Alternative Extraction and Characterization of Nitrogen-Containing Azaphilone Red Pigments and Ergosterol Derivatives from the Marine-Derived Fungal Talaromyces sp. 30570 Strain with Industrial Relevance
Abstract
1. Introduction
2. Materials and Methods
2.1. Submerged Fermentation of Fungal Strain
2.2. Biomass Separation, Extraction and Quantification of the Polyketide-Based Pigments
2.3. HPLC-DAD Analysis
2.4. UHPLC-HR-ESI-MS Analyses
3. Results
3.1. Alternative Extraction and Characterization of Monascus-Like Azaphilone Pigments from the Marine-Derived Talaromyces sp. 30570 Strain
3.2. Influence of the Nutrients Profile on the Production of Monascus-Like Azaphilone Red Pigments by the Marine-Derived Talaromyces sp. 30570 Strain
4. Discussion
4.1. Efficiency and Selectivity of the Alternative Pressurized Liquid Extraction (PLE) of Azaphilone Red Pigments from the Mycelial Cells of the Marine-Derived Talaromyces sp. 30570
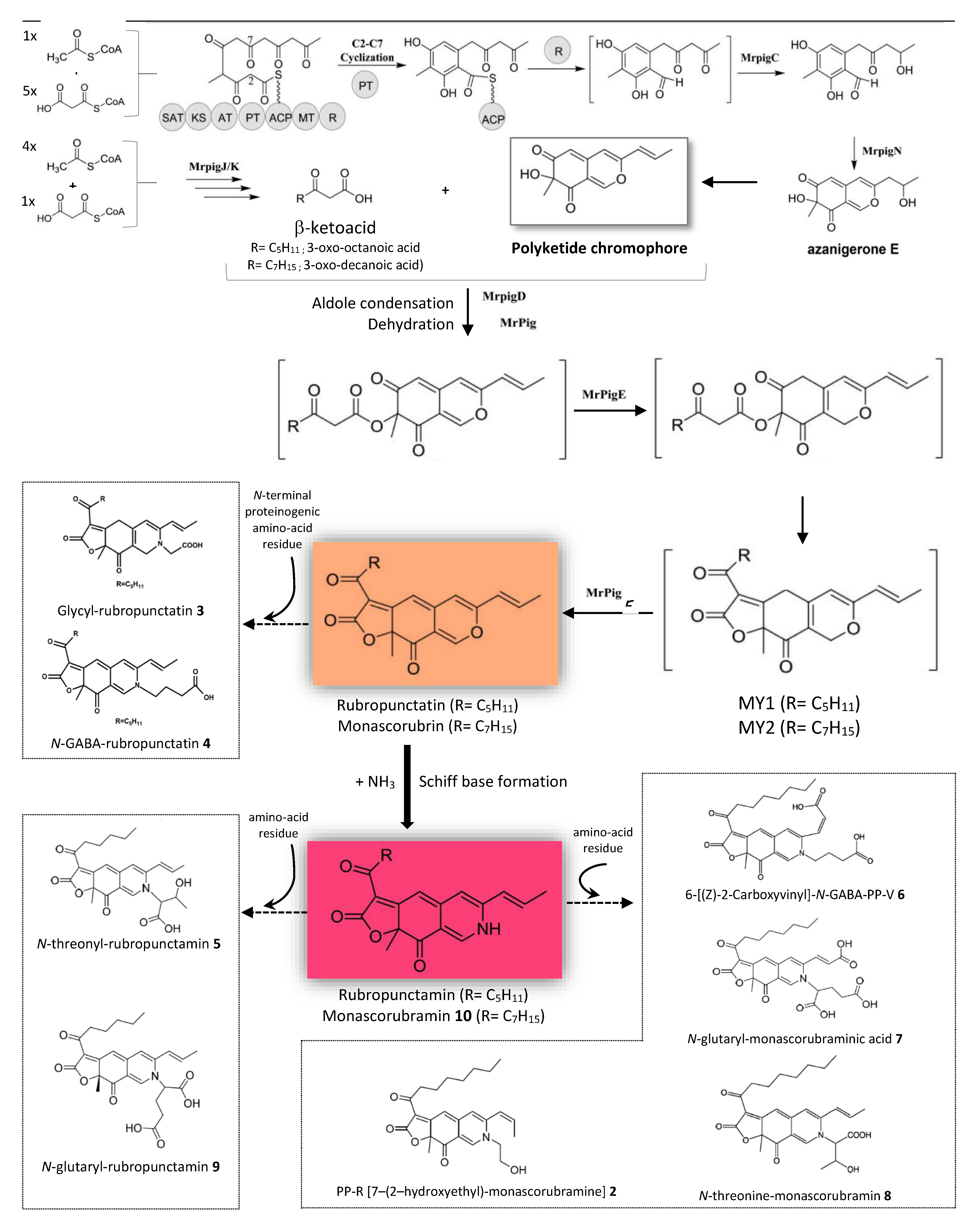
4.2. Putative Metabolic Pathway for the Production of Derivatives of Nitrogen-Containing Monascus-Like Azaphilone Red Pigments in the Marine-Derived Talaromyces sp. 30570
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed]
- Dufossé, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.S.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotech. 2014, 26, 56–61. [Google Scholar] [CrossRef]
- Akilandeswari, P.; Pradeep, B.V. Exploration of industrially important pigments from soil fungi. Appl. Microbiol. Biotechnol. 2016, 100, 1631–1643. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites. Reference Series in Phytochemistry; Merillon, J.M., Ramawat, K.G., Eds.; Springer: Cham, Switzerland, 2017; pp. 499–568. [Google Scholar]
- Fouillaud, M.; Venkatachalam, M.; Girard-Valenciennes, E.; Caro, Y.; Dufossé, L. Anthraquinones and derivatives from marine-derived fungi: Structural diversity and selected biological activities. Mar. Drugs 2016, 14, 64. [Google Scholar] [CrossRef]
- Caro, Y.; Anamale, L.; Fouillaud, M.; Laurent, P.; Petit, T.; Dufossé, L. Natural hydroxyanthraquinoid pigments as potent food grade colorants: An overview. Nat. Prod. Bioprospect. 2012, 2, 174–193. [Google Scholar] [CrossRef]
- Arikan, E.B.; Canli, O.; Caro, Y.; Dufossé, L.; Dizge, N. Production of bio-based pigments from food processing industry by-products (apple, pomegranate, black carrot, red beet pulps) using Aspergillus carbonarius. J. Fungi 2020, 6, 240. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.S.; Thrane, U.; Meyer, A.S. Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol. 2010, 28, 300–307. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef]
- Jannel, S.; Caro, Y.; Bermudes, M.; Petit, T. Novel insights into the biotechnological production of Haematococcus pluvialis-derived astaxanthin: Advances and key challenges to allow its industrial use as novel food ingredient. J. Mar. Sci. Eng. 2020, 8, 789. [Google Scholar] [CrossRef]
- Jůzlová, P.; Martínková, L.; Křen, V. Secondary metabolites of the fungus Monascus: A review. J. Ind. Microbiol. 1996, 16, 163–170. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Karki, S.; Chiu, S.H.; Kim, H.J.; Suh, J.W.; Nam, B.; Yoon, Y.-M.; Chen, C.-C.; Kwon, H.-J. Genetic localization and in vivo characterization of a Monascus azaphilone pigment biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2013, 97, 6337–6345. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, J.; Kato, J.; Oishi, K.; Fujimoto, Y. PP-R, 7-(2-hydroxyethyl)-monascorubramine, a red pigment produced in the mycelia of Penicillium sp. AZ. J. Biosci. Bioeng. 2001, 91, 44–47. [Google Scholar] [CrossRef]
- Mapari, S.A.; Meyer, A.S.; Thrane, U.; Frisvad, J.C. Identification of potentially safe promising fungal cell factories for the production of polyketide natural food colorants using chemotaxonomic rationale. Microb. Cell Fact. 2009, 8, 24. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PLoS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Woo, P.C.; Lam, C.-W.; Tam, E.W.T.; Lee, K.-C.; Yung, K.K.Y.; Leung, C.K.F.; Sze, K.-H.; Lau, S.K.P.; Yuen, K.-Y. The biosynthetic pathway for a thousand-year-old natural food colorant and citrinin in Penicillium marneffei. Sci. Rep. 2014, 4, 6728. [Google Scholar] [CrossRef]
- Gomes, D.C.; Takahashi, J.A. Sequential fungal fermentation-biotransformation process to produce a red pigment from sclerotiorin. Food Chem. 2016, 210, 355–361. [Google Scholar] [CrossRef]
- Morales-Oyervides, L.; Ruiz-Sánchez, J.P.; Oliveira, J.C.; Sousa-Gallagher, M.J.; Méndez-Zavala, A.; Giuffrida, D.; Dufossé, L.; Montañez, J. Biotechnological approaches for the production of natural colorants by Talaromyces/Penicillium: A review. Biotechnol. Adv. 2020, 43, 107601. [Google Scholar] [CrossRef]
- Chen, W.; Chen, R.; Liu, Q.; He, Y.; He, K.; Ding, X.; Kang, L.; Guo, X.; Xie, N.; Zhou, Y.; et al. Orange, red, yellow: Biosynthesis of azaphilone pigments in Monascus fungi. Chem. Sci. 2017, 8, 4917–4925. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Y.; Molnár, I.; Chen, F. Nature and nurture: Confluence of pathway determinism with metabolic and chemical serendipity diversifies: Monascus azaphilone pigments. Nat. Prod. Rep. 2019, 36, 561–572. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Huang, Y.; Xin, Q.; Wang, Z. Diversifying of chemical structure of native Monascus pigments. Front. Microbiol. 2018, 9, 3143. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.R.; Adhikari, N. An overview on common organic solvents and their toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Lebeau, J.; Venkatachalam, M.; Fouillaud, M.; Petit, T.; Vinale, F.; Dufossé, L.; Caro, Y. Production and new extraction method of polyketide red pigments produced by ascomycetous fungi from terrestrial and marine habitats. J. Fungi 2017, 3, 34. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Clerc, P.; Dufossé, L.; Caro, Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotechnol. Prog. 2019, 35, e2738. [Google Scholar] [CrossRef]
- Martins, F.S.; Borges, L.L.; Paula, J.R.; Conceição, E.C. Impact of different extraction methods on the quality of Dipteryx alata extracts. Rev. Bras. Farmacogn. 2013, 23, 521–526. [Google Scholar] [CrossRef]
- Fouillaud, M.; Venkatachalam, M.; Llorente, M.; Magalon, H.; Cuet, P.; Dufossé, L. Biodiversity of pigmented fungi isolated from marine environment in La Réunion island, Indian Ocean: New resources for colored metabolites. J. Fungi 2017, 3, 36. [Google Scholar] [CrossRef]
- Klitgaard, A.; Iversen, A.; Andersen, M.R.; Larsen, T.O.; Frisvad, J.C.; Nielsen, K.F. Aggressive dereplication using UHPLC-DAD-QTOF: Screening extracts for up to 3000 fungal secondary metabolites. Anal. Bioanal. Chem. 2014, 406, 1933–1943. [Google Scholar] [CrossRef]
- Rasmussen, K.B. Talaromyces atroroseus: Genome Sequencing, Monascus Pigments and Azaphilone Gene Cluster Evolution. Ph.D. Thesis, Technical University of Danemark, Kongens Lyngby, Danemark, 2015. [Google Scholar]
- Izawa, S.; Harada, N.; Watanabe, T.; Kotokawa, N.; Yamamoto, A.; Hayatsu, H.; Arimoto-Kobayashi, S. Inhibitory effects of food-coloring agents derived from Monascus on the mutagenicity of heterocyclic amines. J. Agric. Food Chem. 1997, 45, 3980–3984. [Google Scholar] [CrossRef]
- Yuliana, A.; Singgih, M.; Julianti, E.; Blanc, P.J. Derivates of azaphilone Monascus pigments. Biocatal. Agric. Biotechnol. 2017, 9, 183–194. [Google Scholar] [CrossRef]
- Mukherjee, G.; Singh, S.K. Purification and characterization of a new red pigment from Monascus purpureus in submerged fermentation. Process. Biochem. 2011, 46, 188–192. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A.; et al. Partial characterization of the pigments produced by the marine-derived fungus Talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compos. Anal. 2018, 67, 38–47. [Google Scholar]
- Hajjaj, H.; Klaebe, A.; Loret, M.O.; Tzedakis, T.; Goma, G.; Blanc, P.J. Production and Identification of N-Glucosylrubropunctamine and N-Glucosylmonascorubramine from Monascus ruber and occurrence of electron donor-acceptor complexes in these red pigments. Appl. Environ. Microbiol. 1997, 63, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Kim, C.; Kim, K.; Shin, C.S. Color characteristics of Monascus pigments derived by fermentation with various amino acids. J. Agric. Food Chem. 2003, 51, 1302–1306. [Google Scholar] [CrossRef]
- Slominski, A.; Semak, I.; Zjawiony, J.; Wortsman, J.; Gandy, M.N.; Li, J.; Zbytek, B.; Li, W.; Tuckey, R.C. Enzymatic metabolism of ergosterol by cytochrome P450scc to biologically active 17α,24-dihydroxyergosterol. Chem. Biol. 2005, 12, 931–939. [Google Scholar] [CrossRef][Green Version]
- Dame, Z.T.; Silima, B.; Gryzenhout, M.; van Ree, T. Bioactive compounds from the endophytic fungus Fusarium proliferatum. Nat. Prod. Res. 2016, 30, 1301–1304. [Google Scholar] [CrossRef]
- Lebeau, J.; Petit, T.; Dufossé, L.; Caro, Y. Putative metabolic pathway for the bioproduction of bikaverin and intermediates thereof in the wild Fusarium oxysporum LCP531 strain. AMB Express 2019, 9, 186. [Google Scholar] [CrossRef]
- Ogihara, J.; Oishi, K. Effect of ammonium nitrate on the production of PP-V and monascorubrin homologues by Penicillium sp. AZ. J. Biosci. Bioeng. 2002, 93, 54–59. [Google Scholar] [CrossRef]
- Arai, T.; Umemura, S.; Ota, T.; Ogihara, J.; Kato, J.; Kasumi, T. Effects of inorganic nitrogen sources on the production of PP-V [(10Z)-12-carboxyl-monascorubramine] and the expression of the nitrate assimilation gene cluster by Penicillium sp. AZ, Biosci. Biotechnol. Biochem. 2012, 76, 120–124. [Google Scholar] [CrossRef][Green Version]
- Padmavathi, T.; Prabhudessai, T. A solid liquid state culture method to stimulate Monascus pigments by intervention of different substrates. Int. Res. J. Biol. Sci. 2013, 2, 22–29. [Google Scholar]
- Liew, F.; Martin, M.E.; Tappel, R.C.; Heijstra, B.D.; Mihalcea, C.; Köpke, M. Gas Fermentation—A flexible platform for commercial scale production of low-carbon-fuels and chemicals from waste and renewable feedstocks. Front. Microbiol. 2016, 7, 694. [Google Scholar] [CrossRef] [PubMed]
- Vega, G.C.; Sohn, J.; Voogt, J.; Nilsson, A.E.; Birkved, M.; Olsen, S.I. Insights from combining techno-economic and life cycle assessment—A case study of polyphenol extraction from red wine pomace. Resour. Conserv. Recycl. 2020, 100045. [Google Scholar] [CrossRef]
- Gao, J.-M.; Yang, S.-X.; Qin, J.-C. Azaphilones: Chemistry and biology. Chem. Rev. 2013, 113, 4755–4811. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tao, H.; Chen, W.; Yang, B.; Zhou, X.; Luo, X.; Liu, Y. Recent advances in the chemistry and biology of azaphilones. RSC Adv. 2020, 10, 10197–10220. [Google Scholar] [CrossRef]
- Tolborg, G.; Ødum, A.S.R.; Isbrandt, T.; Larsen, T.O.; Workman, M. Unique processes yielding pure azaphilones in Talaromyces atroroseus. Appl. Microbiol. Biotechnol. 2020, 104, 603–613. [Google Scholar] [CrossRef]
- Isbrandt, T.; Tolborg, G.; Ødum, A.; Workman, M.; Larsen, T.O. Atrorosins: A new subgroup of Monascus pigments from Talaromyces atroroseus. Appl. Microbiol. Biotechnol. 2020, 104, 615–622. [Google Scholar] [CrossRef]
- Peterson, S.W.; Jurjevic, Z. The Talaromyces pinophilus species complex. Fungal Biol. 2019, 123, 745–762. [Google Scholar] [CrossRef]
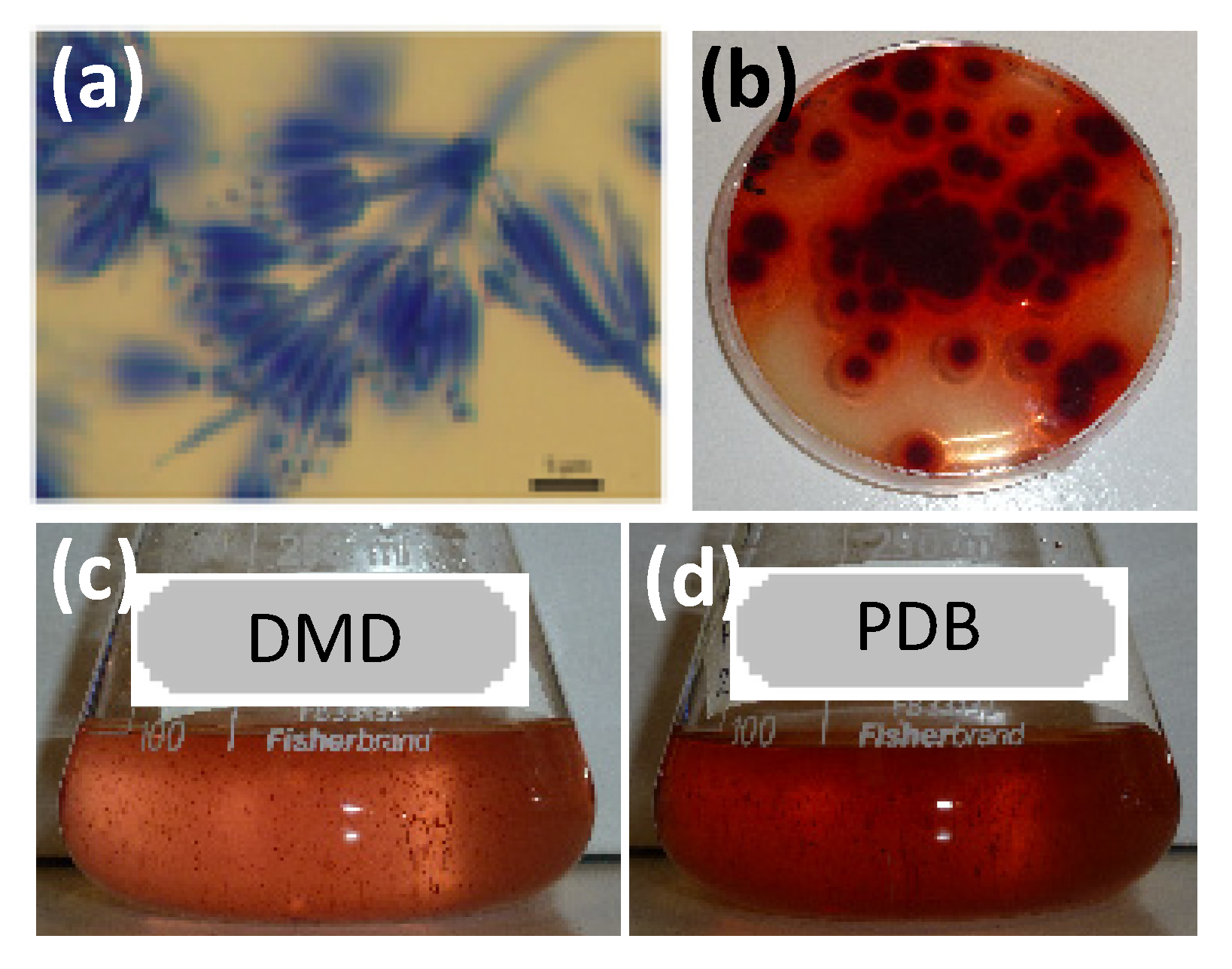
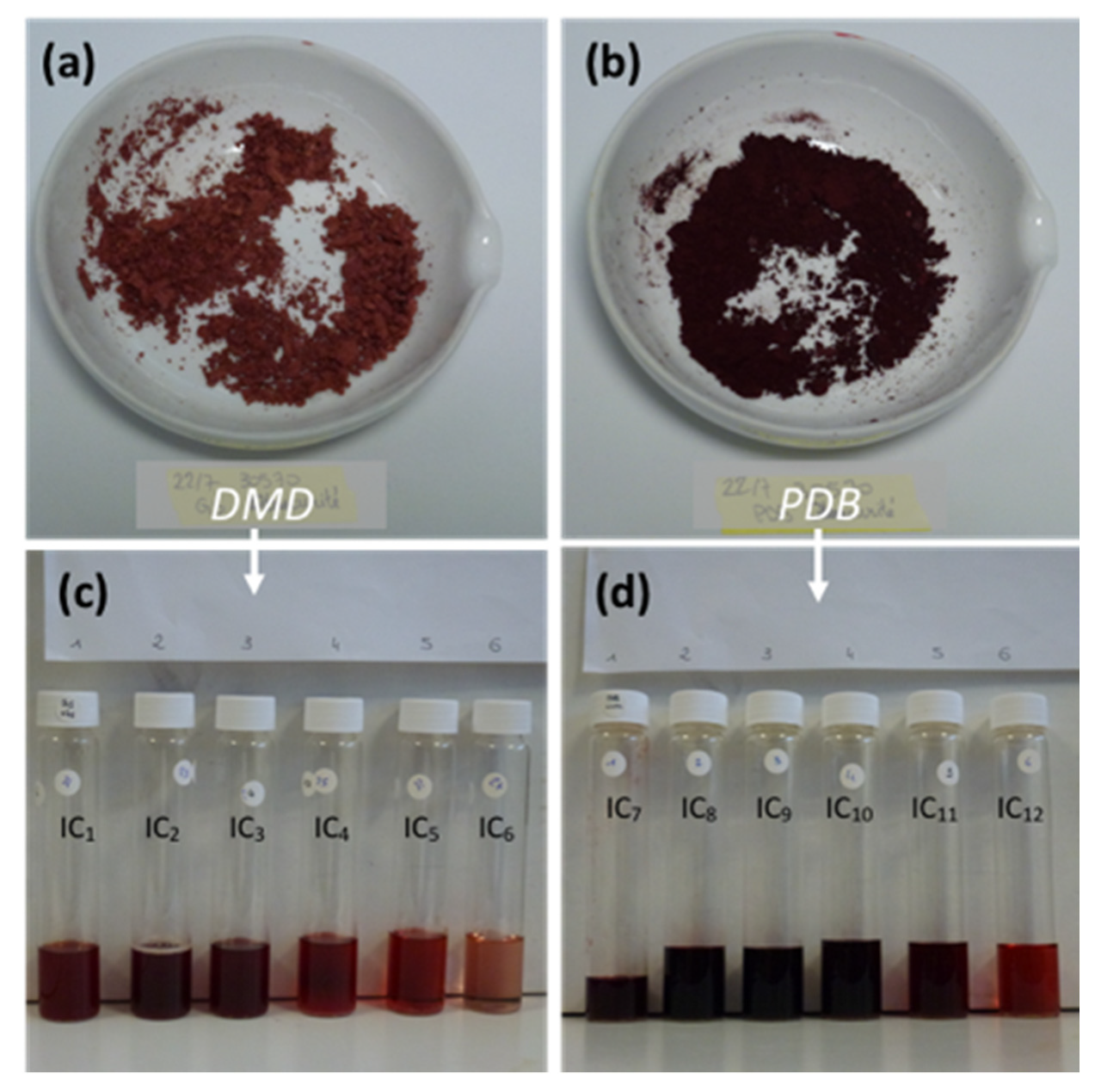
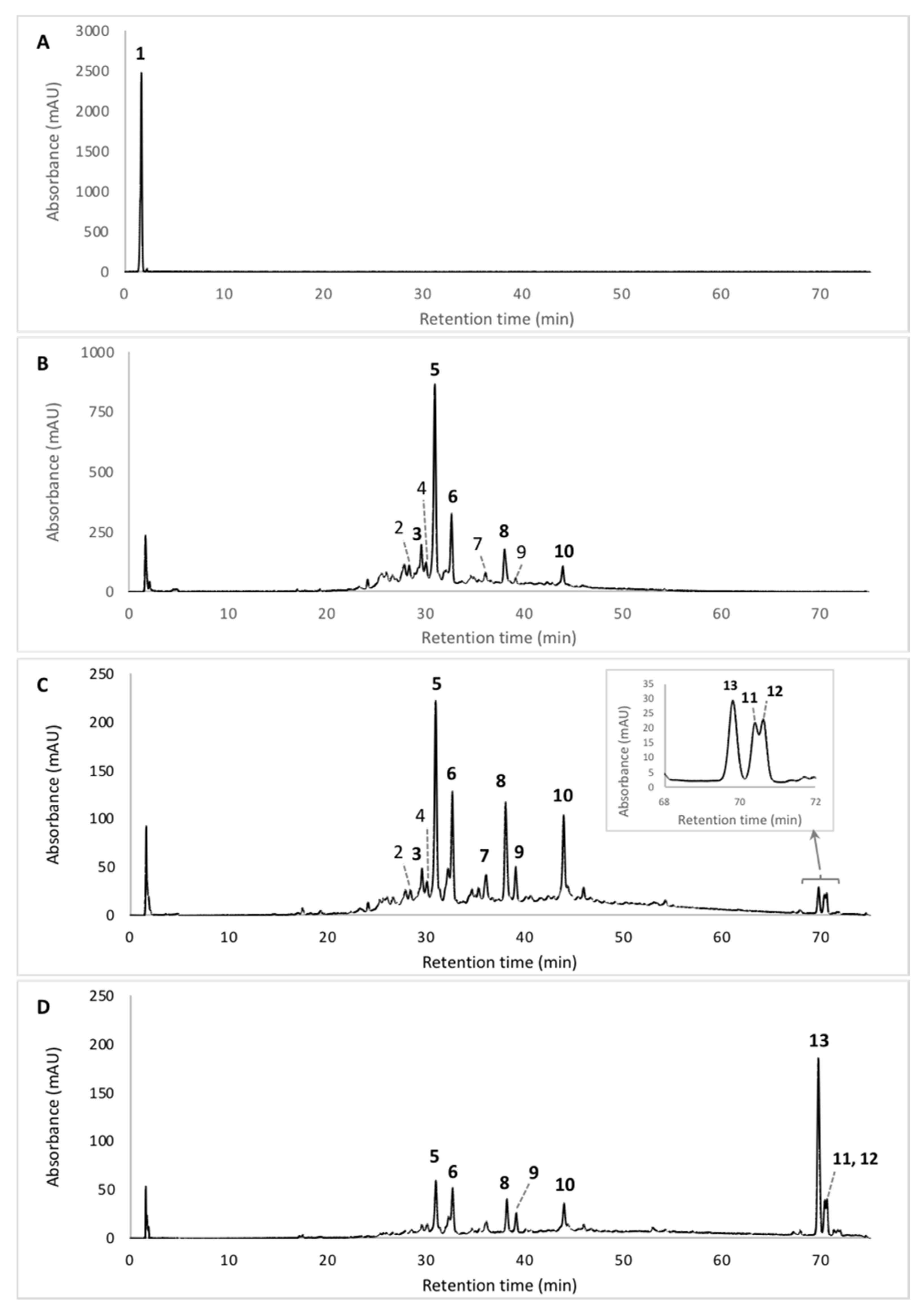

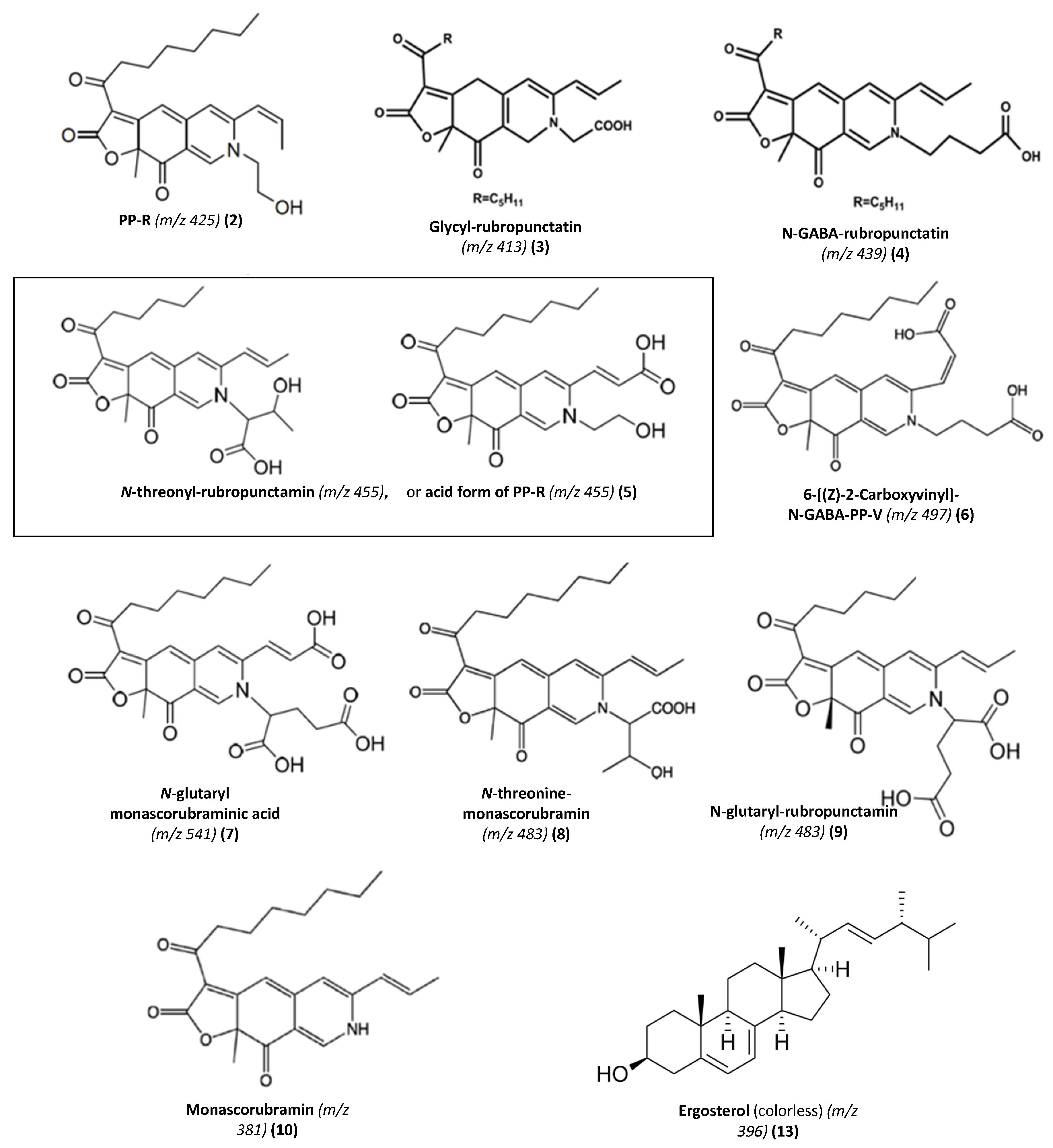
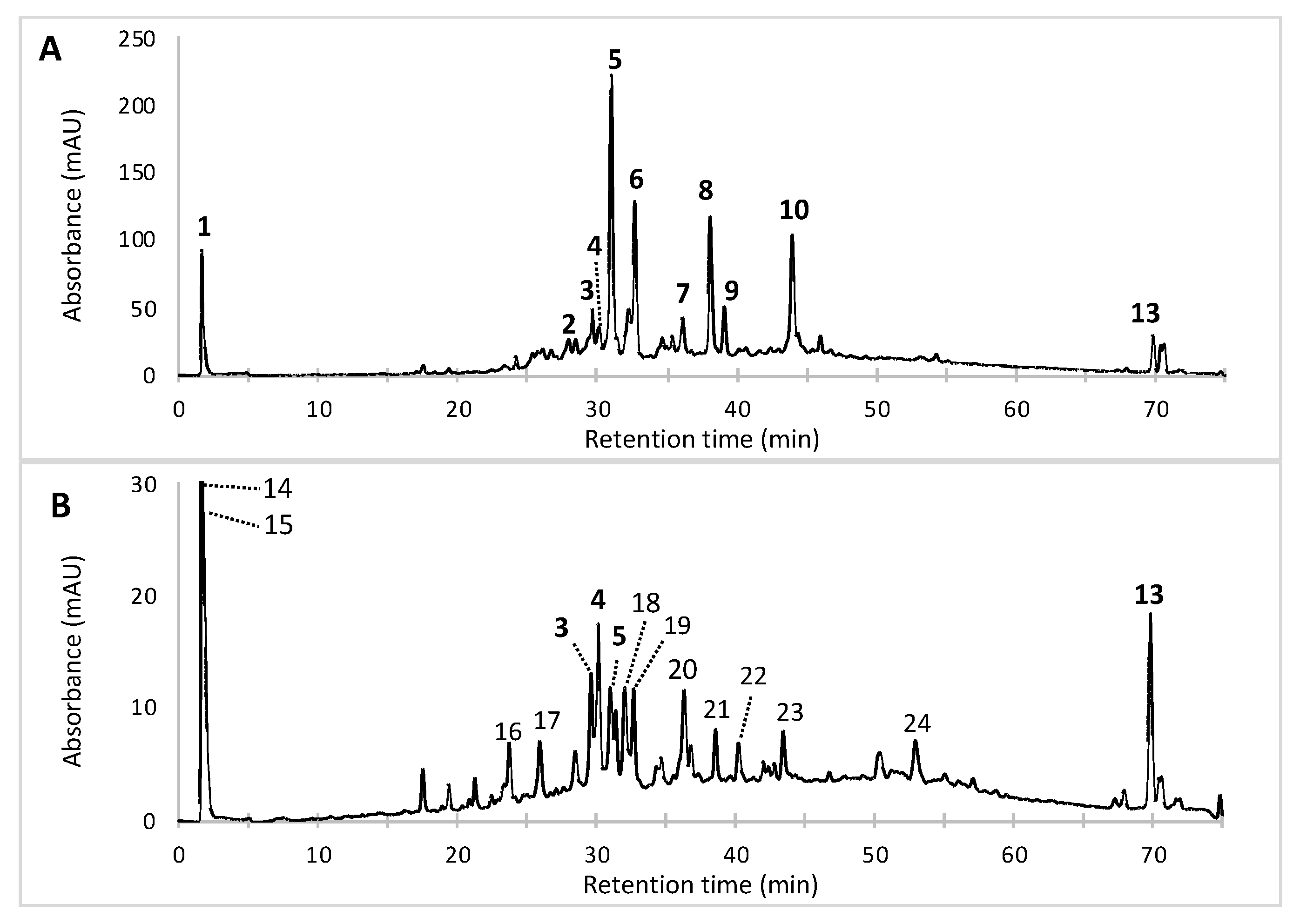
| No | Rt. (min) | UV-Vis λmax (nm) | Observed Peak HR-ESI MS (m/z) | Tentative Identification (Identified or Assumed Compounds) | Proposed Molecular Formula | Monoisotopic Mass in Da (Mass Error) (1) | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | 1.71 | 201, 216, 244, 276, 423, 514 | n.d. | Diglycoside derivative of a Monascus-like azaphilone red pigment (n.i.) | n.d. | n.d. | - |
| 2 | 28.52 | 192, 245, 274, 421, 515 | 488.1820 [M + CAN + Na]+ | PP-R [7-(2-hydroxyethyl)-monascorubramin] | C25H31NO5 | 425.22 (0.0380) | [14,15,16] |
| 3 | 29.60 | 193, 245, 274, 421, 518 | 416.1960 [M + H]+ | Glycyl-rubropunctatin | C23H27NO6 | 413.18 (2.0160) | [31,32,33] |
| 4 | 30.15 | 193, 245, 274, 426, 515 | 440.1936 [M + H]+ | N-GABA-rubropunctatin (GABA: γ-aminobutyric acid) | C25H29NO6 | 439.51 (0.3164) | [20] |
| 5 | 30.97 | 195, 245, 274, 424, 520 | 456.1543 [M + H]+ | N-threonyl-rubropunctamin (or acid form of PP-R) (presumed) | C25H29N07 | 455.20 (0.0457) | [25,30] |
| 6 | 32.66 | 193, 218, 250, 287, 424, 546 | 498.1665 [M + H]+ | 6-[(Z)-2-Carboxyvinyl]-N-GABA-PP-V | C27H31N08 | 497.54 (0.3735) | [28,34] |
| 7 | 36.11 | 196, 247, 288, 422, 522 | 542.1598 [M + H]+ | N-glutaryl-monascorubraminic acid | C28H31N010 | 541.20 (0.038) | [30] |
| 8 | 38.04 | 193, 246, 273, 426, 521 | 484.1910 [M+H]+ | N-threonine-monascorubramin | C27H33N07 | 483.55 (0.0402) | [34] |
| 9 | 39.10 | 193, 216, 250, 277, 426, 532 | 484.5110 [M + H]+ 546.1556 [M + CAN + Na]+ | N-glutaryl-rubropunctamin | C26H29N08 | 483.51 (0.0010) | [15,34,35,36] |
| 10 | 43.95 | 193, 245, 272, 424, 519 | 381.1198 [M + H]+ | Monascorubramin | C23H27NO4 | 381.19 (1.0702) | [15,16] |
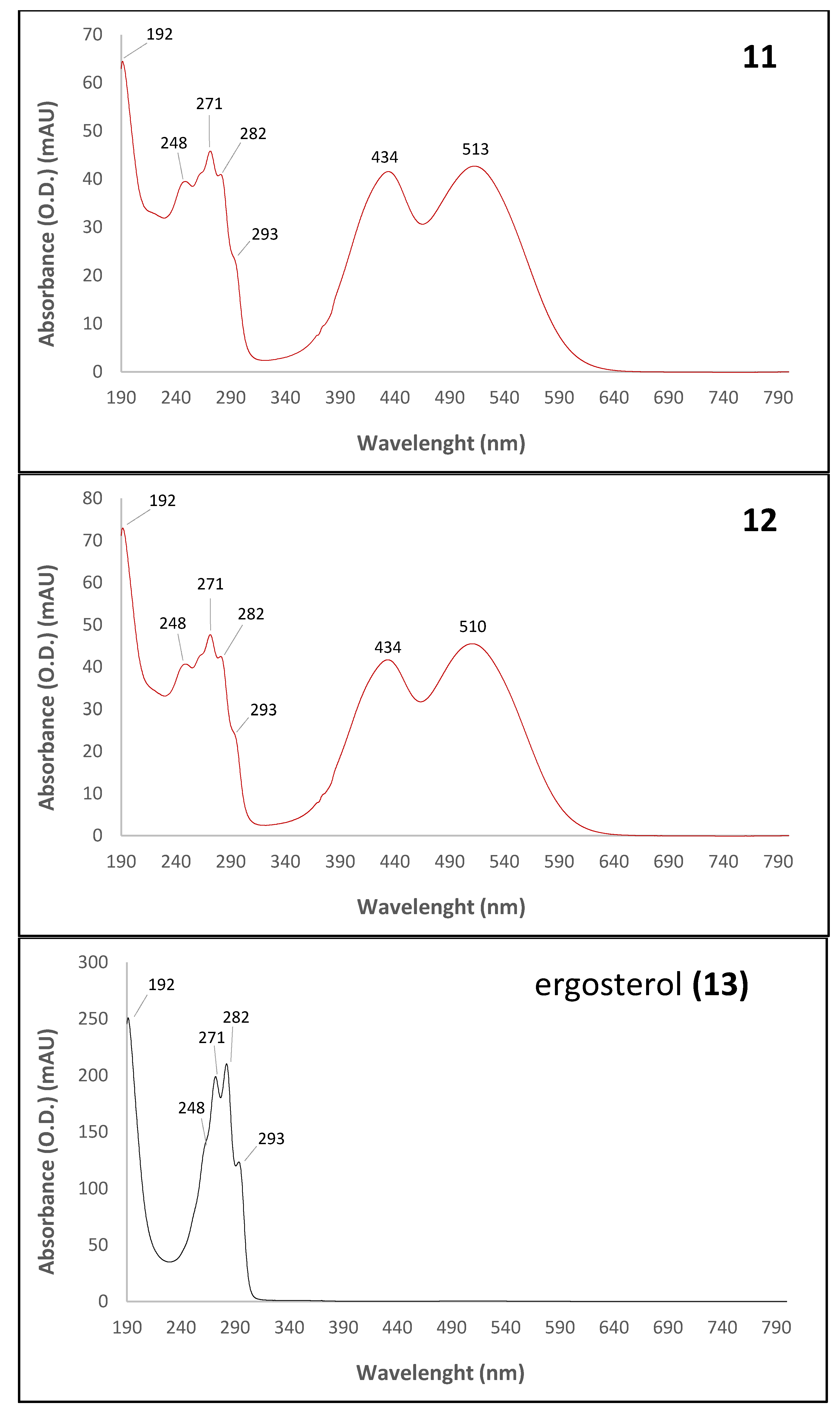
| 11 | 70.40 | 192, 248, 271, 282, 293, 434, 513 | n.d. | Derivative of a Monascus-like azaphilone red pigment (n.i.) | n.d. | n.d | - |
| 12 | 70.64 | 192, 248, 271, 282, 293, 434, 510 | n.d. | Derivative of a Monascus-like azaphilone red pigment (n.i.) | n.d. | n.d. | - |
| 13 | 69.78 | 192, 248, 271, 282, 293 | 393.2693 | Ergosterol (colorless compound) | C28H44O | 396.65 (0.3807) | [37,38] |
| No | Rt. (min) | UV-Vis λmax (nm) (Bold: λmax in Visible) | Tentative Identification (Identified or Assumed Compounds) | Polyketide-Based Compounds Content (meqv.L−1) | |
|---|---|---|---|---|---|
| in PDB (1) | in DMD (1) | ||||
| 1 | 1.71 | 201, 216, 244, 276, 423, 514 | Diglycoside derivative of a Monascus-like azaphilone red pigment | 124.8 ± 5.0 | - |
| 2 | 28.52 | 192, 245, 274, 421, 515 | PP-R [7-(2-hydroxyethyl)-monascorubramin] | 6.7 ± 0.4 | - |
| 3 | 29.60 | 193, 245, 274, 421, 518 | Glycyl-rubropunctatin | 22.1 ± 1.3 | 7.3 ± 0.3 |
| 4 | 30.15 | 193, 245, 274, 426, 515 | N-GABA-rubropunctatin (GABA: γ-aminobutyric acid) | 8.0 ± 0.3 | 4.9 ± 0.4 |
| 5 | 30.97 | 195, 245, 274, 424, 520 | N-threonyl-rubropunctamin (or acid form of PP-R) | 83.4 ± 4.1 | 9.0 ± 0.8 |
| 6 | 32.66 | 193, 218, 250, 287, 424, 546 | 6-[(Z)-2-Carboxyvinyl]-N-GABA-PP-V | 33.5 ± 1.3 | - |
| 7 | 36.11 | 196, 247, 288, 422, 522 | N-glutaryl-monascorubraminic acid | 7.3 ± 0.4 | - |
| 8 | 38.04 | 193, 246, 273, 426, 521 | N-threonine-monascorubramin | 24.3 ± 0.6 | - |
| 9 | 39.10 | 193, 216, 250, 277, 426, 532 | N-glutaryl-rubropunctamin | 5.7 ± 0.2 | - |
| 10 | 43.95 | 193, 245, 272, 424, 519 | Monascorubramin | 19.6 ± 0.9 | - |
| 11 | 70.40 | 192, 248, 271, 282, 293, 434, 513 | Derivative of a Monascus-like azaphilone red pigment (n.i.) | 4.1 ± 0.2 | - |
| 12 | 70.64 | 192, 248, 271, 282, 293, 434, 510 | Derivative of a Monascus-like azaphilone red pigment (n.i.) | 4.2 ± 0.4 | - |
| 13 | 69.78 | 192, 248, 271, 282, 293 | Ergosterol (colorless compound) | 24.0 ± 1.0 | 73.8 ± 1.9 |
| 14 | 1.63 | 198, 260 | Colorless compound (n.i.) | - | 31.1 ± 1.2 |
| 15 | 2.01 | 196, 258 | Colorless compound (n.i.) | - | 6.1 ± 0.3 |
| 16 | 23.79 | 203, 256, 298 | Colorless compound (n.i.) | - | 6.3 ± 0.3 |
| 17 | 25.93 | 196, 264, 278, 479 | Yellow pigment (n.i.) | - | 5.2 ± 0.2 |
| 18 | 32.03 | 193, 252, 294, 428, 546 | Purple-red pigment (n.i.) | - | 5.0 ± 0.3 |
| 19 | 32.73 | 192, 220, 246, 289, 415, 546 | Purple-red pigment (n.i.) | - | 3.8 ± 0.3 |
| 20 | 36.30 | 193, 260, 274 | Colorless compound (n.i.) | - | 2.7 ± 0.2 |
| 21 | 38.57 | 192, 211, 243, 391 | Colorless compound (n.i.) | - | 1.5 ± 0.2 |
| 22 | 40.21 | 210, 292, 421 | Colorless compound (n.i.) | - | 0.4 ± 0.1 |
| 23 | 43.43 | 192, 280, 409, 431 | Yellow pigment (n.i.) | - | 0.4 ± 0.1 |
| 24 | 52.93 | 192, 248, 271, 282, 293, 414 | Yellow pigment (n.i.) | - | 17.4 ± 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebeau, J.; Petit, T.; Fouillaud, M.; Dufossé, L.; Caro, Y. Alternative Extraction and Characterization of Nitrogen-Containing Azaphilone Red Pigments and Ergosterol Derivatives from the Marine-Derived Fungal Talaromyces sp. 30570 Strain with Industrial Relevance. Microorganisms 2020, 8, 1920. https://doi.org/10.3390/microorganisms8121920
Lebeau J, Petit T, Fouillaud M, Dufossé L, Caro Y. Alternative Extraction and Characterization of Nitrogen-Containing Azaphilone Red Pigments and Ergosterol Derivatives from the Marine-Derived Fungal Talaromyces sp. 30570 Strain with Industrial Relevance. Microorganisms. 2020; 8(12):1920. https://doi.org/10.3390/microorganisms8121920
Chicago/Turabian StyleLebeau, Juliana, Thomas Petit, Mireille Fouillaud, Laurent Dufossé, and Yanis Caro. 2020. "Alternative Extraction and Characterization of Nitrogen-Containing Azaphilone Red Pigments and Ergosterol Derivatives from the Marine-Derived Fungal Talaromyces sp. 30570 Strain with Industrial Relevance" Microorganisms 8, no. 12: 1920. https://doi.org/10.3390/microorganisms8121920
APA StyleLebeau, J., Petit, T., Fouillaud, M., Dufossé, L., & Caro, Y. (2020). Alternative Extraction and Characterization of Nitrogen-Containing Azaphilone Red Pigments and Ergosterol Derivatives from the Marine-Derived Fungal Talaromyces sp. 30570 Strain with Industrial Relevance. Microorganisms, 8(12), 1920. https://doi.org/10.3390/microorganisms8121920









