findlay, V. Gill disease of marine fish caused by infection with Neoparamoeba pemaquidensis. J. Fish Dis. 2002, 24, 497–507. [Google Scholar] [CrossRef]
- Adams, M.B.; Nowak, B.F. Amoebic gill disease: Sequential pathology in cultured Atlantic salmon, Salmo salar L. J. Fish Dis. 2003, 26, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.D.; Powell, M.D. The viscosity and glycoprotein biochemistry of salmonid mucus varies with species, salinity and the presence of amoebic gill disease. J. Comp. Physiol. B 2005, 175, 1–11. [Google Scholar] [CrossRef]
- Taylor, R.S.; Slinger, J.; Camargo Lima, P.; English, C.J.; Maynard, B.T.; Samsing, F.; McCulloch, R.; Quezada-Rodriguez, P.R.; Wynne, J.W. Evaluation of sodium percarbonate as a bath treatment for amoebic gill disease in Atlantic salmon. Aquac. Res. 2020. [Google Scholar] [CrossRef]
- Taylor, R.S.; Muller, W.J.; Cook, M.T.; Kube, P.D.; Elliott, N.G. Gill observations in Atlantic salmon (Salmo salar, L.) during repeated amoebic gill disease (AGD) field exposure and survival challenge. Aquaculture 2009, 290, 1–8. [Google Scholar] [CrossRef]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef]
- Hayes, C.A.; Karlsson, N.G.; Struwe, W.B.; Lisacek, F.; Rudd, P.M.; Packer, N.H.; Campbell, M.P. UniCarb-DB: A database resource for glycomic discovery. Bioinformatics 2011, 27, 1343–1344. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Schulz, B.L.; Packer, N.H. Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MSn. J. Am. Soc. Mass Spectrom. 2004, 15, 659–672. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef]
- Wong-Madden, S.T.; Landry, D. Purification and characterization of novel glycosidases from the bacterial genus Xanthomonas. Glycobiology 1995, 5, 19–28. [Google Scholar] [CrossRef] [PubMed]
- de Hoon, M.J.; Imoto, S.; Nolan, J.; Miyano, S. Open source clustering software. Bioinformatics 2004, 20, 1453–1454. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, N.; Vanbeselaere, J.; Chang, L.-Y.; Yu, S.-Y.; Ducrocq, L.; Harduin-Lepers, A.; Kurata, J.; Aoki-Kinoshita, K.F.; Sato, C.; Khoo, K.-H.; et al. Systems glycomics of adult zebrafish identifies organ-specific sialylation and glycosylation patterns. Nat. Commun. 2018, 9, 4647. [Google Scholar] [CrossRef] [PubMed]
- Sharba, S.; Navabi, N.; Padra, M.; Persson, J.A.; Quintana-Hayashi, M.P.; Gustafsson, J.K.; Szeponik, L.; Venkatakrishnan, V.; Sjoling, A.; Nilsson, S.; et al. Interleukin 4 induces rapid mucin transport, increases mucus thickness and quality and decreases colitis and Citrobacter rodentium in contact with epithelial cells. Virulence 2019, 10, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Johansson, M.E.; Hansson, G.C. Microbiology. Keeping bacteria at a distance. Science 2011, 334, 182–183. [Google Scholar] [CrossRef]
- Fujino, K.; Kazama, T.K. The Y System of Skipjack Tuna Blood Groups. Vox Sang. 1968, 14, 383–395. [Google Scholar] [CrossRef]
- Sanders, B.G.; Wright, J.E. Immunogenetic studies in two trout species of the genus Salmo. Ann. N. Y. Acad. Sci. 1962, 97, 116–130. [Google Scholar] [CrossRef]
- Padra, M.; Adamczyk, B.; Benktander, J.; Flahou, B.; Skoog, E.C.; Padra, J.T.; Smet, A.; Jin, C.; Ducatelle, R.; Samuelsson, T.; et al. Helicobacter suis binding to carbohydrates on human and porcine gastric mucins and glycolipids occurs via two modes. Virulence 2018, 9, 898–918. [Google Scholar] [CrossRef]
- Bugaytsova, J.A.; Bjornham, O.; Chernov, Y.A.; Gideonsson, P.; Henriksson, S.; Mendez, M.; Sjostrom, R.; Mahdavi, J.; Shevtsova, A.; Ilver, D.; et al. Helicobacter pylori Adapts to Chronic Infection and Gastric Disease via pH-Responsive BabA-Mediated Adherence. Cell Host Microbe 2017, 21, 376–389. [Google Scholar] [CrossRef]
- Morikis, V.A.; Chase, S.; Wun, T.; Chaikof, E.L.; Magnani, J.L.; Simon, S.I. Selectin catch-bonds mechanotransduce integrin activation and neutrophil arrest on inflamed endothelium under shear flow. Blood 2017, 130, 2101–2110. [Google Scholar] [CrossRef]
- Valdenegro-Vega, V.A.; Crosbie, P.; Bridle, A.; Leef, M.; Wilson, R.; Nowak, B.F. Differentially expressed proteins in gill and skin mucus of Atlantic salmon (Salmo salar) affected by amoebic gill disease. Fish Shellfish Immunol. 2014, 40, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sheng, Y.H.; Every, A.L.; Miles, K.M.; Skoog, E.C.; Florin, T.H.; Sutton, P.; McGuckin, M.A. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009, 5, e1000617. [Google Scholar] [CrossRef] [PubMed]
- Skoog, E.C.; Padra, M.; Aberg, A.; Gideonsson, P.; Obi, I.; Quintana-Hayashi, M.P.; Arnqvist, A.; Linden, S.K. BabA dependent binding of Helicobacter pylori to human gastric mucins cause aggregation that inhibits proliferation and is regulated via ArsS. Sci. Rep. 2017, 7, 40656. [Google Scholar] [CrossRef] [PubMed]
- Skoog, E.C.; Sjoling, A.; Navabi, N.; Holgersson, J.; Lundin, S.B.; Linden, S.K. Human Gastric Mucins Differently Regulate Helicobacter pylori Proliferation, Gene Expression and Interactions with Host Cells. PLoS ONE 2012, 7, e36378. [Google Scholar] [CrossRef]
- Pickard, J.M.; Chervonsky, A.V. Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015, 194, 5588–5593. [Google Scholar] [CrossRef]
- Ermund, A.; Meiss, L.N.; Rodriguez-Pineiro, A.M.; Bahr, A.; Nilsson, H.E.; Trillo-Muyo, S.; Ridley, C.; Thornton, D.J.; Wine, J.J.; Hebert, H.; et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem. Biophys. Res. Commun. 2017, 492, 331–337. [Google Scholar] [CrossRef]
- Marcos-López, M.; Calduch-Giner, J.A.; Mirimin, L.; MacCarthy, E.; Rodger, H.D.; O’Connor, I.; Sitjà-Bobadilla, A.; Pérez-Sánchez, J.; Piazzon, M.C. Gene expression analysis of Atlantic salmon gills reveals mucin 5 and interleukin 4/13 as key molecules during amoebic gill disease. Sci. Rep. 2018, 8, 13689. [Google Scholar] [CrossRef]
- Sharba, S.; Venkatakrishnan, V.; Padra, M.; Winther, M.; Gabl, M.; Sundqvist, M.; Wang, J.; Forsman, H.; Linden, S.K. Formyl peptide receptor 2 orchestrates mucosal protection against Citrobacter rodentium infection. Virulence 2019, 10, 610–624. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Linden, S.K.; Alwan, A.H.; Scholte, B.J.; Hansson, G.C.; Sjovall, H. Carbachol-induced colonic mucus formation requires transport via NKCC1, K channels and CFTR. Pflügers Arch.-Eur. J. Physiol. 2014, 467, 1403–1415. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Navabi, N.; Rodriguez-Pineiro, A.M.; Alomran, A.H.; Premaratne, P.; Fernandez, H.R.; Banerjee, D.; Sjovall, H.; Hansson, G.C.; Linden, S.K. Dynamic changes in mucus thickness and ion secretion during Citrobacter rodentium infection and clearance. PLoS ONE 2013, 8, e84430. [Google Scholar] [CrossRef]
- Roberts, S.D.; Powell, M.D. Oral L-cysteine ethyl ester (LCEE) reduces amoebic gill disease (AGD) in Atlantic salmon Salmo salar. Dis. Aquat. Organ. 2005, 66, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Powell, M.D.; Ransome, J.; Barney, M.; Duijf, R.M.M.; Flik, G. Effect of Dietary Inclusion of N-Acetyl Cysteine on Mucus Viscosity and Susceptibility of Rainbow Trout, Oncorhynchus mykiss, and Atlantic Salmon, Salmo salar, to Amoebic Gill Disease. J. World Aquac. Soc. 2007, 38, 435–442. [Google Scholar] [CrossRef]
- Huynh, H.Q.; Couper, R.T.; Tran, C.D.; Moore, L.; Kelso, R.; Butler, R.N. N-acetylcysteine, a novel treatment for Helicobacter pylori infection. Dig. Dis. Sci. 2004, 49, 1853–1861. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benktander, J.; Padra, J.T.; Maynard, B.; Birchenough, G.; Botwright, N.A.; McCulloch, R.; Wynne, J.W.; Sharba, S.; Sundell, K.; Sundh, H.; et al. Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon. Microorganisms 2020, 8, 1871. https://doi.org/10.3390/microorganisms8121871
Benktander J, Padra JT, Maynard B, Birchenough G, Botwright NA, McCulloch R, Wynne JW, Sharba S, Sundell K, Sundh H, et al. Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon. Microorganisms. 2020; 8(12):1871. https://doi.org/10.3390/microorganisms8121871
Chicago/Turabian StyleBenktander, John, János T. Padra, Ben Maynard, George Birchenough, Natasha A. Botwright, Russel McCulloch, James W. Wynne, Sinan Sharba, Kristina Sundell, Henrik Sundh, and et al. 2020. "Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon" Microorganisms 8, no. 12: 1871. https://doi.org/10.3390/microorganisms8121871
APA StyleBenktander, J., Padra, J. T., Maynard, B., Birchenough, G., Botwright, N. A., McCulloch, R., Wynne, J. W., Sharba, S., Sundell, K., Sundh, H., & Lindén, S. K. (2020). Gill Mucus and Gill Mucin O-glycosylation in Healthy and Amebic Gill Disease-Affected Atlantic Salmon. Microorganisms, 8(12), 1871. https://doi.org/10.3390/microorganisms8121871