Combination of Colistin and Azidothymidine Demonstrates Synergistic Activity against Colistin-Resistant, Carbapenem-Resistant Klebsiella pneumoniae
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolates
2.2. Antimicrobial Susceptibility
2.3. Synergistic Analysis
(MIC of drug B tested in combination)/(MIC of drug B tested alone).
2.4. In Vivo Study
2.5. Polymerase Chain Reaction Detection
2.6. Statistical Analyses
3. Results
3.1. Distribution of Resistance Mechanisms and In Vitro Susceptibilities
3.2. Checkerboard Analysis and In Vivo C. elegans Toxicity and Killing Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marston, H.D.; Dixon, D.M.; Knisely, J.M.; Palmore, T.N.; Fauci, A.S. Antimicrobial Resistance. JAMA 2016, 316, 1193–1204. [Google Scholar] [CrossRef] [PubMed]
- Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 11 November 2020).
- 2019 AR Threats Report. Available online: https://www.cdc.gov/drugresistance/biggest-threats.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fdrugresistance%2Fbiggest_threats.html (accessed on 11 November 2020).
- Kelly, A.M.; Mathema, B.; Larson, E.L. Carbapenem-resistant Enterobacteriaceae in the community: A scoping review. Int. J. Antimicrob. Agents 2017, 50, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Logan, L.K.; Weinstein, R.A. The epidemiology of carbapenem-resistant Enterobacteriaceae: The impact and evolution of a global menace. J. Infect. Dis. 2017, 215, S28–S36. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Fisher, J.F. Epidemiological expansion, structural studies, and clinical challenges of new beta-lactamases from gram-negative bacteria. Annu. Rev. Microbiol. 2011, 65, 455–478. [Google Scholar] [CrossRef] [PubMed]
- Lasko, M.J.; Nicolau, D.P. Carbapenem-resistant Enterobacterales: Considerations for treatment in the era of new antimicrobials and evolving enzymology. Curr. Infect. Dis. Rep. 2020, 22, 6. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Goodman, K.E.; Harris, A.D.; Tekle, T.; Roberts, A.; Taiwo, A.; Simner, P.J. Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin. Infect. Dis. 2017, 64, 257–264. [Google Scholar] [CrossRef]
- Chang, Y.T.; Siu, L.K.; Wang, J.T.; Wu, T.L.; Chen, Y.H.; Chuang, Y.C.; Lin, J.C.; Lu, P.L. Resistance mechanisms and molecular epidemiology of carbapenem-nonsusceptible Escherichia coli in Taiwan, 2012–2015. Infect. Drug Resist. 2019, 12, 2113–2123. [Google Scholar] [CrossRef]
- Rodriguez-Bano, J.; Gutierrez-Gutierrez, B.; Machuca, I.; Pascual, A. Treatment of infections caused by extended-spectrum-beta-lactamase-, ampC-, and carbapenemase-producing Enterobacteriaceae. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef]
- Rojas, L.J.; Salim, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Marshall, S.; Rudin, S.D.; et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: Laboratory detection and impact on mortality. Clin. Infect. Dis. 2017, 64, 711–718. [Google Scholar] [CrossRef]
- Keith, B.R.; White, G.; Wilson, H.R. In vivo efficacy of zidovudine (3′-azido-3′-deoxythymidine) in experimental gram-negative-bacterial infections. Antimicrob. Agents Chemother. 1989, 33, 479–483. [Google Scholar] [CrossRef]
- Herrmann, J.L.; Lagrange, P.H. Intracellular activity of zidovudine (3′-azido-3′-deoxythymidine, AZT) against Salmonella typhimurium in the macrophage cell line J774-2. Antimicrob. Agents Chemother. 1992, 36, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Peyclit, L.; Baron, S.A.; Yousfi, H.; Rolain, J.M. Zidovudine: A salvage therapy for mcr-1 plasmid-mediated colistin-resistant bacterial infections? Int. J. Antimicrob. Agents 2018, 52, 11–13. [Google Scholar] [CrossRef] [PubMed]
- Hind, C.K.; Dowson, C.G.; Sutton, J.M.; Jackson, T.; Clifford, M.; Garner, R.C.; Czaplewski, L. Evaluation of a library of FDA-approved drugs for their ability To potentiate antibiotics against multidrug-resistant Gram-negative pathogens. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.M.S.; Sioson, J.S.P.; Yap, J.M.; Ng, F.M.; Ching, H.S.V.; Teo, J.W.P.; Jureen, R.; Hill, J.; Chia, C.S.B. Repurposing zidovudine in combination with tigecycline for treating carbapenem-resistant Enterobacteriaceae infections. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.K.; Ma, L.; Chan, M.C.; Lin, Y.T.; Fung, C.P.; Wu, T.L.; Chuang, Y.C.; Lu, P.L.; Wang, J.T.; Lin, J.C.; et al. Carbapenem nonsusceptible Klebsiella pneumoniae in Taiwan: Dissemination and increasing resistance of carbapenemase producers during 2012–2015. Sci. Rep. 2018, 8, 8468. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: Thirtieth Informational Supplement; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020; Document M100-S30 CLSI. [Google Scholar]
- Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 11 November 2020).
- Tseng, S.P.; Wang, S.F.; Ma, L.; Wang, T.Y.; Yang, T.Y.; Siu, L.K.; Chuang, Y.C.; Lee, P.S.; Wang, J.T.; Wu, T.L.; et al. The plasmid-mediated fosfomycin resistance determinants and synergy of fosfomycin and meropenem in carbapenem-resistant Klebsiella pneumoniae isolates in Taiwan. J. Microbiol. Immunol. Infect. 2017, 50, 653–661. [Google Scholar] [CrossRef]
- Yang, T.Y.; Wang, S.F.; Lin, J.E.; Griffith, B.T.S.; Lian, S.H.; Hong, Z.D.; Lin, L.; Lu, P.L.; Tseng, S.P. Contributions of insertion sequences conferring colistin resistance in Klebsiella pneumoniae. Int. J. Antimicrob. Agents 2020, 55, 105894. [Google Scholar] [CrossRef]
- Kakuda, T.N.; Page, L.M.; Anderson, P.L.; Henry, K.; Schacker, T.W.; Rhame, F.S.; Acosta, E.P.; Brundage, R.C.; Fletcher, C.V. Pharmacological basis for concentration-controlled therapy with zidovudine, lamivudine, and indinavir. Antimicrob. Agents Chemother. 2001, 45, 236–242. [Google Scholar] [CrossRef][Green Version]
- Lutgring, J.D. Carbapenem-resistant Enterobacteriaceae: An emerging bacterial threat. Semin. Diagn. Pathol. 2019, 36, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Porreca, A.M.; Sullivan, K.V.; Gallagher, J.C. The epidemiology, evolution, and treatment of KPC-producing organisms. Curr. Infect. Dis. Rep. 2018, 20, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Colistin and tigecycline resistance in carbapenem-resistant Enterobacteriaceae: Checkmate to our last line of defense. Infect. Control. Hosp. Epidemiol. 2016, 37, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Qamar, S.; Shaheen, N.; Shakoor, S.; Farooqi, J.; Jabeen, K.; Hasan, R. Frequency of colistin and fosfomycin resistance in carbapenem-resistant Enterobacteriaceae from a tertiary care hospital in Karachi. Infect. Drug Resist. 2017, 10, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, Q.L.; Shen, Y.; Zhang, Y.; Yang, J.W.; Shu, L.B.; Zhou, H.W.; Wang, Y.; Wang, B.; Zhang, R.; et al. Rapid increase in prevalence of carbapenem-resistant Enterobacteriaceae (CRE) and emergence of colistin resistance gene mcr-1 in CRE in a hospital in Henan, China. J. Clin. Microbiol. 2018, 56. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Chen, L.; Tang, Y.W.; Kreiswirth, B.N. Emergence of the mcr-1 colistin resistance gene in carbapenem-resistant Enterobacteriaceae. Lancet Infect. Dis. 2016, 16, 287–288. [Google Scholar] [CrossRef]
- Malchione, M.D.; Torres, L.M.; Hartley, D.M.; Koch, M.; Goodman, J.L. Carbapenem and colistin resistance in Enterobacteriaceae in Southeast Asia: Review and mapping of emerging and overlapping challenges. Int. J. Antimicrob. Agents 2019, 54, 381–399. [Google Scholar] [CrossRef]
- Doleans-Jordheim, A.; Bergeron, E.; Bereyziat, F.; Ben-Larbi, S.; Dumitrescu, O.; Mazoyer, M.A.; Morfin, F.; Dumontet, C.; Freney, J.; Jordheim, L.P. Zidovudine (AZT) has a bactericidal effect on enterobacteria and induces genetic modifications in resistant strains. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 1249–1256. [Google Scholar] [CrossRef]
- Jordheim, L.P.; Durantel, D.; Zoulim, F.; Dumontet, C. Advances in the development of nucleoside and nucleotide analogues for cancer and viral diseases. Nat. Rev. Drug Discov. 2013, 12, 447–464. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Liu, P.; Dai, S.H.; Sun, J.; Liu, Y.H.; Liao, X.P. Activity of tigecycline or colistin in combination with zidovudine against Escherichia coli harboring tet(X) and mcr-1. Antimicrob. Agents Chemother. 2020. [Google Scholar] [CrossRef]
- Lin, Y.W.; Abdul Rahim, N.; Zhao, J.; Han, M.L.; Yu, H.H.; Wickremasinghe, H.; Chen, K.; Wang, J.; Paterson, D.L.; Zhu, Y.; et al. Novel polymyxin combination with the antiretroviral zidovudine exerts synergistic killing against NDM-producing multidrug-resistant Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2019, 63, e02176-18. [Google Scholar] [CrossRef]
- Falagas, M.E.; Voulgaris, G.L.; Tryfinopoulou, K.; Giakkoupi, P.; Kyriakidou, M.; Vatopoulos, A.; Coates, A.; Hu, Y.; Colistin—Azidothymidine Hellenic Study Group. Synergistic activity of colistin with azidothymidine against colistin-resistant Klebsiella pneumoniae clinical isolates collected from inpatients in Greek hospitals. Int. J. Antimicrob. Agents 2019, 53, 855–858. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, Y.; Coates, A. Azidothymidine produces synergistic activity in combination with colistin against antibiotic-resistant Enterobacteriaceae. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Elwell, L.P.; Ferone, R.; Freeman, G.A.; Fyfe, J.A.; Hill, J.A.; Ray, P.H.; Richards, C.A.; Singer, S.C.; Knick, V.B.; Rideout, J.L.; et al. Antibacterial activity and mechanism of action of 3′-azido-3′-deoxythymidine (BW A509U). Antimicrob. Agents Chemother. 1987, 31, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Loose, M.; Naber, K.G.; Hu, Y.; Coates, A.; Wagenlehner, F.M.E. Serum bactericidal activity of colistin and azidothymidine combinations against mcr-1-positive colistin-resistant Escherichia coli. Int. J. Antimicrob. Agents 2018, 52, 783–789. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.K.; Reyes-Ortega, F.; Velkov, T.; Li, J. Antibiotic-non-antibiotic combinations for combating extremely drug-resistant Gram-negative ‘superbugs’. Essays Biochem. 2017, 61, 115–125. [Google Scholar] [PubMed]
- Wattanagoon, Y.; Na Bangchang, K.; Hoggard, P.G.; Khoo, S.H.; Gibbons, S.E.; Phiboonbhanakit, D.; Karbwang, J.; Back, D.J. Pharmacokinetics of zidovudine phosphorylation in human immunodeficiency virus-positive thai patients and healthy volunteers. Antimicrob. Agents Chemother. 2000, 44, 1986–1989. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.M.; Meenhorst, P.L.; ten Napel, C.H.; Mulder, J.W.; Neef, C.; Koks, C.H.; Bult, A.; Beijnen, J.H. Pharmacokinetic variability of zidovudine in HIV-infected individuals: Subgroup analysis and drug interactions. AIDS 1994, 8, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Drew, R.H.; Weller, S.; Gallis, H.A.; Walmer, K.A.; Bartlett, J.A.; Blum, M.R. Bioequivalence assessment of zidovudine (Retrovir) syrup, solution, and capsule formulations in patients infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 1989, 33, 1801–1803. [Google Scholar] [CrossRef]
- Hoetelmans, R.M.; Burger, D.M.; Meenhorst, P.L.; Beijnen, J.H. Pharmacokinetic individualisation of zidovudine therapy. Current state of pharmacokinetic-pharmacodynamic relationships. Clin. Pharmacokinet. 1996, 30, 314–327. [Google Scholar] [CrossRef]
- Lewin, C.S.; Allen, R.; Amyes, S.G. Zidovudine-resistance in Salmonella typhimurium and Escherichia coli. J. Antimicrob. Chemother. 1990, 25, 706–708. [Google Scholar] [CrossRef]
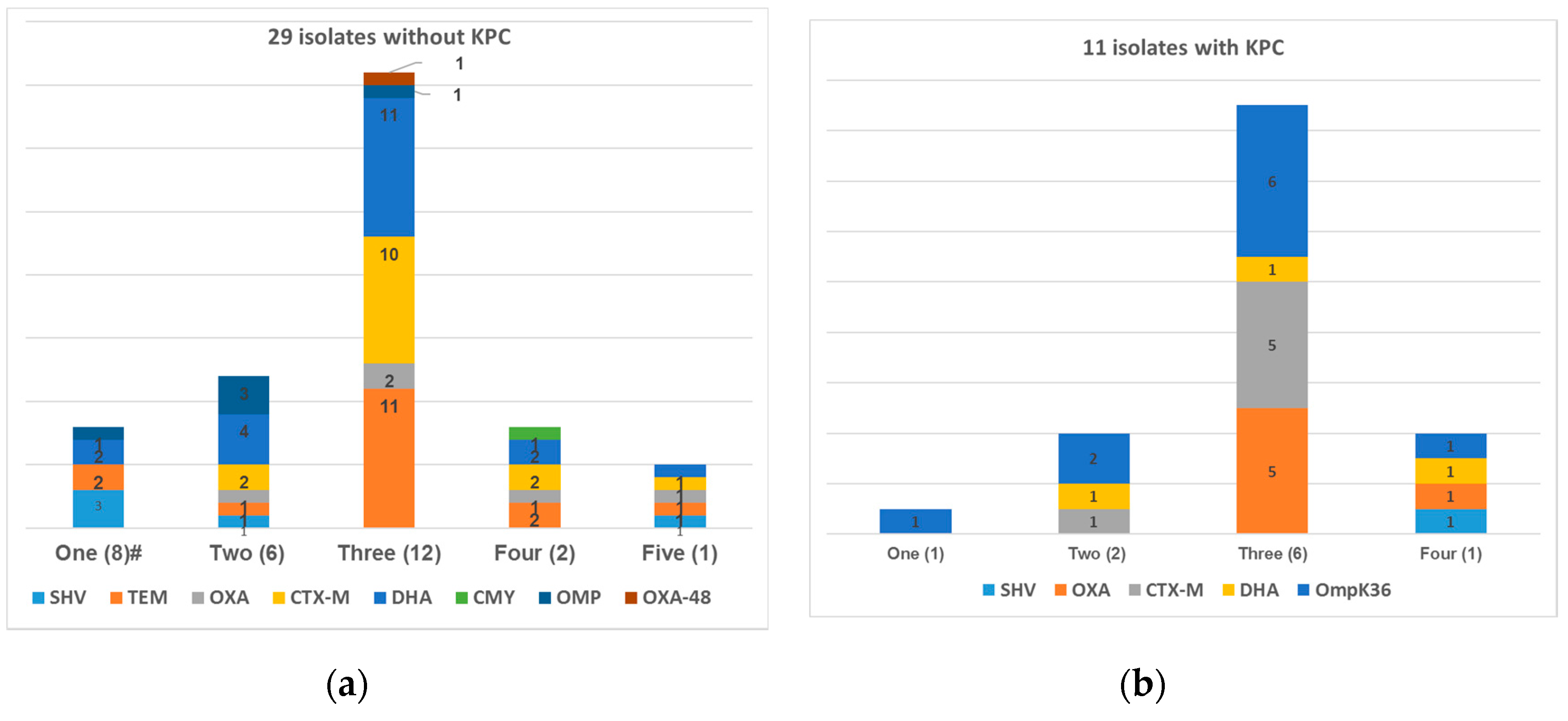
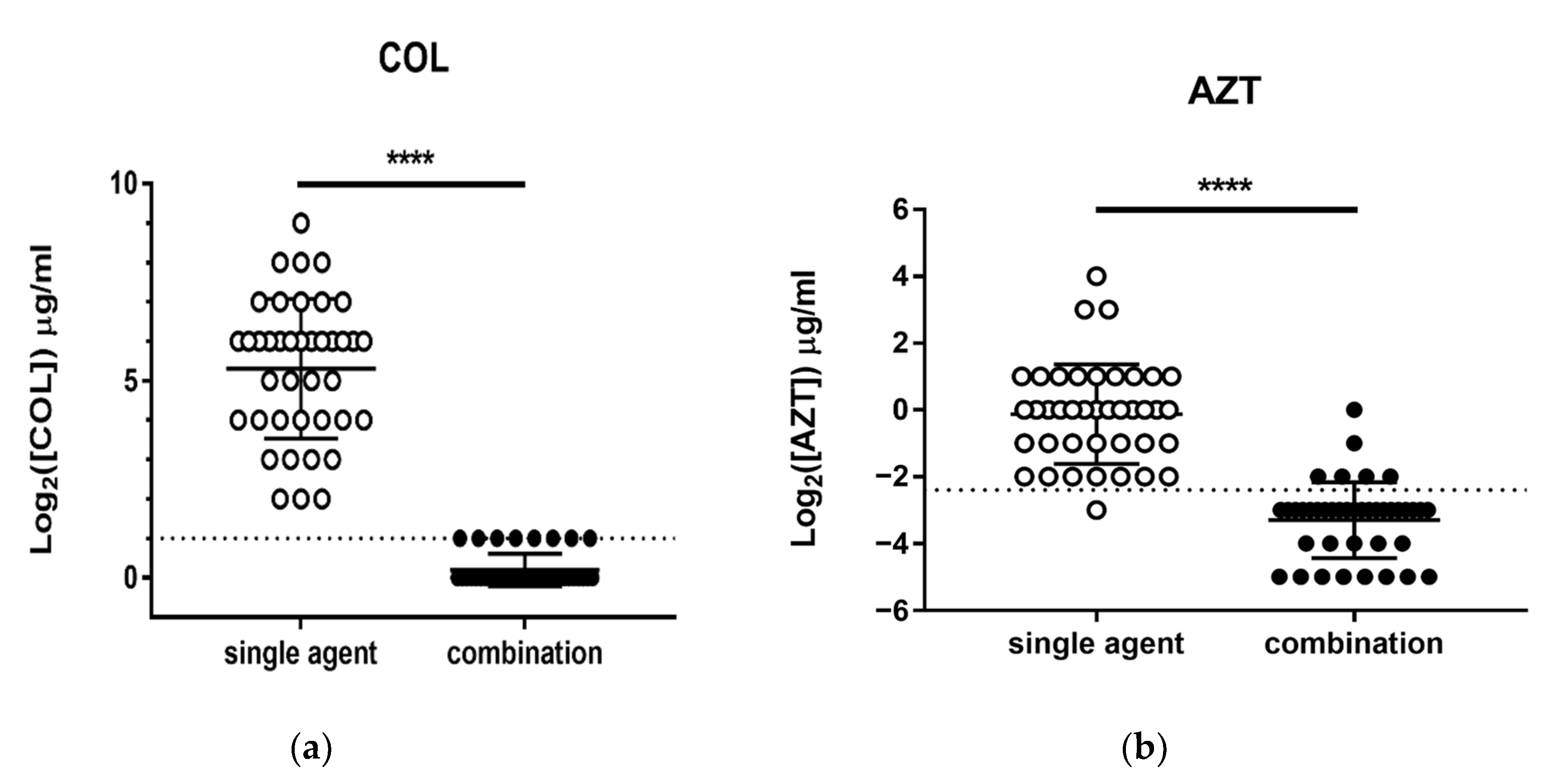
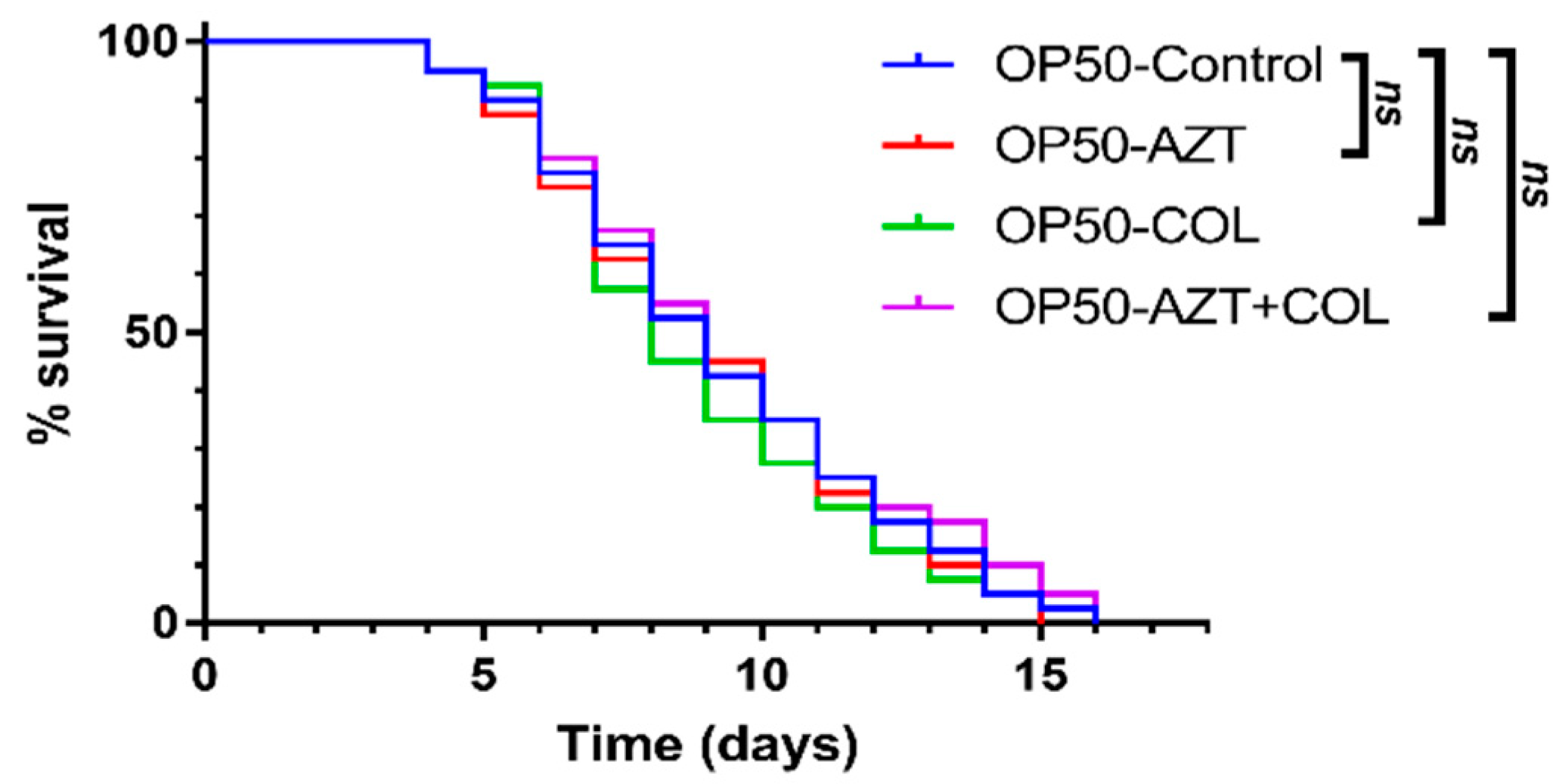
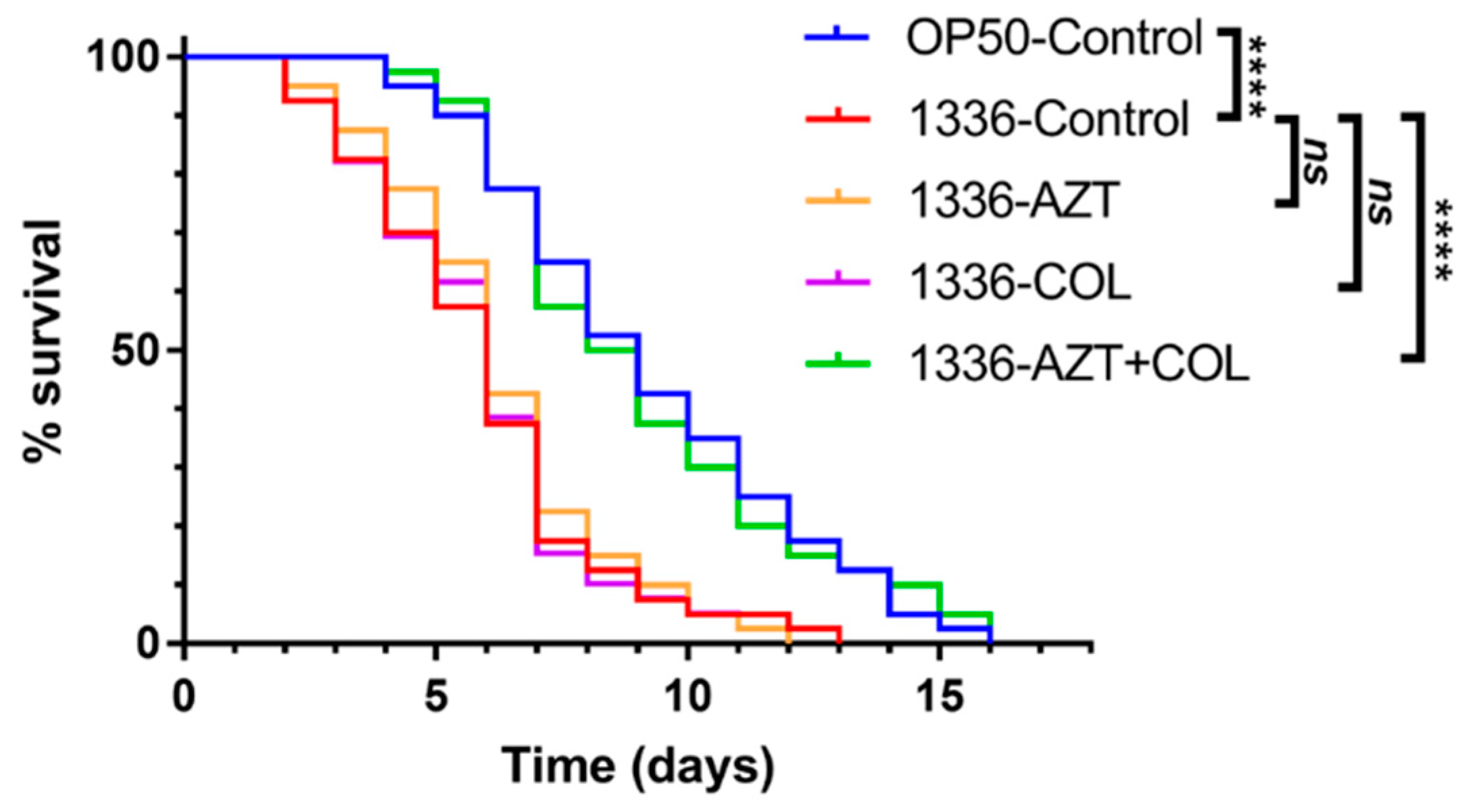
| Source | No. of Isolates |
|---|---|
| Urine | 11 |
| Sputum | 10 |
| Stool | 4 |
| Pus/wound | 3 |
| Abscess | 3 |
| Blood | 2 |
| Endotracheal | 2 |
| Ascites | 2 |
| Bile | 2 |
| Drainage | 1 |
| Antimicrobial Agents | Antibiotic Susceptibility | ||
|---|---|---|---|
| S | I | R | |
| Ampicillin | 0.0% | 0.0% | 100.0% |
| Ceftazidime | 0.0% | 2.5% | 97.5% |
| Cefazolin | 0.0% | 0.0% | 100.0% |
| Cefepime | 5.0% | 7.5% | 87.5% |
| Cefoxitin | 2.5% | 2.5% | 95.0% |
| Ceftriaxone | 0.0% | 0.0% | 100.0% |
| Cefotaxime | 2.5% | 0.0% | 97.5% |
| Imipenem | 2.5% | 10.0% | 87.5% |
| Meropenem | 7.5% | 0.0% | 92.5% |
| Doripenem | 7.5% | 2.5% | 90.0% |
| Ertapenem | 0.0% | 2.5% | 97.5% |
| Aztreonam | 7.5% | 2.5% | 90.0% |
| Piperacillin/Tazobactam | 5.0% | 0.0% | 95.0% |
| Tigecycline | 87.5% | 10.0% | 2.5% |
| Ciprofloxacin | 2.5% | 0.0% | 97.5% |
| Levofloxacin | 2.5% | 0.0% | 97.5% |
| Gentamicin | 32.5% | 0.0% | 67.5% |
| Amikacin | 70.0% | 0.0% | 30.0% |
| Sulfamethoxazole-Trimethoprim | 12.5% | 0.0% | 87.5% |
| Agents | MIC (μg/mL) | MIC in Combination (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Range | MIC50 | MIC75 | MIC90 | Range | MIC50 | MIC75 | MIC90 | |
| Colistin | 4–512 | 64 | 64 | 128 | 1–2 | 1 | 1 | 2 |
| AZT | 0.125–16 | 1 | 2 | 2 | 0.03125–1 | 0.125 | 0.125 | 0.25 |
| Group | Activity of the Combination | FICI Criteria | Total No. (%) of Isolates | |
|---|---|---|---|---|
| CCRKP (n = 40) | KPC-producer (n = 11) | synergy | ≤0.5 | 11 (100.0%) |
| No interaction | >0.5–4 | 0 | ||
| Antagonism | >4 | 0 | ||
| non-KPC-producer (n = 29) | synergy | ≤0.5 | 29 (100.0%) | |
| No interaction | >0.5–1 | 0 | ||
| Antagonism | >4 | 0 | ||
| Test | Group | Median Survival (Days) | p Value | Risk Ratio | 95% CI | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| In vivo toxicity assay | OP50-Control | 9 | - | 1 | - | - |
| OP50-AZT | 9 | 0.7901 | 0.934 | 0.57 | 1.54 | |
| OP50-COL | 8 | 0.4573 | 0.827 | 0.50 | 1.36 | |
| OP50-AZT + COL | 9 | 0.6177 | 1.13 | 0.69 | 1.87 | |
| In vivo killing assay | OP50-Control | 9 | <0.0001 | 0.27 | 0.16 | 0.47 |
| 1336-Control | 6 | - | 1 | - | - | |
| 1336-AZT | 6 | 0.7254 | 1.097 | 0.65 | 1.84 | |
| 1336-COL | 6 | 0.8561 | 0.953 | 0.56 | 1.61 | |
| 1336-AZT + COL | 8.5 | <0.0001 | 0.288 | 0.17 | 0.50 | |
| Species | Country | No. | Resistance Phenotype | Resistance Mechanism(s) | MIC of AZT (mg/L) | MIC of Colistin (mg/L) | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| Alone | Combination | Alone | Combination | ||||||
| K. pneumoniae | Greece | 100 | Colistin | NA | 0.125–4 | 0.0625–1 | 4–128 | 0.25–16 | [34] |
| E. coli | China | 9 | Colistin and tigecycline | tet(X) and mcr-1 | 0.5–4 | 0.3–1.5 | 4–8 | <0.13–4 | [32] |
| K. pneumoniae, E. coli, E. cloacae | UK | 7 | Carbapenem | blaNDM-1 | 2–4 | 0.25–16 | 0.125–1 | Reduced 32–256 fold | [35] |
| E. coli | UK | 13 | Colistin | mcr-1 | 8–64 | 2–8 | Reduced 4–256 fold | [35] | |
| K. pneumoniae | Taiwan | 40 | Colistin and carbapenem | multiple, blaKPC | 0.125–16 | 0.03125–1 | 4–512 | 1–2 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-T.; Yang, T.-Y.; Lu, P.-L.; Lin, S.-Y.; Wang, L.-C.; Wang, S.-F.; Hsieh, Y.-J.; Tseng, S.-P. Combination of Colistin and Azidothymidine Demonstrates Synergistic Activity against Colistin-Resistant, Carbapenem-Resistant Klebsiella pneumoniae. Microorganisms 2020, 8, 1964. https://doi.org/10.3390/microorganisms8121964
Chang Y-T, Yang T-Y, Lu P-L, Lin S-Y, Wang L-C, Wang S-F, Hsieh Y-J, Tseng S-P. Combination of Colistin and Azidothymidine Demonstrates Synergistic Activity against Colistin-Resistant, Carbapenem-Resistant Klebsiella pneumoniae. Microorganisms. 2020; 8(12):1964. https://doi.org/10.3390/microorganisms8121964
Chicago/Turabian StyleChang, Ya-Ting, Tsung-Ying Yang, Po-Liang Lu, Shang-Yi Lin, Liang-Chun Wang, Sheng-Fan Wang, Ya-Ju Hsieh, and Sung-Pin Tseng. 2020. "Combination of Colistin and Azidothymidine Demonstrates Synergistic Activity against Colistin-Resistant, Carbapenem-Resistant Klebsiella pneumoniae" Microorganisms 8, no. 12: 1964. https://doi.org/10.3390/microorganisms8121964
APA StyleChang, Y.-T., Yang, T.-Y., Lu, P.-L., Lin, S.-Y., Wang, L.-C., Wang, S.-F., Hsieh, Y.-J., & Tseng, S.-P. (2020). Combination of Colistin and Azidothymidine Demonstrates Synergistic Activity against Colistin-Resistant, Carbapenem-Resistant Klebsiella pneumoniae. Microorganisms, 8(12), 1964. https://doi.org/10.3390/microorganisms8121964







