Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Identification of the Fungal Strains
2.3. Cultivation of Mould Colonies
2.4. Viability Staining with Hoechst 33342 + Propidium Iodide
2.5. Toxicity Test Using Biomass Lysates
2.6. Toxic Responses in Old Biomass of Stachybotrys sp. Grown for >20 Years on Paper Board
2.7. Monitoring Guttation
2.8. Monitoring Emission of Surface-Active Compounds
3. Results
3.1. Comparison of Methods Monitoring Metabolic Activity in Fungal Biomass
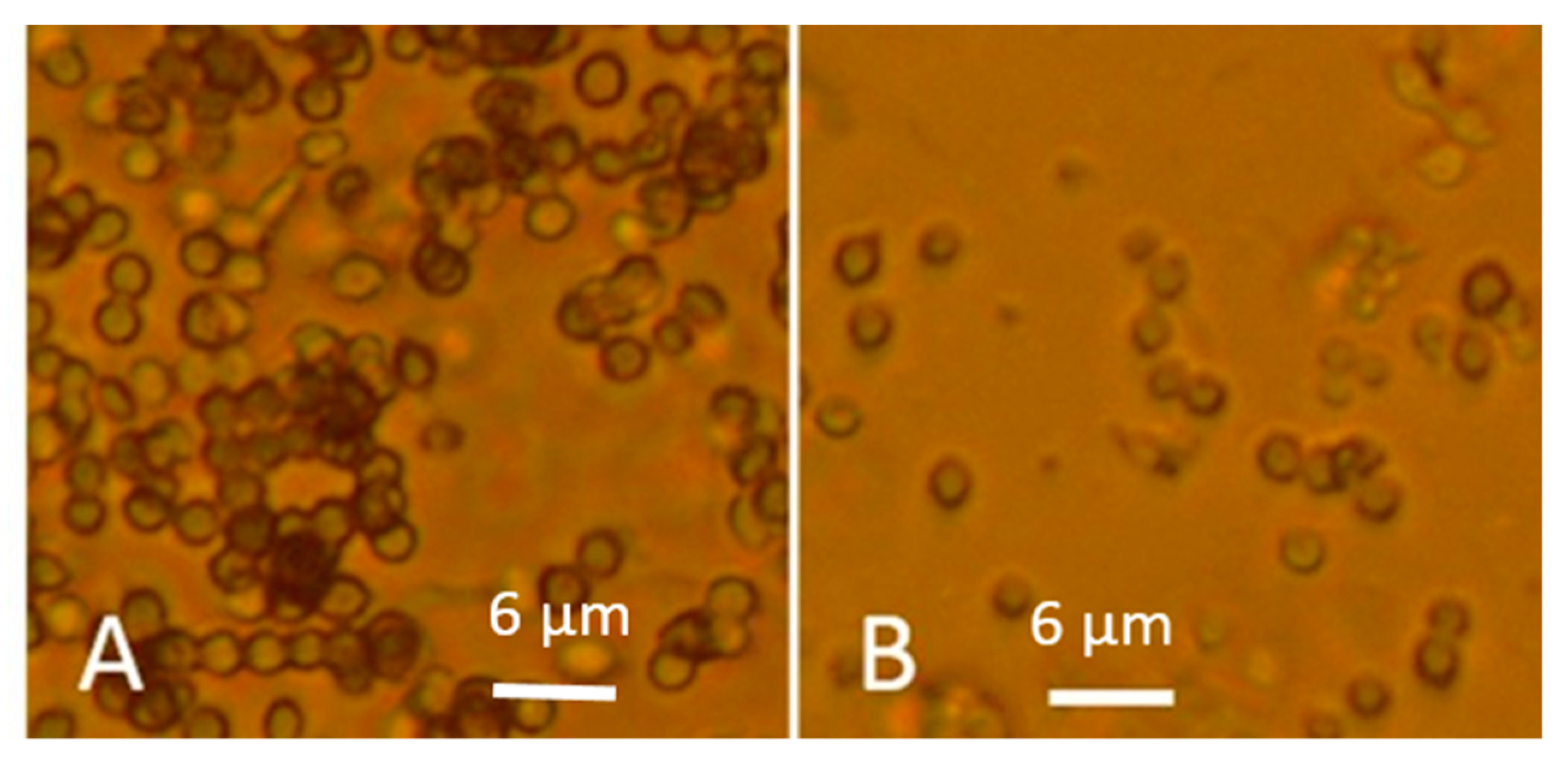
3.1.1. Fluorescence Intensity in Actively Growing and Dormant Fungal Biomass Stained with the Viability Staining by Hoechst 33342 Combined with Propidium Iodide
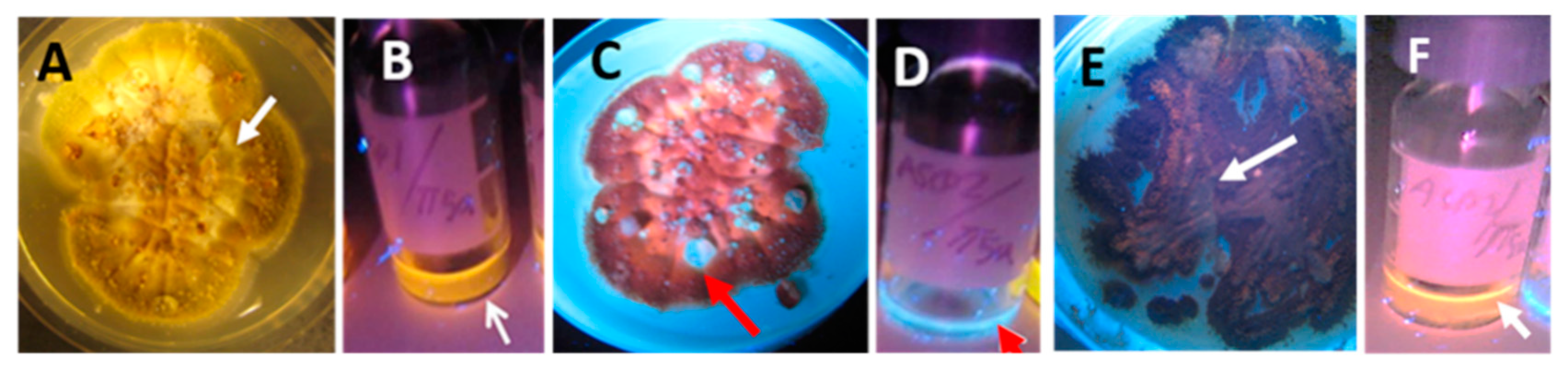
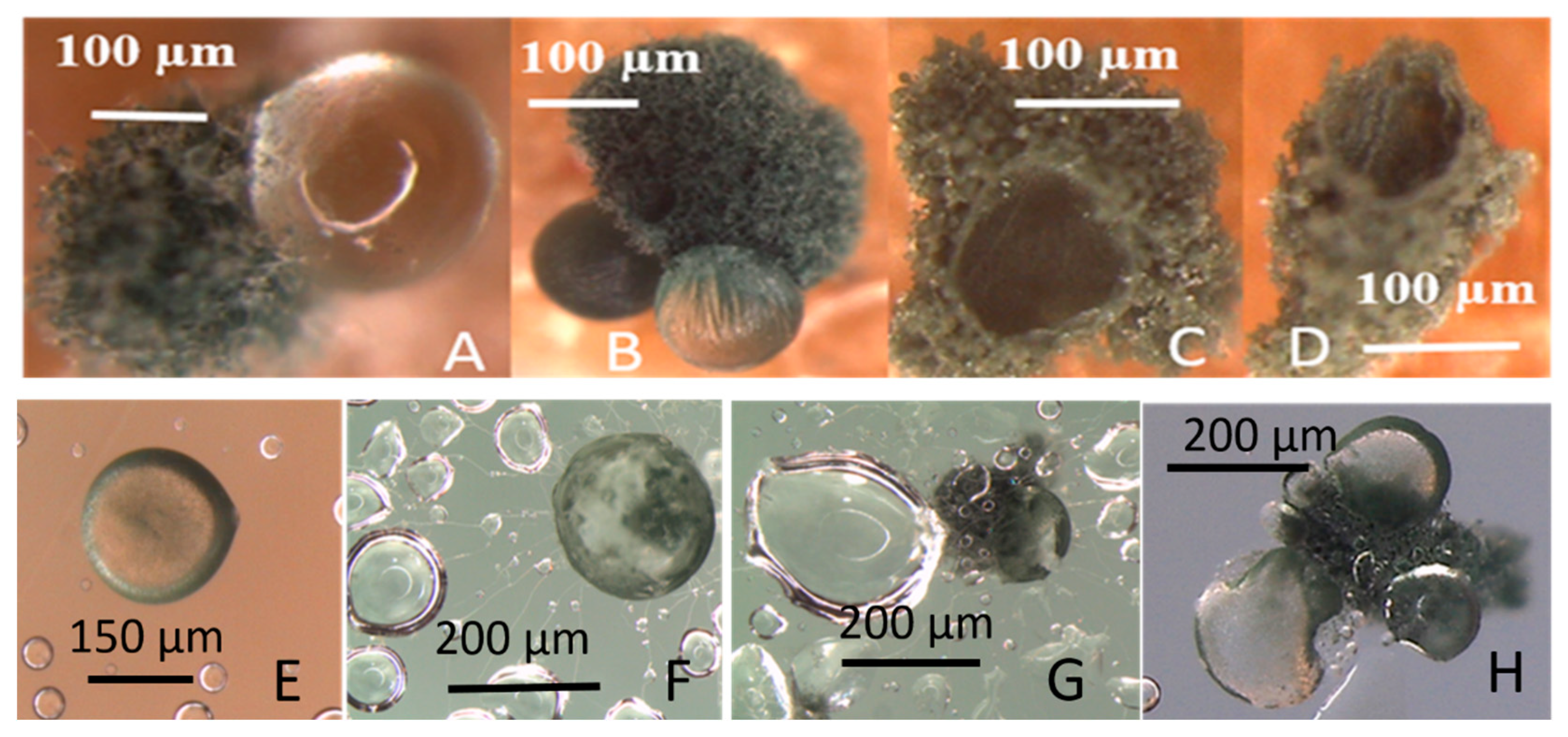
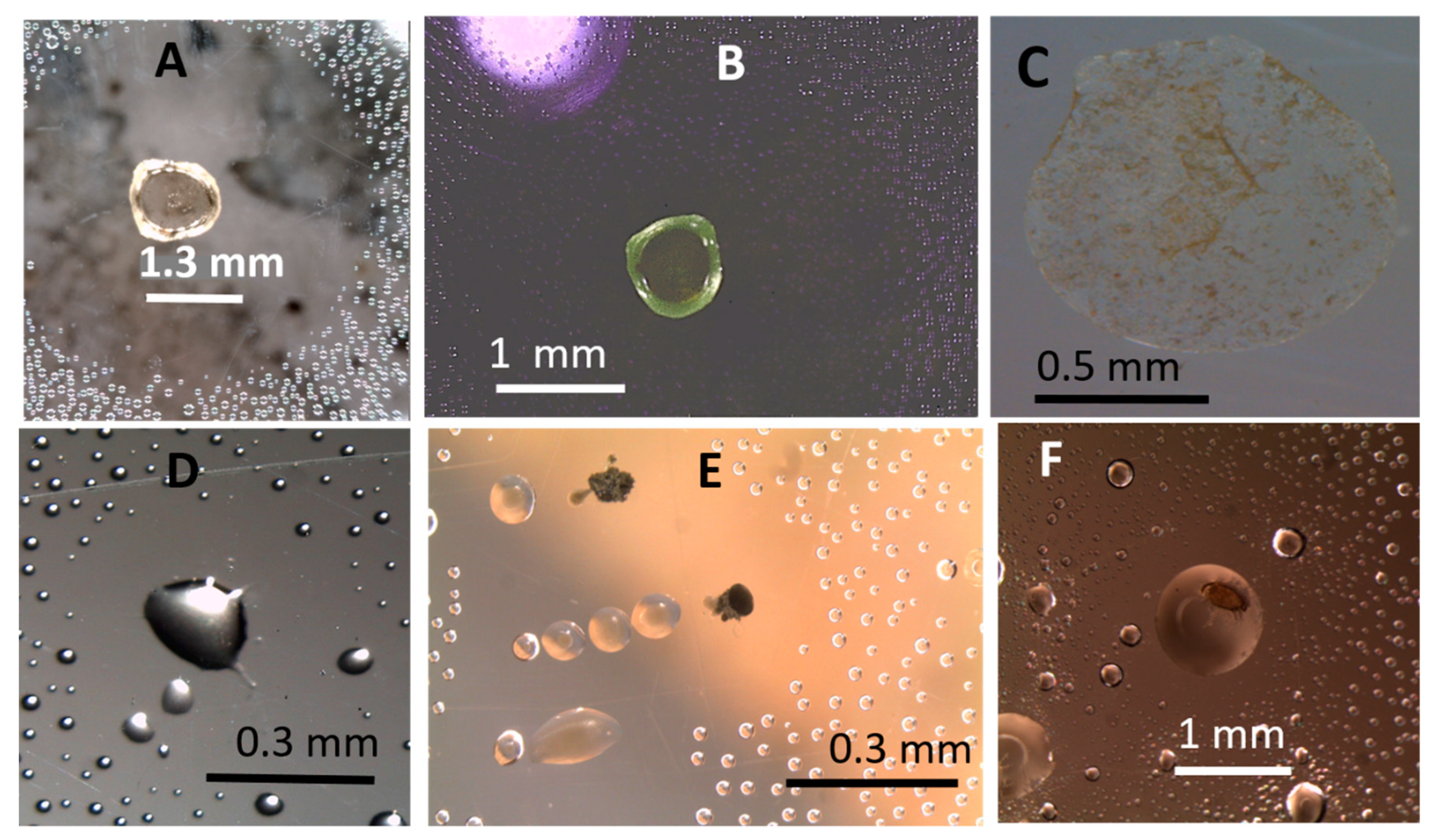
3.1.2. Formation of Guttation Droplets in Fresh and Old Plate-Grown Fungal Cultures
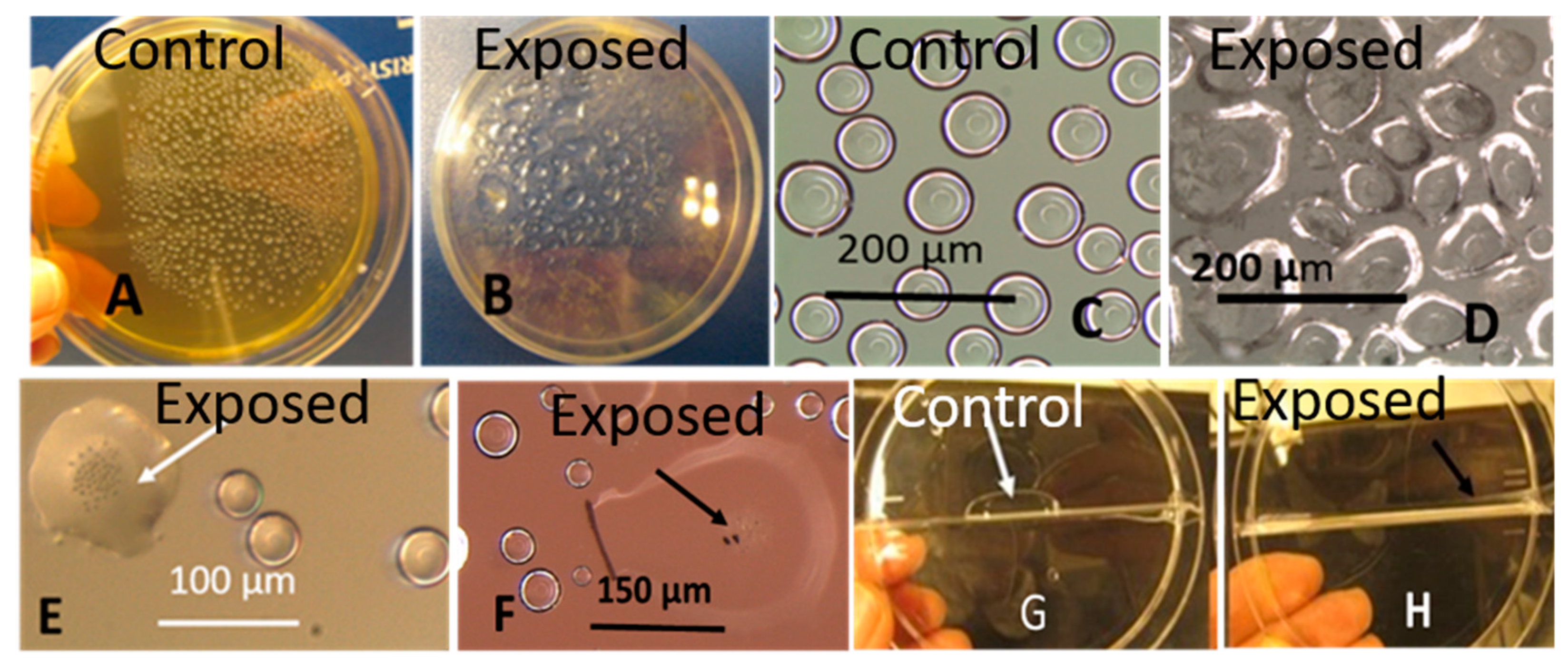
3.1.3. Toxic Response in Bioassays Against Somatic Mammalian Cell Lines (PK-15 and MNA)
Old Biomasses of Stachybotrys sp. Grown on Paperboard and Fresh Biomass Grown on Malt Extract Agar (MEA)
Toxicity of Old Biomasses of Stachybotrys sp. Grown on Paperboard and Fresh Biomass Grown on Malt Extract Agar (MEA)
3.2. Substances Influencing Surface Tension of Water
3.2.1. Actively Growing Fungal Cultures Secrete Substances Decreasing Surface Tension of Water
3.2.2. Fungal Cultures Secrete Hydrophobic Water-Repelling Substances
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adamatzkya, A.; Gandia, A.; Chiolerio, A. Fungal sensing skin. arXiv 2020. [Google Scholar] [CrossRef]
- Meyer, V.; Basenko, E.Y.; Benz, J.P.; Braus, G.H.; Caddick, M.X.; Csukai, M.; de Vries, R.P.; Endy, D.; Frisvad, J.C.; Gunde-Cimerman, N.; et al. Growing a circular economy with fungal biotechnology: A white paper. Fungal Biol. Biotechnol. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Yim, G.; Wang, H.; Davies, J. Antibiotics as signalling molecules. Phil. Trans. R. Soc. B 2007, 362, 1195–1200. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.; Luna, M.; Santiago, A.; Franco, L.; Silva, K.; de Souza, P.; Okada, K.; Albuquerque, C.D.C.; Silva, C.A.A.; Campos-Takaki, G.M. Biosurfactant-and-bioemulsifier produced by a promising Cunninghamella echinulata isolated from Caatinga soil in the northeast of Brazil. Int. J. Mol. Sci. 2014, 15, 15377–15395. [Google Scholar] [CrossRef]
- Qazi, M.; Subhan, M.; Fatima, N.; Ali, M.; Ahmed, S. Role of biosurfactant produced by Fusarium sp. BS-8 in enhanced oil recovery (MEOR) through sand pack column. Int. J. Biosci. Biochem. Bioinform. 2013, 3, 598–604. [Google Scholar] [CrossRef]
- Araújo, H.W.; Andrade, R.F.; Montero-Rodríguez, D.; Rubio-Ribeaux, D.; da Silva, C.A.A.; Campos-Takaki, G.M. Sustainable biosurfactant produced by Serratia marcescens UCP 1549 and its suitability for agricultural and marine bioremediation applications. Microb. Cell Fact. 2019, 18, 2. [Google Scholar] [CrossRef]
- Bayry, J.; Aimanianda, V.; Guijarro, J.I.; Sunde, M.; Latge, J.P. Hydrophobins unique fungal proteins. PLoS Pathog. 2012, 8, e1002700. [Google Scholar] [CrossRef]
- Zhang, J.; Miao, Y.; Rahimi, M.J.; Zhu, H.; Steindorff, A.; Schiessler, S.; Cai, F.; Chenthamara, K.; Xu, Y.; Kubicek, C.; et al. Guttation capsules containing hydrogen peroxide: An evolutionarily conserved NADPH oxidase gains a role in wars between related fungi. Environ. Microbiol. 2019, 21, 2644–2658. [Google Scholar] [CrossRef]
- Cai, F.; Gao, R.; Zhao, Z.; Ding, M.; Jiang, S.; Yagtu, C.; Zhu, H.; Zhang, J.; Ebner, T.; Mayrhofer-Reinhartshuber, M.; et al. Evolutionary compromises in fungal fitness: Hydrophobins can hinder the adverse dispersal of conidiospores and challenge their survival. ISME J. 2020, 14, 2610–2624. [Google Scholar] [CrossRef]
- Parsek, M.R.; Fuqua, C. Biofilms 2003: Emerging themes and challenges in studies of surface-associated microbial life. J. Bacteriol. 2004, 186, 4427–4440. [Google Scholar] [CrossRef]
- Runyanga, T.J.; Chen, Y.; Sun, F. Cell communication and fungal virulence: A review. Res. Rev. Res. J. Biol. 2017, 5, 8–16. [Google Scholar]
- Ramada, M.; Steindorff, A.; Bloch, C., Jr.; Ulhoa, C. Secretome analysis of the mycoparasitic fungus Trichoderma harzianum ALL 42 cultivated in different media supplemented with Fusarium solani cell wall or glucose. Proteomics 2016, 16, 477–490. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, D.; Leynaert, B.; Keirsbulck, M.; Nadif, R. Indoor mould exposure, asthma and rhinitis: Findings from systematic reviews and recent longitudinal studies—A review. Eur. Respir. Rev. 2018, 27, 170–199. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Damp and mould. In Health Risks, Prevent and Remedial Actions; WHO: Copenhagen, Denmark, 2009. [Google Scholar]
- Wang, J.; Pindus, M.; Janson, C.; Sigsgaard, T.; Kim, J.L.; Holm, M.; Sommar, J.; Orru, H.; Gislason, T.; Johannessen, A.; et al. Dampness, mould, onset and remission of adult respiratory symptoms, asthma and rhinitis. Eur. Respir. J. 2019, 56. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Kumagai, K. Observation-based metrics for residential dampness and mold with dose-response relationships to health: A review. Indoor Air 2017, 27, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reponen, T.; Hershey, G. Fungal exposure and asthma: IgE and non-IgE-mediated mechanisms. Curr. Allergy Asthma Rep. 2016, 16, 86. [Google Scholar] [CrossRef]
- Rand, T.; Flemming, J.; Miller, J.; Womiloju, T. Comparison of inflammatory responses in mouse lungs exposed to atranones A and C from Stachybotrys chartarum. J. Toxicol. Environ. Health A 2006, 69, 1239–1251. [Google Scholar] [CrossRef]
- Rand, T.G.; Giles, S.; Flemming, J.; Miller, J.; Puniani, E. Inflammatory and cytotoxic responses in mouse lungs exposed to purified toxins from building isolated Penicillium brevicompactum Dierckx and P. chrysogenum Thom. Toxicol. Sci. 2005, 87, 213–222. [Google Scholar] [CrossRef]
- Kankkunen, P.; Rintahaka, J.; Aalto, A.; Leino, M.; Majuri, M.L.; Alenius, H.; Wolff, H.; Matikainen, S. Trichothecene mycotoxins activate inflammatory response in human macrophages. J. Immunol. 2009, 182, 6418–6425. [Google Scholar] [CrossRef]
- Kankkunen, P.; Valimaki, E.; Rintahaka, J.; Palomäki, J.; Nyman, T.; Alenius, H.; Wolff, H.; Matikainen, S. Trichothecene mycotoxins activate NLRP3 inflammasome through a P2X7 receptor and Src tyrosine kinase dependent pathway. Hum. Immunol. 2014, 75, 134–140. [Google Scholar] [CrossRef]
- Korkalainen, M.; Taubel, M.; Naarala, J.; Kirjavainen, P.; Kistinen, A.; Hyvärinen, A.; Komulainen, H.; Viluksela, M. Synergistic proinflammatory interactions of microbial toxins and structural components characteristic to moisture-damaged buildings. Indoor Air 2017, 27, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Magun, B.; Wood, L. Lung inflammation caused by inhaled toxicants: A review. Int. J. COPD 2016, 11, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Othman, M.R.; Latif, M.T. Early study of surfactants in indoor dust and their connection with street dust. Int. J. Environ. Res. 2009, 3, 403–410. [Google Scholar] [CrossRef]
- Veirup, K.; Wolkoff, P. Linear alkylbenzene sulfonates in indoor floor dust. Sci. Total. Environ. 2003, 300, 51–58. [Google Scholar] [CrossRef]
- Poulsen, L.K.; Clausen, S.K.; Glue, C.; Millner, A.; Nielsen, G.D.; Jinquan, T. Detergents in the indoor environment–what is the evidence for an allergy promoting effect? Known and postulated mechanisms. Toxicology 2002, 152, 79–85. [Google Scholar] [CrossRef]
- Kuhn, D.; Ghannoum, M. Indoor mold, toxigenic fungi, and Stachybotrys chartarum: Infectious disease perspective. Clin. Microbiol. Rev. 2003, 6, 144–172. [Google Scholar] [CrossRef] [PubMed]
- Valtonen, V. Clinical diagnosis of the dampness and mold hypersensitivity syndrome: Review of the literature and suggested diagnostic criteria. Front. Immunol. 2017, 8, 951. [Google Scholar] [CrossRef]
- Tuuminen, T.; Andersson, A.; Hyvönen, S.; Lohi, J.; Vaali, K. Indoor air nontoxicity should be proven with special techniques prior claiming that it may cause a variety of mental disorders. J. Hyg. Environ. Health 2020, 229, 113545. [Google Scholar] [CrossRef]
- Nordin, S. Mechanisms underlying nontoxic indoor air health problems: A review. Int. J. Hyg. Environ. Health 2020, 226, 113489. [Google Scholar] [CrossRef]
- Savelieva, K.; Elovainio, M.; Lampi, J.; Ung-Lanki, S.; Pekkanen, J. Psychosocial factors and indoor environmental quality in respiratory symptom reports of pupils: A cross-sectional study in Finnish schools. BMJ Open 2020, 10, e036873. [Google Scholar] [CrossRef]
- Hurraß, J.; Heinzow, B.; Aurbach, U.; Bergmann, K.-C.; Bufe, A.; Buzina, W.; Cornely, O.A.; Engelhart, S.; Fischer, G.; Gabrio, T.; et al. Medical diagnostics for indoor mold exposure. Int. J. Hyg. Environ. Health 2016, 220, 305–328. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.; Borràs-Santos, A.; Krop, E.; Täubel, M.; Leppänen, H.; Haverinen-Shaughnessy, U.; Pekkanen, J.; Hyvärinen, A.; Doekes, G.; Zock, J.-P.; et al. Dampness, bacterial and fungal components in dust in primary schools and respiratory health in school children across Europe. Occup. Environ. Med. 2014, 71, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The medical effects of mold exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Moses, L.; Morrissey, K.; Sharpe, R.; Taylor, T. Exposure to indoor mouldy odour increases the risk of asthma in older adults living in social housing. Int. J. Environ. Res. Publc. Health 2019, 16, 2600. [Google Scholar] [CrossRef] [PubMed]
- Gareis, M.; Gottschalk, C. Stachybotrys spp. and the guttation phenomenon. Mycotox. Res. 2014, 30, 151–159. [Google Scholar] [CrossRef]
- Castagnoli, E.; Marik, T.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018, 125, 1408–1422. [Google Scholar] [CrossRef]
- Salo, M.J.; Marik, T.; Mikkola, R.; Andersson, M.A.; Kredics, L.; Salonen, H.; Kurnitski, J. Penicillium expansum strain isolated from indoor building material was able to grow on gypsum board and emitted guttation droplets containing chaetoglobosins and communesins A, B and D. J. Appl. Microbiol. 2019, 127, 1135–1147. [Google Scholar] [CrossRef]
- Zhao, G.; Lin, X.; Dou, W.; Tian, S.; Deng, S.; Shi, J. Use of the smart tongue to monitor mold growth and discriminate between four mold species grown in liquid media. Anal. Chim. Acta 2011, 690, 240–247. [Google Scholar] [CrossRef]
- Castagnoli, E.; Salo, J.; Toivonen, M.S.; Marik, T.; Mikkola, R.; Kredics, L.; Vicente-Carrillo, A.; Nagy, S.; Andersson, M.T.; Andersson, M.A.; et al. An evaluation of boar spermatozoa as a biosensor for the detection of sublethal and lethal toxicity. Toxins 2018, 10, 463. [Google Scholar] [CrossRef]
- Salo, M.J.; Marik, T.; Bencsik, O.; Mikkola, R.; Kredics, L.; Szekeres, A.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Screening mold colonies by using two toxicity assays revealed indoor strains of Aspergillus calidoustus producing ophiobolins G and K. Toxins 2019, 11, 683. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food and Air-Borne Fungi, 7th ed.; Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2004. [Google Scholar]
- Kirchhoff, C.; Cypionka, H. Propidium ion enters viable cells with high membrane potential during live-dead staining. J. Microbiol. Methods 2017, 142, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Yang, J.; Guan, M.; Ji, W.; Li, Y.; Rens, W. Single UV excitation of Hoechst 33342 and propidium iodide for viability assessment of rhesus monkey spermatozoa using flow cytometry. Arch. Androl. 2005, 51, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Salo, J.; Kedves, O.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Kurnitski, J.; Salonen, H. Detection of Chaetomium globosum, Ch. cochliodes and Ch. rectangulare during the diversity tracking of mycotoxin-producing Chaetomium-like isolates obtained in buildings in Finland. Toxins 2020, 12, 443. [Google Scholar] [CrossRef] [PubMed]
- Bencsik, O.; Papp, T.; Berta, M.; Zana, A.; Forgó, P.; Dombi, G.; Andersson, M.; Salkinoja-Salonen, M.; Vágvölgyi, C.; Szekeres, A. Ophiobolin A from Bipolaris oryzae perturbs motility and membrane integrities of porcine sperm and induces cell death on mammalian somatic cell lines. Toxins 2014, 6, 2857–2871. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, R.; Andersson, M.A.; Kredics, L.; Grigoriev, P.A.; Sundell, N.; Salkinoja-Salonen, M.S. 20-residue and 11-residue peptaibols from the fungus Trichoderma longibrachiatum are synergistic in forming Na+/K+-permeable channels and adverse action towards mammalian cells. FEBS J. 2012, 279, 4172–4190. [Google Scholar] [CrossRef]
- Castagnoli, E.; Andersson, M.; Mikkola, R.; Kurnitski, J.; Salonen, H. Airborne toxicity of a non-ionic alcohol ethoxylate surfactant and wetting agent used in cleaning chemicals. In Proceedings of the 15th Conference of the International Society of Indoor Air Quality & Climate (ISIAQ), Philadelphia, PA, USA, 22–27 July 2018; p. 373. [Google Scholar]
- Vornanen-Winqvist, C.; Järvi, K.; Toomla, S.; Ahmed, K.; Andersson, M.A.; Mikkola, R.; Marik, T.; Kredics, L.; Salonen, H.; Kurnitski, J. Ventilation positive pressure intervention effect on indoor air quality in a school building with moisture problems. Int. J. Environ. Res. Public Health 2018, 15, 230. [Google Scholar] [CrossRef]
- Sterling, M.; Rogers, C.; Levetin, E. An evaluation of two methods used for microscopic analysis of airborne fungal spore concentrations from the Burkard Spore Trap. Aerobiologia 1999, 15, 9–18. [Google Scholar] [CrossRef]
- Andersson, M.A.; Nikulin, M.; Köljalg, U.; Andersson, M.C.; Rainey, F.; Reijula, K.; Hintikka, E.L.; Salkinoja-Salonen, M. Bacteria, molds, and toxins in water-damaged building materials. Appl. Environ. Microbiol. 1997, 63, 387–393. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Smedsgaard, J. Fungal metabolite screening: Database of 474 mycotoxins and fungal metabolites for dereplication by standardised liquid chromatography-UV-mass spectrometry methodology. J. Chromatogr. A 2003, 100, 111–136. [Google Scholar] [CrossRef]
- Holme, J.; Hägerhed-Engman, L.; Mattsson, J.; Sundell, J.; Bornehag, C.-G. Culturable mold in indoor air and its association with moisture-related problems and asthma and allergy among Swedish children. Indoor Air 2010, 20, 329–340. [Google Scholar] [CrossRef]
- Meklin, T.; Haugland, R.A.; Reponen, T.; Varma, M.; Lummus, Z.; Bernstein, D.; Wymer, L.J.; Vesper, S.J. Quantitative PCR analysis of house dust can reveal abnormal mold conditions. J. Environ. Monit. 2004, 6, 615–620. [Google Scholar] [CrossRef] [PubMed]
- Haines, S.R.; Siegel, J.A.; Dannemiller, K.C. Modeling microbial growth in carpet dust exposed to diurnal variations in relative humidity using the “Time-of-Wetness” framework. Indoor Air 2020, 100, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Byrne, S.; Larsen, L.S.; Sigsgaard, T.; Thorne, P.S.; Larsson, L.; Sebastian, A.; Bornehag, C.G. Residential culturable fungi, (1-3, 1-6)-β-d-glucan, and ergosterol concentrations in dust are not associated with asthma, rhinitis, or eczema diagnoses in children. Indoor Air 2014, 24, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Tirkkonen, J.; Taubel, M.; Hirvonen, M.-R.; Leppaänen, H.; Lindsley, W.; Bean, T.; Chen, B.; Hyvaärinen, A.; Huttunen, K. Evaluation of sampling methods for toxicological testing of indoor air particulate matter. Inhal. Toxicol. 2016, 28, 500–507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nordberg, M.-E.; Täubel, M.; Jalava, P.; BéruBé, K.; Tervahauta, A.; Hyvärinen, A.; Huttunen, K. Human airway construct model is suitable for studying transcriptome changes associated with indoor air particulate matter toxicity. Indoor Air 2020, 30, 433–444. [Google Scholar] [CrossRef]
- Corada-Fernández, C.; Lara-Martín, P.A.; Candela, L.; González-Mazo, E. Tracking sewage derived contamination in riverine settings by analysis of synthetic surfactants. J. Environ. Monit. 2011, 13, 2010. [Google Scholar] [CrossRef]
- Zoller, U. Estuarine and coastal zone marine pollution by the nonionic alkylphenol ethoxylates endocrine disrupters: Is there a potential ecotoxicological problem? Environ. Int. 2006, 32, 269–272. [Google Scholar] [CrossRef]
- Vicente-Carrillo, A.; Edebert, I.; Garside, H.; Cotgreave, I.; Rigler, R.; Loitto, V.; Magnusson, K.E.; Rodríguez-Martínez, H. Boar spermatozoa successfully predict mitochondrial modes of toxicity: Implications for drug toxicity testing and the 3R principles. Toxicol. Vitr. 2015, 29, 582–591. [Google Scholar] [CrossRef]
- Ajao, C.; Andersson, M.A.; Teplova, V.V.; Nagy, S.; Gahmberg, C.G.; Andersson, L.C.; Hautaniemi, M.; Kakasi, B.; Roivainen, M.; Salkinoja-Salonen, M. Mitochondrial toxicity of triclosan on mammalian cells. Toxicol. Rep. 2015, 2, 624–637. [Google Scholar] [CrossRef]
- Azizullah, A.; Häder, D.-P. Advanced methods and applications—A comparison of commonly used and commercially available bioassays for aquatic ecosystems. In Bioassays, Chapter 17; Elsevier: Amsterdam, The Netherlands, 2018; pp. 347–368. [Google Scholar] [CrossRef]
- Carlsson, G.; Pohl, J.; Athanassiadis, I.; Norrgren, L.; Weiss, J. Thyroid disruption properties of three indoor dust chemicals tested in silurana tropicalis tadpoles. J. Appl. Toxicol. 2019, 39, 1248–1256. [Google Scholar] [CrossRef]
- Li, S.; Yang, Z.; Hu, D.; Cao, L.; He, Q. Understanding building-occupant-microbiome interactions toward healthy built environments: A review. Front. Environ. Sci. Eng. 2021, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Kruglov, A.G.; Andersson, M.A.; Mikkola, R.; Roivainen, M.; Kredics, L.; Saris, N.-E.; Salkinoja-Salonen, M.S. Novel mycotoxin from Acremonium exuviarum is a powerful inhibitor of the mitochondrial respiratory chain complex III. Chem. Res. Toxicol. 2009, 9, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Mikkola, R.; Raulio, M.; Kredics, L.; Maijala, P.; Salkinoja-Salonen, M.S. Acrebol, a novel toxic peptaibol produced by an Acremonium exuviarum indoor isolate. J. Appl. Microbiol. 2009, 106, 909–923. [Google Scholar] [CrossRef] [PubMed]
- Amuzie, C.; Harkem, J.; Pestka, J. Tissue distribution and proinflammatory cytokine induction by the trichothecene deoxynivalenol in the mouse: Comparison of nasal vs. oral exposure. Toxicology 2008, 248, 39–44. [Google Scholar] [CrossRef]
- Kenne, G.J.; Chakraborty, P.; Chanda, A. Modeling toxisome protrusions in filamentous fungi. JSM Environ. Sci. Ecol. 2014, 2, 1010–1012. [Google Scholar]
- Kistler, H.; Broz, K. Cellular compartmentalization of secondary metabolism. Front. Microbiol. 2015, 6, 68. [Google Scholar] [CrossRef]
- Huttunen, K.; Wlodarczyk, A.J.; Tirkkonen, J.; Mikkonen, S.; Täubel, M.; Krop, E.; Jacobs, J.; Pekkanen, J.; Heederik, D.; Zock, J.P.; et al. Oxidative capacity and hemolytic activity of settled dust from moisture-damaged schools. Indoor Air 2019, 29, 299–307. [Google Scholar] [CrossRef]

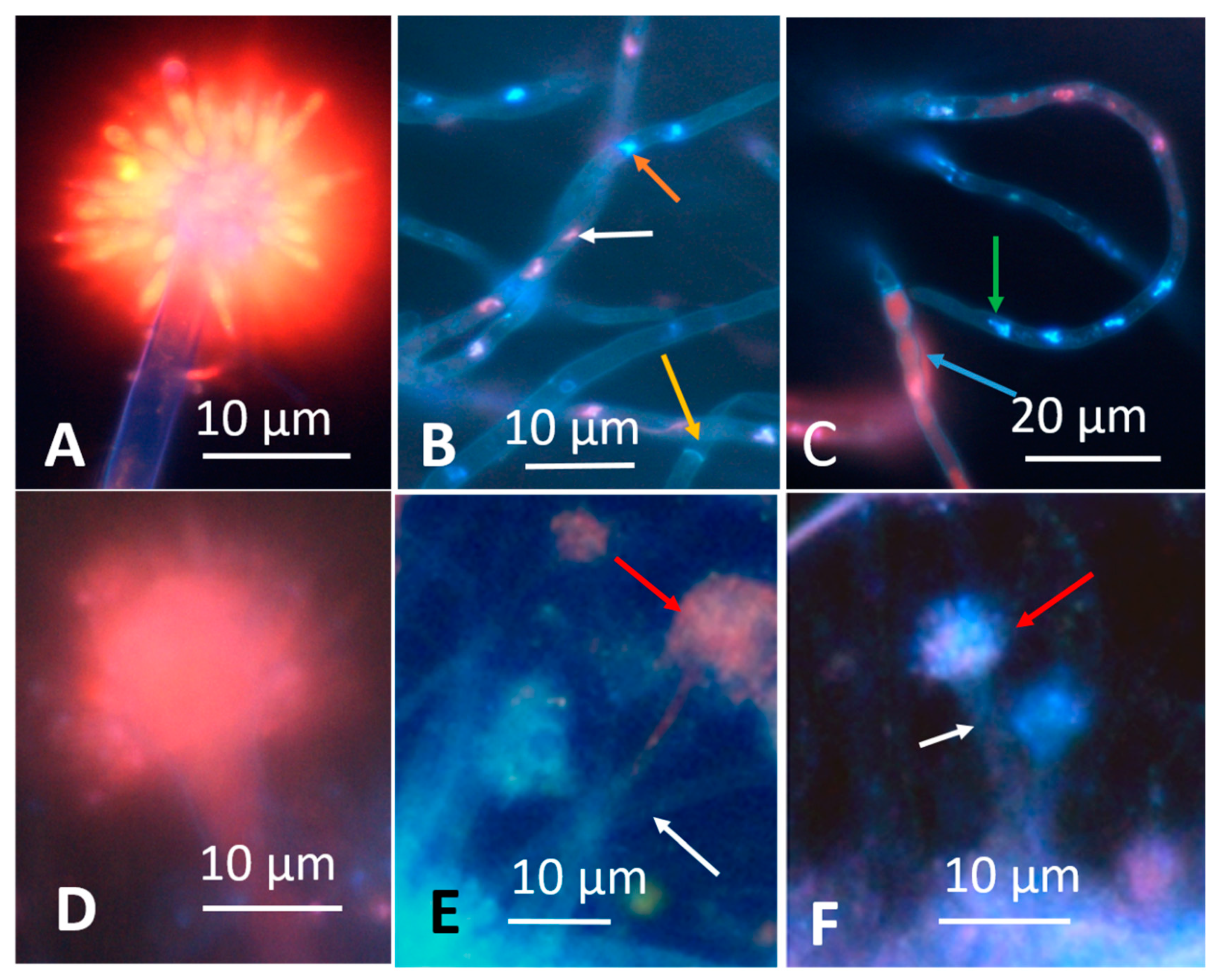
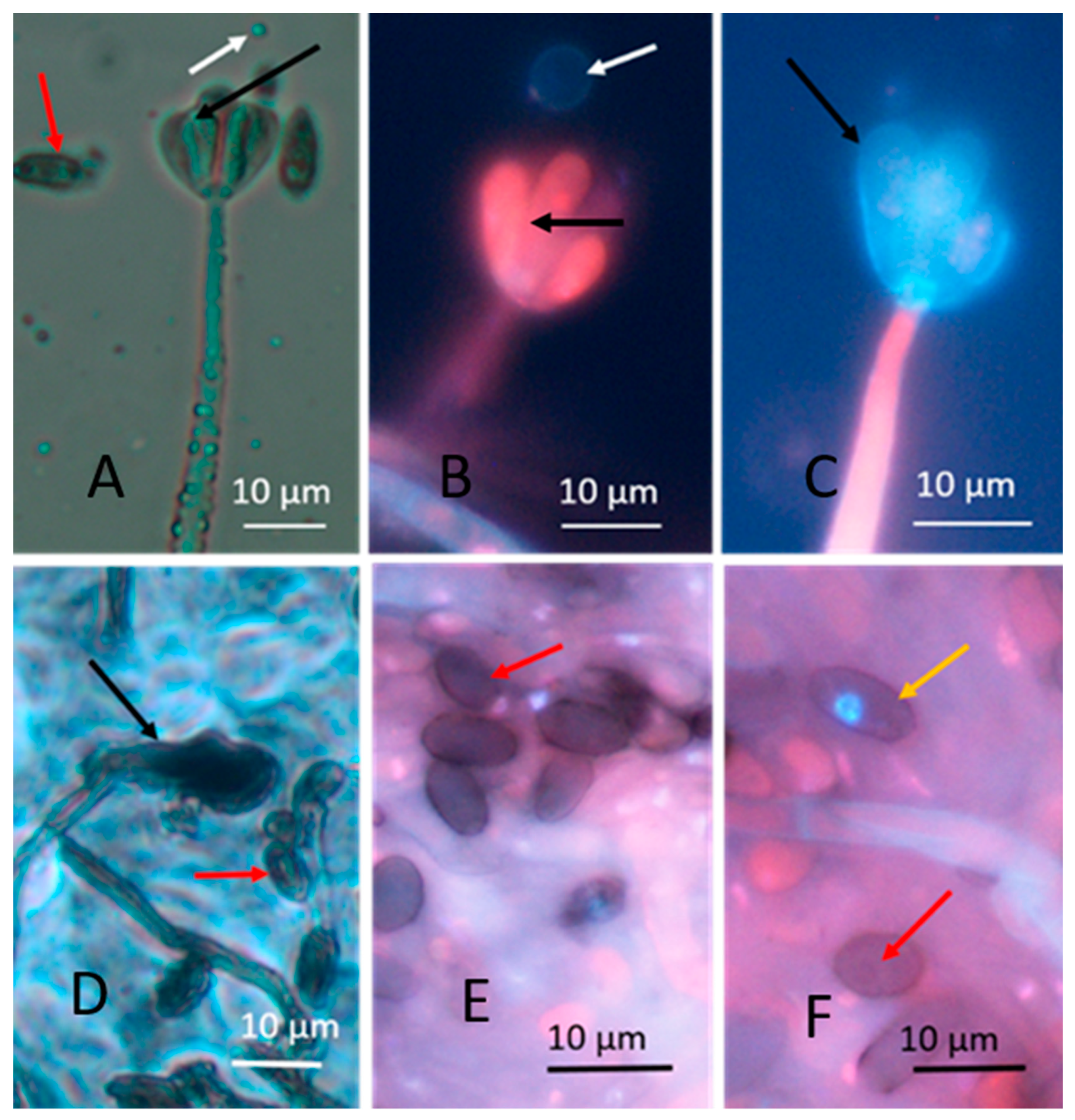
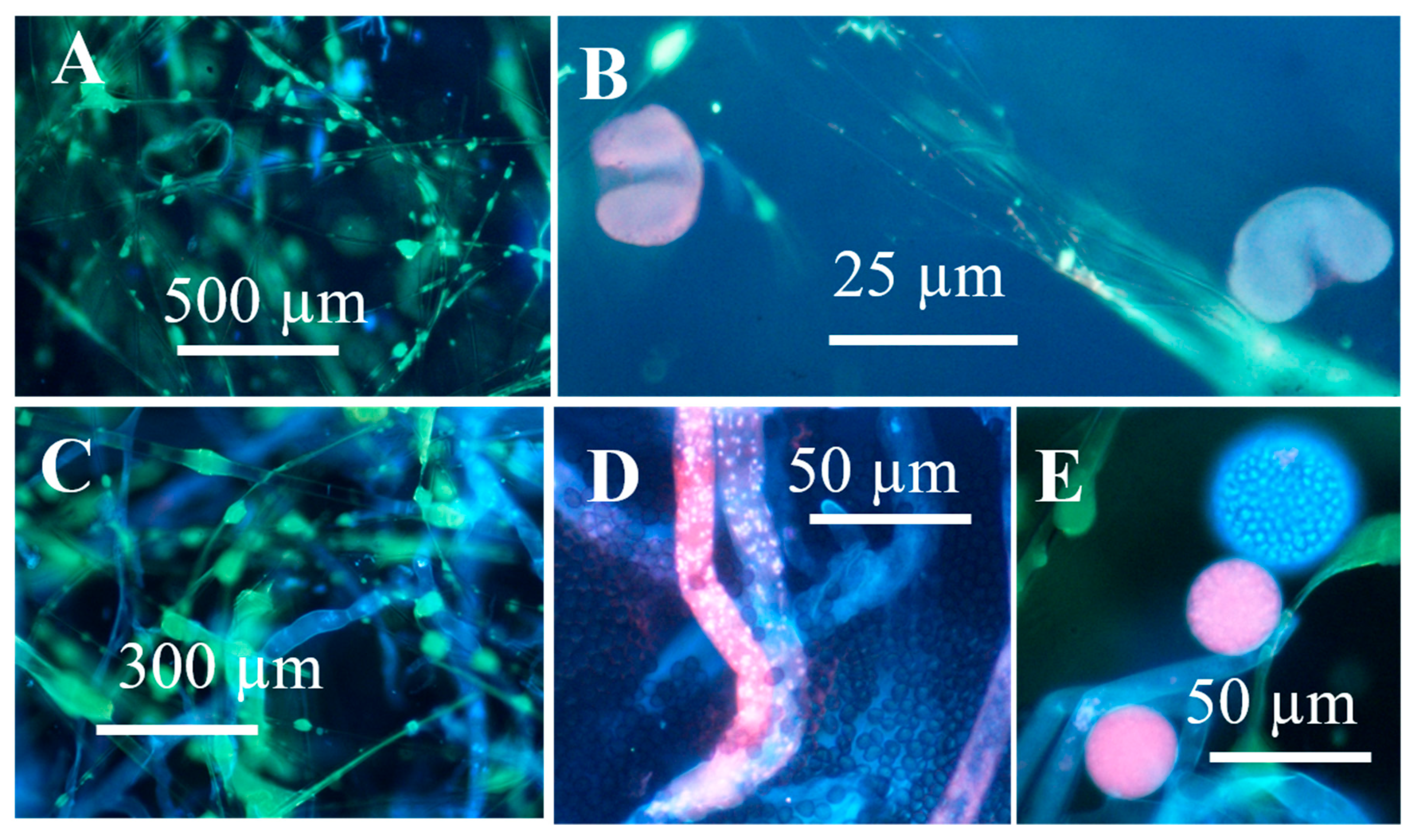
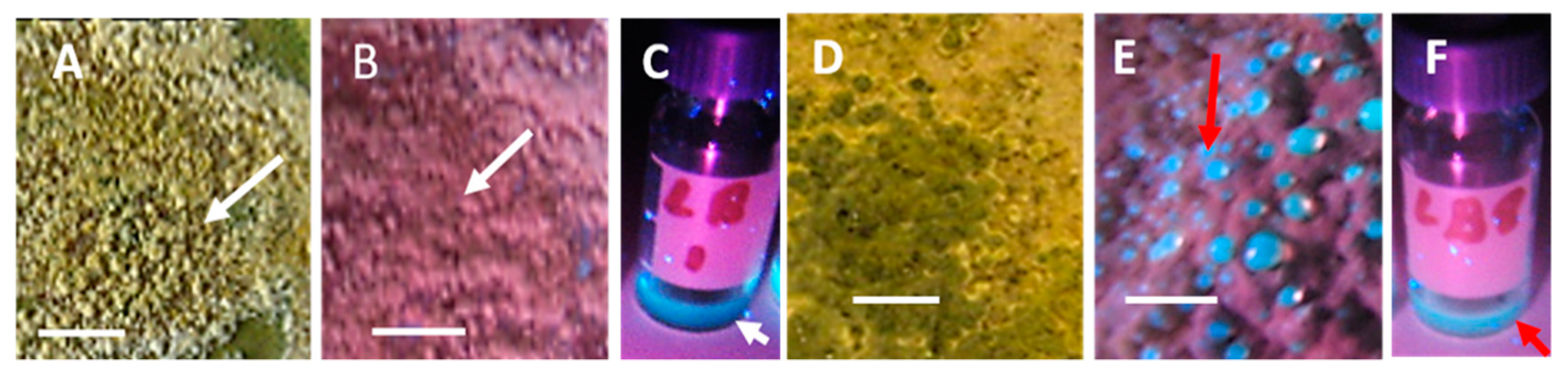

| Strain Code | Origin | Sample Site | Identified * | Biomass Lysates | Reference | ||
|---|---|---|---|---|---|---|---|
| Toxicity | Fluorescence | ||||||
| BSMI | ICP | ||||||
| Aspergillus section Versicolores unable to grow at 37 °C | |||||||
| Asp2/TH | Settled dust | School, Vantaa | Morphotype | − | + | Orange | This study |
| GAS226 | Settled dust | Office, Helsinki | DSMZ | − | + | Orange | [38,41,45] |
| MH25 | Settled dust | University, Espoo | Morphotype | − | + | Orange | [41] |
| MH35 | Settled dust | University, Espoo | Morphotype | − | + | Orange | [41] |
| SL/3 | Fall out plate | Apartment, Helsinki | DSMZ | − | + | Orange | [37,38,41,45] |
| Chaetomium globosum unable to grow at 37 °C | |||||||
| MH5 | Settled dust | University, Espoo | tef1α (MT498108) | + | + | Blue-green | [45] |
| MO9 | Settled dust | Piggery, Orimattila | tef1α (MT498106) | + | + | Blue-green | [45] |
| 2b/26 | Settled dust | Apartment, Vantaa | tef1α (MT498110) | + | + | Blue-green | [45] |
| 2c/26 | Settle d dust | Apartment, Vantaa | tef1α (MW310244) | + | + | Blue-green | [45] |
| Trichoderma atroviride unable to grow at 37 °C | |||||||
| H1/226 | Fallout plate | Office, Helsinki | tef1α (MH176994) ITS (KM853017) | + | (+) | Blue | [37] |
| T1/SKK | Exhaust filter | School, Vantaa | ITS (MW306736) | + | (+) | Blue | [42,49] |
| T7/SKK | Exhaust filter | School, Vantaa | ITS (MW306737) | + | (+) | Blue | [42,49] |
| 8/AM | Exhaust filter | University, Espoo | tef1α (MH176996) ITS (MH158553) | + | (+) | Blue | [37] |
| 14/AM | Exhaust filter | University, Espoo | tef1α (MH176997) ITS (MH158554) | + | (+) | Blue | [37] |
| Tri/335 | Settled dust | University, Espoo | tef1α (MH176998) | + | (+) | Blue | [37] |
| KIV10 | Andersen impactor | School, Lahti | tef1α (MH176999) | + | (+) | Blue | [37] |
| Trichoderma trixiae unable to grow at 37 °C | |||||||
| LB1 | Settled dust | Apartment, Helsinki | tef1α (MH177001) ITS (MH158556) | + | (+) | Blue | [37] |
| NJ14 | Settled dust | Ice Rink, Nivala | tef1α (MH177002) ITS (MH158557) | + | (+) | Blue | [37] |
| NJ22 | Settled dust | Ice Rink, Nivala | tef1α (MH177003) ITS (MH158558) | + | (+) | Blue | [37] |
| Penicillium expansum unable to grow at 37 °C | |||||||
| Rcp61 | Cork liner | University, Espoo | ITS (MK201596), WI | (+) | + | Blue | [38] |
| Rhizopus sp. grew at 37 °C | |||||||
| MKA1 | Exhaust filter | School, Vantaa | Morphotype | − | − | Blue | This study |
| Stachybotrys sp. unable to grow at 37 °C | |||||||
| HJ5 | Paperboard | Apartment, Mäntsälä | Morphotype | (+) | + | Blue | This study |
| EC50 µg Fungal Biomass (Wet Weight) mL−1 | ||||
|---|---|---|---|---|
| Age of Biomass | 2 Weeks | 1 Year | ||
| PK-15 | MNA | PK-15 | MNA | |
| Trichoderma atroviride | ||||
| T1/SKK | 100 | 100 | 2500 | 2500 |
| T7/SKK | 130 | 130 | 1500 | 1500 |
| H1/226 | 100 | 100 | 5000 | 5000 |
| Aspergillus versicolor | ||||
| SL/3 | 100 | >320 | ||
| Chaetomium globosum | ||||
| 9/MO | 30 | 600 | ||
| 2b/26 | 40 | 2500 | ||
| 2c/26 | 60 | 400 | ||
| MH5 | 50 | 600 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, M.A.; Salo, J.; Kedves, O.; Kredics, L.; Druzhinina, I.; Kurnitski, J.; Salonen, H. Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates. Microorganisms 2020, 8, 1940. https://doi.org/10.3390/microorganisms8121940
Andersson MA, Salo J, Kedves O, Kredics L, Druzhinina I, Kurnitski J, Salonen H. Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates. Microorganisms. 2020; 8(12):1940. https://doi.org/10.3390/microorganisms8121940
Chicago/Turabian StyleAndersson, Maria A., Johanna Salo, Orsolya Kedves, László Kredics, Irina Druzhinina, Jarek Kurnitski, and Heidi Salonen. 2020. "Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates" Microorganisms 8, no. 12: 1940. https://doi.org/10.3390/microorganisms8121940
APA StyleAndersson, M. A., Salo, J., Kedves, O., Kredics, L., Druzhinina, I., Kurnitski, J., & Salonen, H. (2020). Bioreactivity, Guttation and Agents Influencing Surface Tension of Water Emitted by Actively Growing Indoor Mould Isolates. Microorganisms, 8(12), 1940. https://doi.org/10.3390/microorganisms8121940








