Marine Bacteria Display Different Escape Mechanisms When Facing Their Protozoan Predators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial and Protozoan Strains
2.2. Bacterial Labeling
2.3. Bacterial and Amoeba Enumeration in NSS
2.4. Bacterial Survival during A. castellanii Co-Incubation
2.5. Cytotoxicity Assay against A. castellanii
2.6. Bacterial Localization within A. castellanii Using CLSM
2.7. Colocalization Studies with Intracellular Organelles
2.8. Bacterial Localization within A. castellanii Using Transmission Electron Microscopy (TEM)
2.9. Biofilm Formation
2.10. Amoeba Against Bacterial Biofilm
2.11. Evaluation of the Presence of Planktonic Bacteria
2.12. Data Extraction from Images and Statistics
3. Results
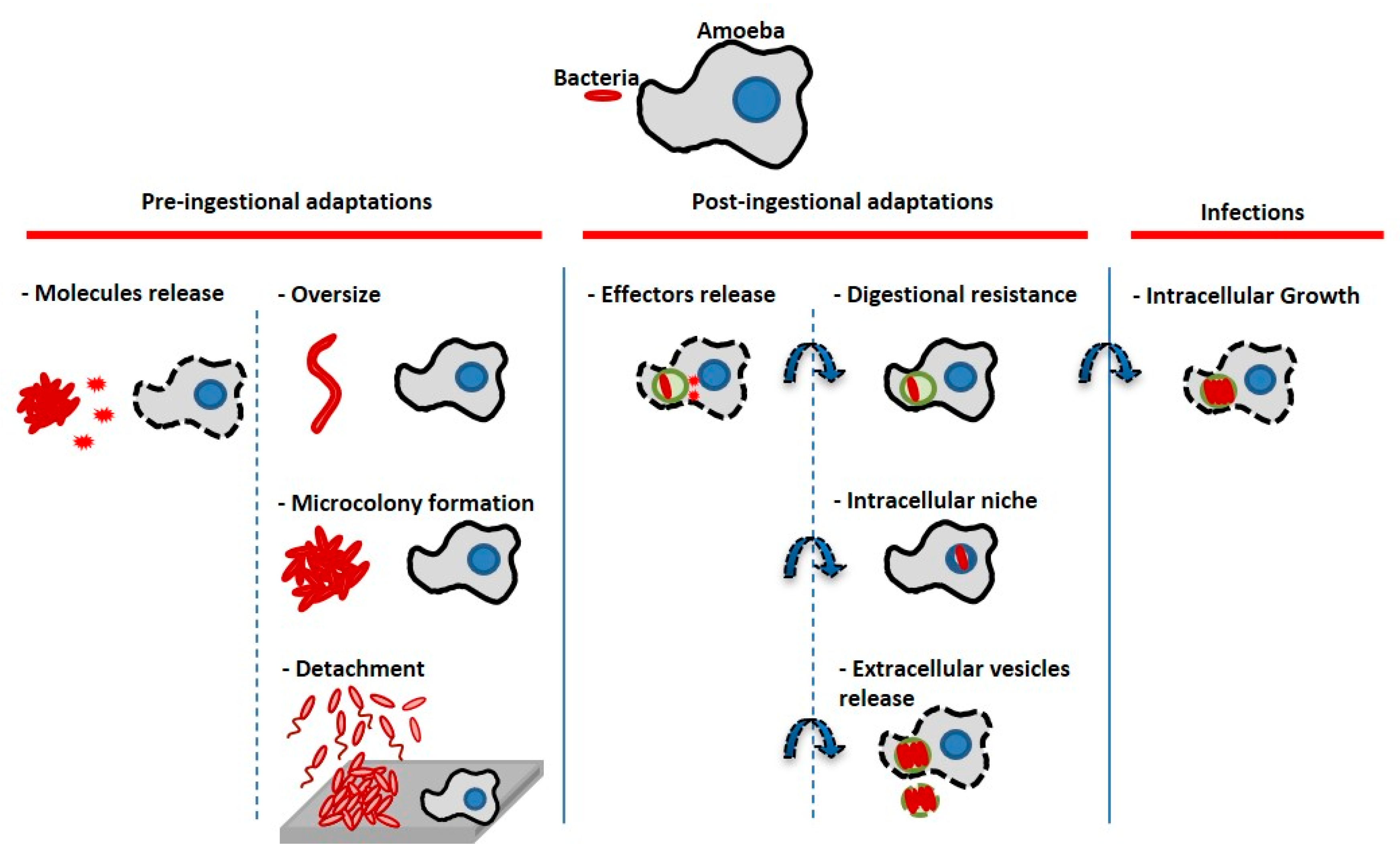
3.1. The Bacterial Strains Are Not All Digested and Follow Different Intracellular Paths within A. castellanii
3.1.1. A. castellanii interact with the Marine Bacteria TC4, TC5, TC10, and TC11
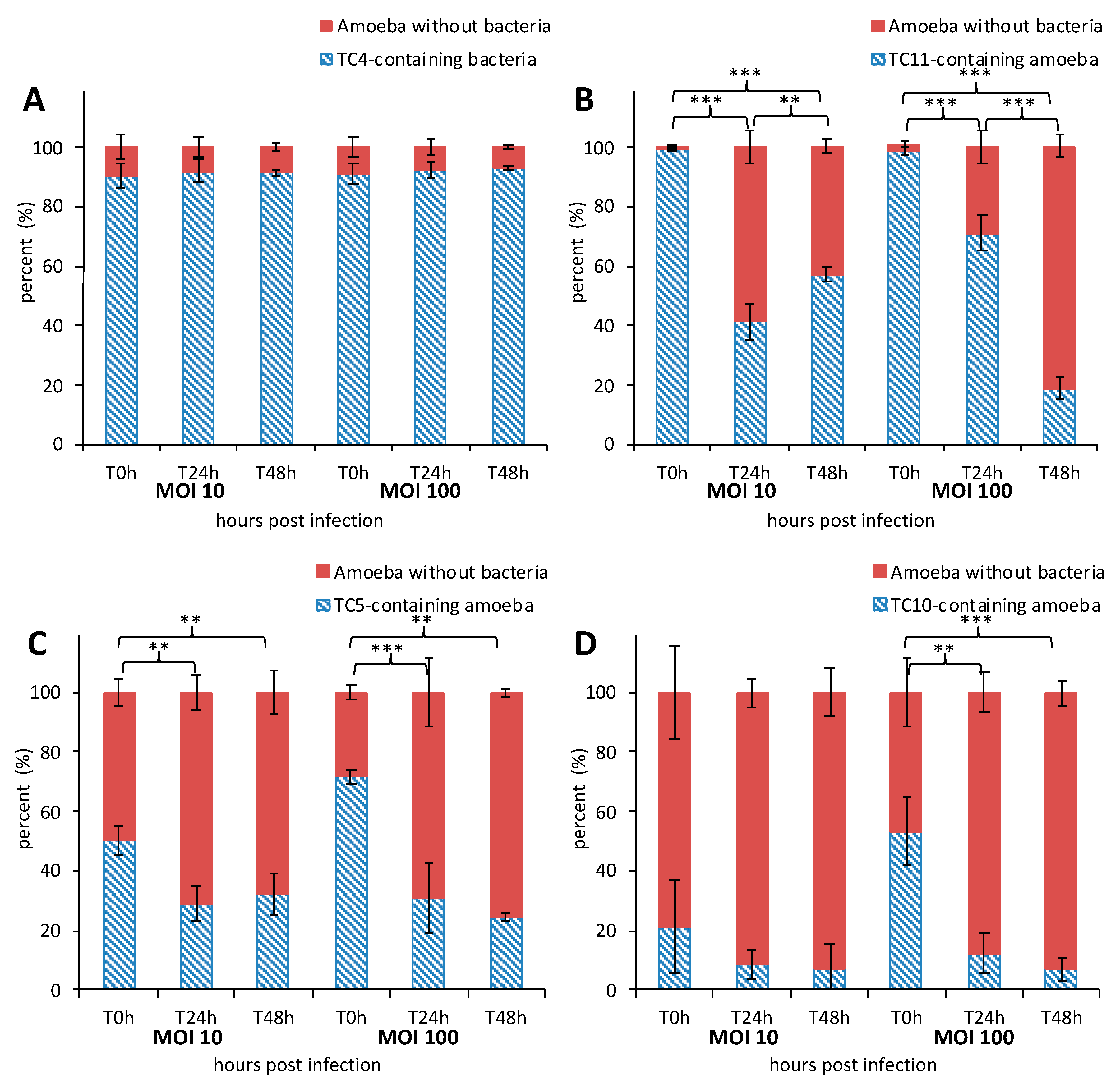
3.1.2. All the Marine Bacteria Are Phagocytized in Different Proportion and Persist Over 48 h within A. castellanii
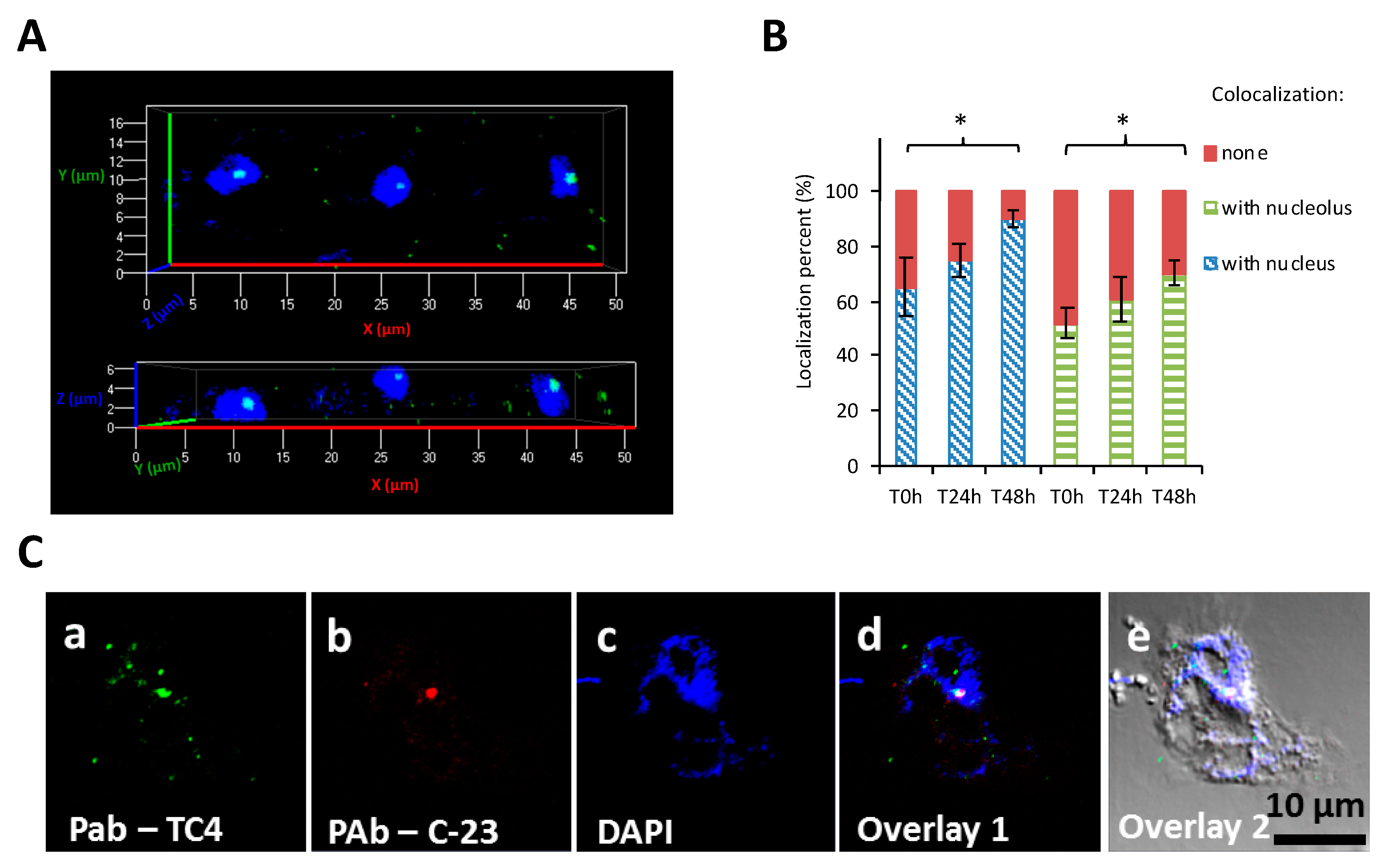
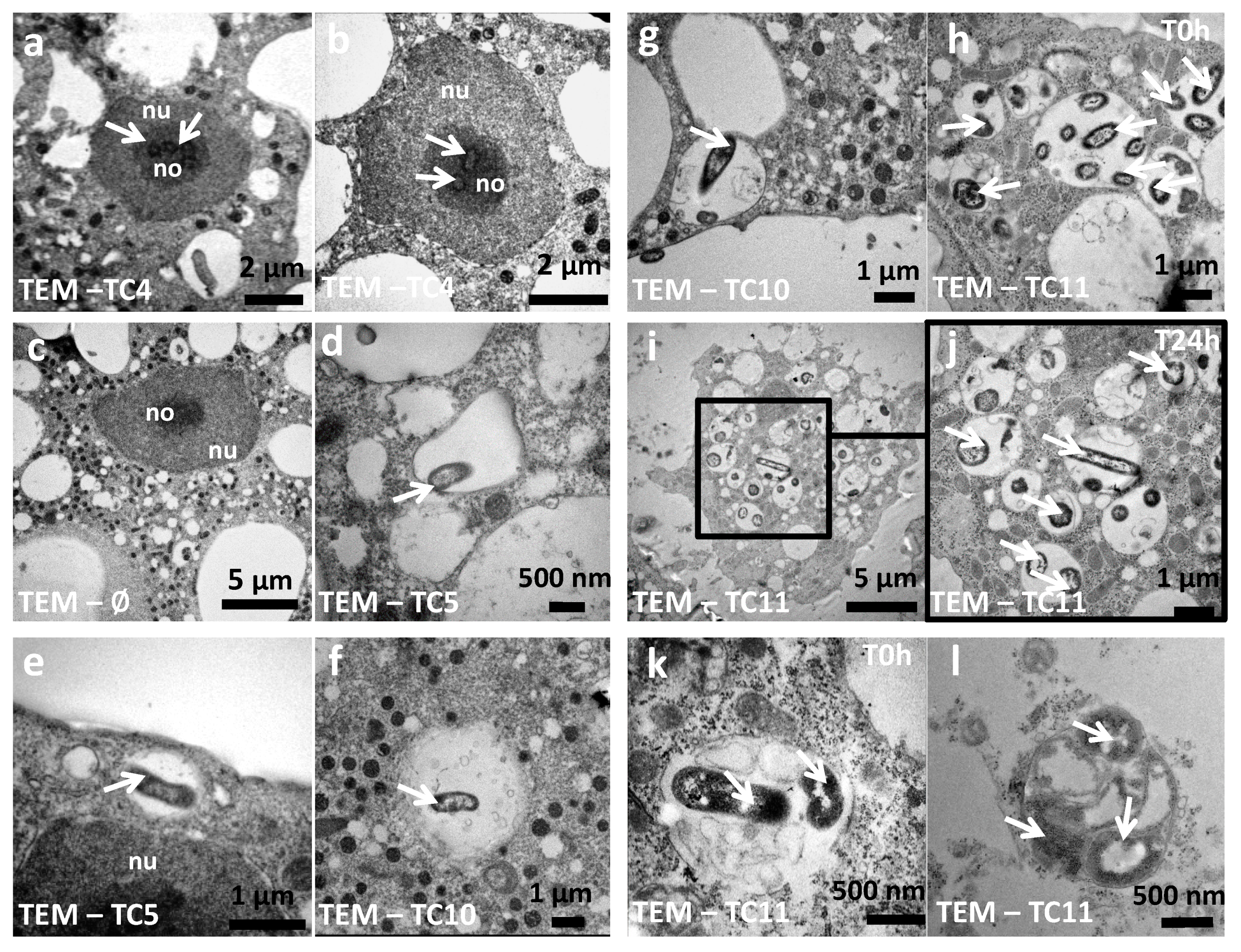
3.1.3. TC4 Is Located within the Nucleolar Compartment
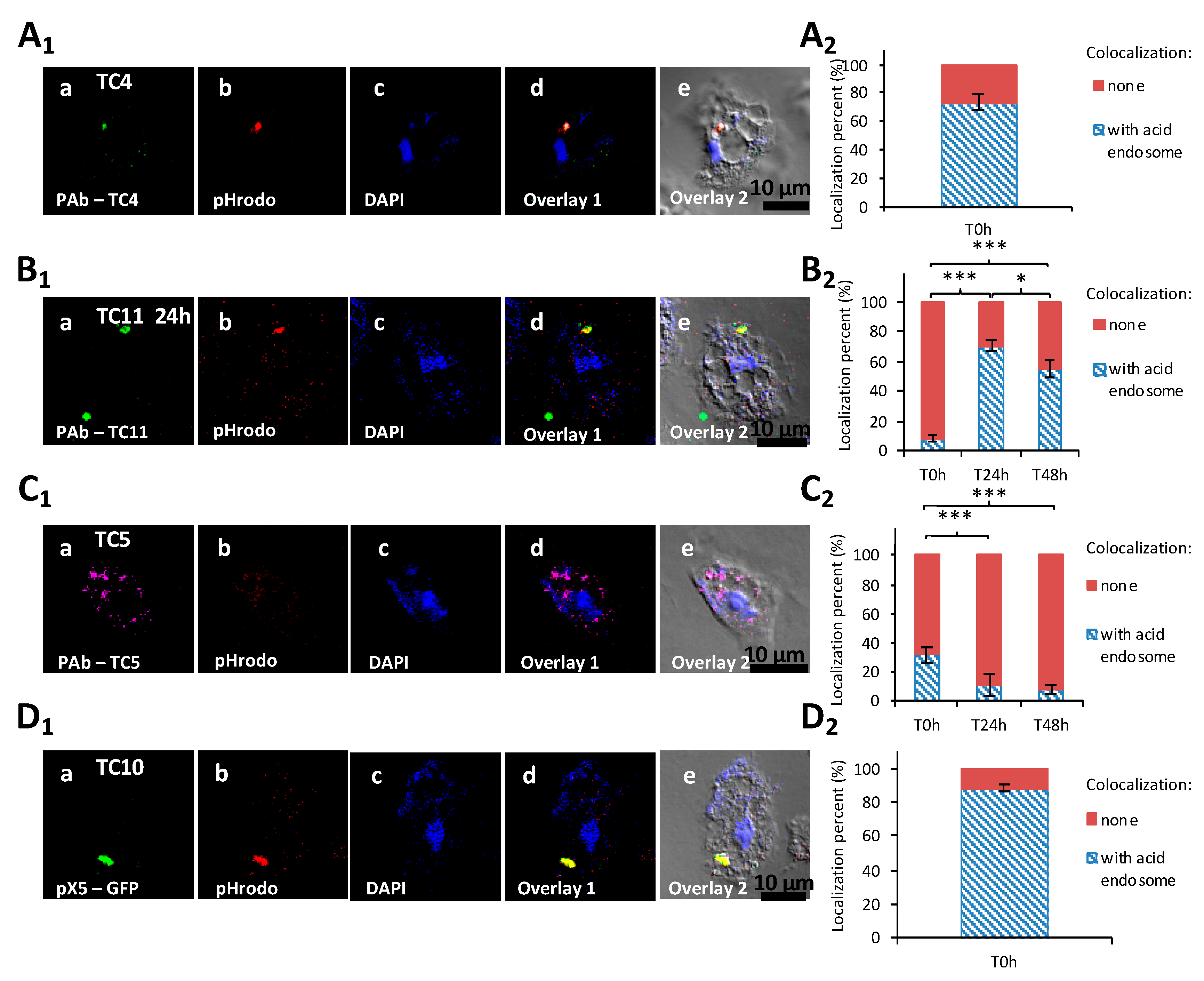
3.1.4. TC5, TC10, and TC11 are Localized in the Cell Cytoplasm and TC11 Appears to Be Expelled Outside the Protozoan Host at 24 h
3.1.5. The Bacterial Strains Follow Different Intracellular Paths within A. castellanii
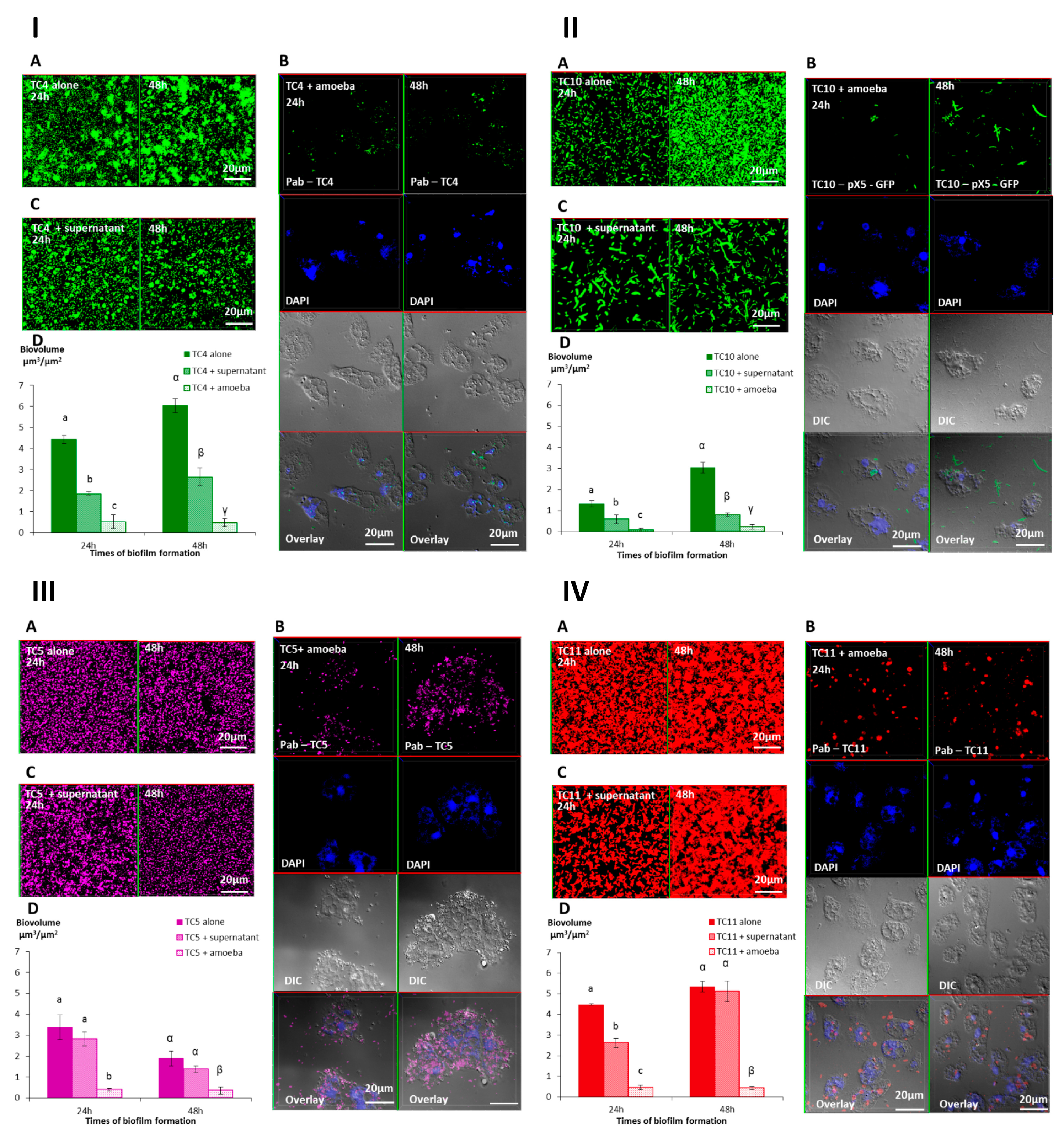
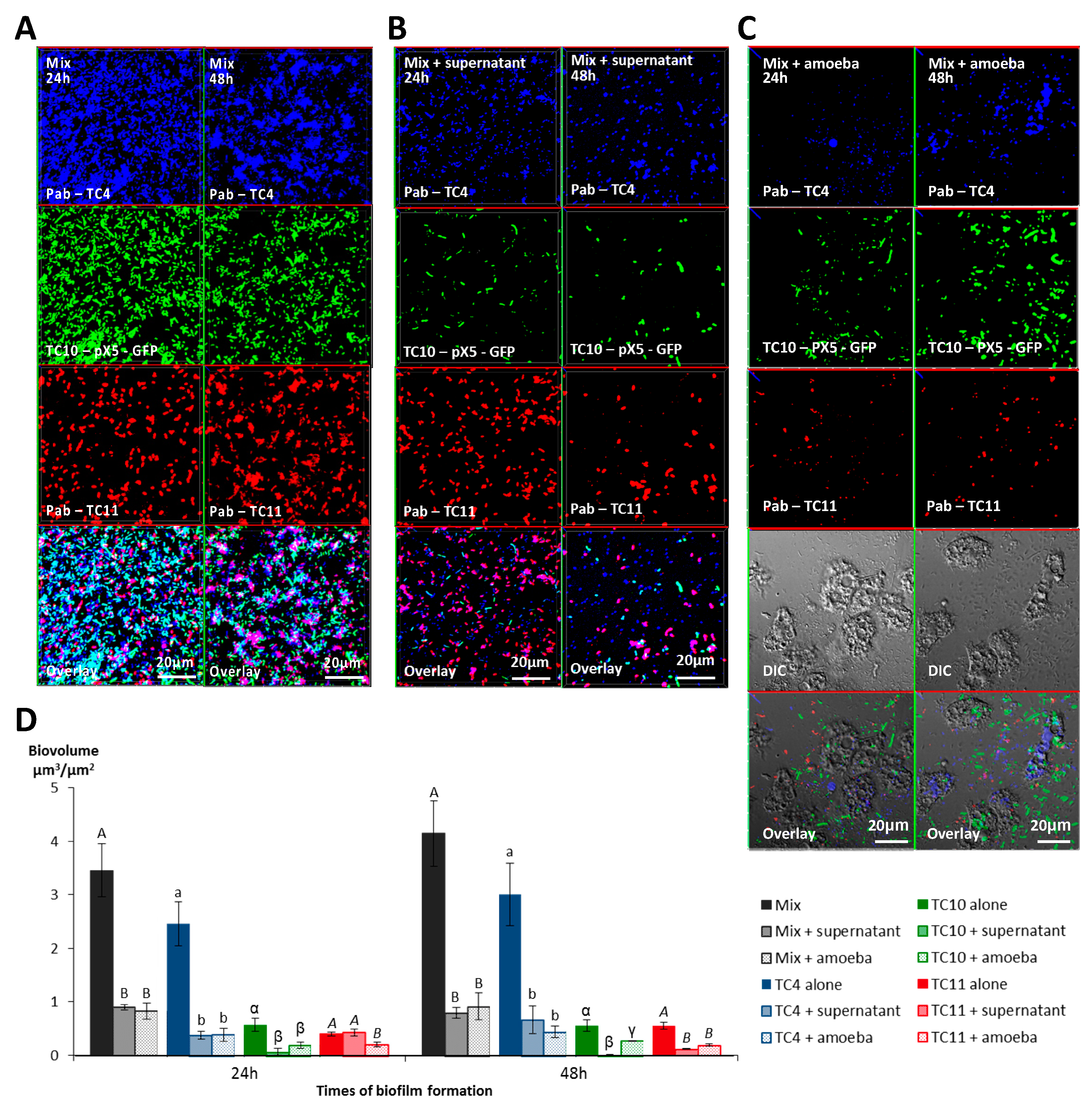
3.2. The Bacterial Biofilms Are Not Grazed Upon by A. castellanii
3.2.1. The Four Bacteria Are Able to form a Biofilm in NSS
3.2.2. A. castellanii Induces Biofilm Detachment from the Surface
3.2.3. The Supernatant of A. castellanii Induces Biofilm Detachment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matz, C.; Webb, J.S.; Schupp, P.J.; Phang, S.Y.; Penesyan, A.; Egan, S.; Steinberg, P.; Kjelleberg, S. Marine Biofilm Bacteria Evade Eukaryotic Predation by Targeted Chemical Defense. PLoS ONE 2008, 3, e2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherr, E.B.; Sherr, B.F. Significance of predation by protists in aquatic microbial food webs. Antonie Van Leeuwenhoek 2002, 81, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Matz, C.; Jurgens, K. High motility reduces grazing mortality of planktonic bacteria. Appl. Environ. Microbiol. 2005, 71, 921–929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jousset, A.; Rochat, L.; Scheu, S.; Bonkowski, M.; Keel, C. Predator-Prey Chemical Warfare Determines the Expression of Biocontrol Genes by Rhizosphere-Associated Pseudomonas Fluorescens. Appl. Environ. Microbiol. 2010, 76, 5263–5268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matz, C.; Kjelleberg, S. Off the hook--how bacteria survive protozoan grazing. Trends Microbiol. 2005, 13, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Brown, M.R. Trojan horses of the microbial world: Protozoa and the survival of bacterial pathogens in the environment. Microbiology 1994, 140, 1253–1259. [Google Scholar] [CrossRef] [Green Version]
- Molmeret, M.; Horn, M.; Wagner, M.; Santic, M.; Abu Kwaik, Y. Amoebae as training grounds for intracellular bacterial pathogens. Appl. Environ. Microbiol. 2005, 71, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Rowbotham, T.J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 1980, 33, 1179–1183. [Google Scholar] [CrossRef] [Green Version]
- Espinoza-Vergara, G.; Noorian, P.; Silva-Valenzuela, C.A.; Raymond, B.B.A.; Allen, C.; Hoque, M.M.; Sun, S.; Johnson, M.S.; Pernice, M.; Kjelleberg, S.; et al. Vibrio cholerae residing in food vacuoles expelled by protozoa are more infectious in vivo. Nat. Microbiol. 2019, 4, 2466–2474. [Google Scholar] [CrossRef]
- Van der Henst, C.; Scrignari, T.; Maclachlan, C.; Blokesch, M. An intracellular replication niche for Vibrio cholerae in the amoeba Acanthamoeba castellanii. ISME J. 2016, 10, 897–910. [Google Scholar] [CrossRef] [Green Version]
- Van der Henst, C.; Vanhove, A.S.; Drebes Dorr, N.C.; Stutzmann, S.; Stoudmann, C.; Clerc, S.; Scrignari, T.; Maclachlan, C.; Knott, G.; Blokesch, M. Molecular insights into Vibrio cholerae’s intra-amoebal host-pathogen interactions. Nat. Commun. 2018, 9, 3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, F.; Horn, M. Intranuclear bacteria: Inside the cellular control center of eukaryotes. Trends Cell Biol. 2015, 25, 339–346. [Google Scholar] [CrossRef] [PubMed]
- De Vargas, C.; Audic, S.; Henry, N.; Decelle, J.; Mahe, F.; Logares, R.; Lara, E.; Berney, C.; Le Bescot, N.; Probert, I.; et al. Ocean plankton. Eukaryotic plankton diversity in the sunlit ocean. Science 2015, 348, 1261605. [Google Scholar] [CrossRef] [Green Version]
- Camps, M.; Briand, J.F.; Guentas-Dombrowsky, L.; Culioli, G.; Bazire, A.; Blache, Y. Antifouling activity of commercial biocides vs. natural and natural-derived products assessed by marine bacteria adhesion bioassay. Marine Pollut. Bull. 2011, 62, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Brian-Jaisson, F.; Ortalo-Magne, A.; Guentas-Dombrowsky, L.; Armougom, F.; Blache, Y.; Molmeret, M. Identification of bacterial strains isolated from the Mediterranean Sea exhibiting different abilities of biofilm formation. Microb. Ecol. 2014, 68, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Favre, L.; Ortalo-Magne, A.; Greff, S.; Perez, T.; Thomas, O.P.; Martin, J.C.; Culioli, G. Discrimination of Four Marine Biofilm-Forming Bacteria by LC-MS Metabolomics and Influence of Culture Parameters. J. Proteome Res. 2017, 16, 1962–1975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Kirat-Chatel, S.; Puymege, A.; Duong, T.H.; Van Overtvelt, P.; Bressy, C.; Belec, L.; Dufrene, Y.F.; Molmeret, M. Phenotypic Heterogeneity in Attachment of Marine Bacteria toward Antifouling Copolymers Unraveled by AFM. Front. Microbiol. 2017, 8, 1399. [Google Scholar] [CrossRef]
- Guillonneau, R.; Baraquet, C.; Bazire, A.; Molmeret, M. Multispecies Biofilm Development of Marine Bacteria Implies Complex Relationships through Competition and Synergy and Modification of Matrix Components. Front Microbiol. 2018, 9, 1960. [Google Scholar] [CrossRef] [Green Version]
- Sawyer, T.K. Marine amebae from clean and stressed bottom sediments of the atlantic ocean and gulf of Mexico. J. Protozool. 1980, 27, 13–32. [Google Scholar] [CrossRef]
- Page, F.C. Marine Gymnamoebae; Institute of Terestrial Ecology: Cambridge, UK, 1983. [Google Scholar]
- Fernandez, M.C.A.; Crespo, E.P.; Mallen, M.M.; Ares, M.P.M.P.; Casas, M.L.C. Marine Amoebae from Waters of Northwest Spain, with Comments on a Potentially Pathogenic Euryhaline Species. J. Protozool. 1989, 36, 239–241. [Google Scholar] [CrossRef]
- Rogerson, A.; Laybourn-Parry, J. The abundance of marine naked amoebae in the water column of the Clyde estuary. Estuarine Coastal Shelf Sci. 1992, 34, 187–196. [Google Scholar] [CrossRef]
- Hilbi, H.; Weber, S.S.; Ragaz, C.; Nyfeler, Y.; Urwyler, S. Environmental predators as models for bacterial pathogenesis. Environ. Microbiol. 2007, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Laskowski-Arce, M.A.; Orth, K. Acanthamoeba castellanii Promotes the Survival of Vibrio parahaemolyticus. Appl. Environ. Microbiol. 2008, 74, 7183–7188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Batlle, M.; Martin-Rodriguez, A.J.; Lopez-Arencibia, A.; Sifaoui, I.; Liendo, A.R.; Bethencourt Estrella, C.J.; Garcia Mendez, A.B.; Chiboub, O.; Hajaji, S.; Valladares, B.; et al. In vitro interactions of Acanthamoeba castellanii Neff and Vibrio harveyi. Exp. Parasitol. 2017, 183, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Mårdén, P.; Tunlid, A.; Malmcrona-Friberg, K.; Odham, G.; Kjelleberg, S. Physiological and morphological changes during short term starvation of marine bacterial islates. Arch. Microbiol. 1985, 142, 326–332. [Google Scholar] [CrossRef]
- Taylor-Mulneix, D.L.; Bendor, L.; Linz, B.; Rivera, I.; Ryman, V.E.; Dewan, K.K.; Wagner, S.M.; Wilson, E.F.; Hilburger, L.J.; Cuff, L.E.; et al. Bordetella bronchiseptica exploits the complex life cycle of Dictyostelium discoideum as an amplifying transmission vector. PLoS Biol. 2017, 15, e2000420. [Google Scholar] [CrossRef]
- Lampe, E.O.; Brenz, Y.; Herrmann, L.; Repnik, U.; Griffiths, G.; Zingmark, C.; Sjöstedt, A.; Winther-Larsen, H.C.; Hagedorn, M. Dissection of Francisella-Host Cell Interactions in Dictyostelium discoideum. Appl. Environ. Microbiol. 2016, 82, 1586–1598. [Google Scholar] [CrossRef] [Green Version]
- Allombert, J.; Vianney, A.; Laugier, C.; Petry, S.; Hébert, L. Survival of taylorellae in the environmental amoeba Acanthamoeba castellanii. BMC Microbiol. 2014, 14, 69. [Google Scholar] [CrossRef] [Green Version]
- Kicka, S.; Trofimov, V.; Harrison, C.; Ouertatani-Sakouhi, H.; McKinney, J.; Scapozza, L.; Hilbi, H.; Cosson, P.; Soldati, T. Establishment and Validation of Whole-Cell Based Fluorescence Assays to Identify Anti-Mycobacterial Compounds Using the Acanthamoeba castellanii—Mycobacterium marinum Host-Pathogen System. PLoS ONE 2014, 9, e87834. [Google Scholar] [CrossRef] [Green Version]
- Harrison, C.F.; Kicka, S.; Trofimov, V.; Berschl, K.; Ouertatani-Sakouhi, H.; Ackermann, N.; Hedberg, C.; Cosson, P.; Soldati, T.; Hilbi, H. Exploring Anti-Bacterial Compounds against Intracellular Legionella. PLoS ONE 2013, 8, e74813. [Google Scholar] [CrossRef]
- Delafont, V.; Samba-Louaka, A.; Cambau, E.; Bouchon, D.; Moulin, L.; Hechard, Y. Mycobacterium llatzerense, a waterborne Mycobacterium, that resists phagocytosis by Acanthamoeba castellanii. Sci. Rep. 2017, 7, 46270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano-Amado, D.; Herrera-Solorio, A.M.; Valdés, J.; Alemán-Lazarini, L.; Almaraz-Barrera, M.d.J.; Luna-Rivera, E.; Vargas, M.; Hernández-Rivas, R. Identification of repressive and active epigenetic marks and nuclear bodies in Entamoeba histolytica. Parasit. Vectors 2016, 9, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayek, M.; Baraquet, C.; Lami, R.; Blache, Y.; Molmeret, M. The Marine Bacterium Shewanella woodyi Produces C8-HSL to Regulate Bioluminescence. Microb. Ecol. 2020, 79, 865–881. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Sharma, D.; Bisht, D.; Khan, A.U. Identification of factors involved in Enterococcus faecalis biofilm under quercetin stress. Microb. Pathog. 2019, 126, 205–211. [Google Scholar] [CrossRef]
- Heydorn, A.; Nielsen, A.T.; Hentzer, M.; Sternberg, C.; Givskov, M.; Ersbøll, B.K.; Molin, S. Quantification of biofilm structures by the novel computer program comstat. Microbiology 2000, 146, 2395–2407. [Google Scholar] [CrossRef] [Green Version]
- Matz, C.; Moreno, A.M.; Alhede, M.; Manefield, M.; Hauser, A.R.; Givskov, M.; Kjelleberg, S. Pseudomonas aeruginosa uses type III secretion system to kill biofilm-associated amoebae. ISME J. 2008, 2, 843–852. [Google Scholar] [CrossRef]
- Robino, E.; Poirier, A.C.; Amraoui, H.; Le Bissonnais, S.; Perret, A.; Lopez-Joven, C.; Auguet, J.C.; Rubio, T.P.; Cazevieille, C.; Rolland, J.L.; et al. Resistance of the oyster pathogen Vibrio tasmaniensis LGP32 against grazing by Vannella sp. marine amoeba involves Vsm and CopA virulence factors. Environ. Microbiol. 2019. [Google Scholar] [CrossRef]
- Schulz, F.; Lagkouvardos, I.; Wascher, F.; Aistleitner, K.; Kostanjšek, R.; Horn, M. Life in an unusual intracellular niche: A bacterial symbiont infecting the nucleus of amoebae. ISME J. 2014, 8, 1634–1644. [Google Scholar] [CrossRef] [Green Version]
- Mengue, L.; Regnacq, M.; Aucher, W.; Portier, E.; Hechard, Y.; Samba-Louaka, A. Legionella pneumophila prevents proliferation of its natural host Acanthamoeba castellanii. Sci. Rep. 2016, 6, 36448. [Google Scholar] [CrossRef] [Green Version]
- Samba-Louaka, A.; Pereira, J.M.; Nahori, M.A.; Villiers, V.; Deriano, L.; Hamon, M.A.; Cossart, P. Listeria monocytogenes dampens the DNA damage response. PLoS Pathog. 2014, 10, e1004470. [Google Scholar] [CrossRef]
- Sanchez-Vizuete, P.; Orgaz, B.; Aymerich, S.; Le Coq, D.; Briandet, R. Pathogens protection against the action of disinfectants in multispecies biofilms. Front Microbiol. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kuwahara, H.; Fujita, K.; Noda, S.; Kihara, K.; Yamada, A.; Ohkuma, M.; Hongoh, Y. Intranuclear verrucomicrobial symbionts and evidence of lateral gene transfer to the host protist in the termite gut. ISME J. 2014, 8, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Rautian, M.S.; Wackerow-Kouzova, N.D. Phylogenetic placement of two previously described intranuclear bacteria from the ciliate Paramecium bursaria (Protozoa, Ciliophora): ‘Holospora acuminata’ and ‘Holospora curviuscula’. Int. J. Syst. Evol. Microbiol. 2013, 63, 1930–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehari, Y.T.; Hayes, B.J.; Redding, K.S.; Mariappan, P.V.; Gunderson, J.H.; Farone, A.L.; Farone, M.B. Description of ‘Candidatus Berkiella aquae’ and ‘Candidatus Berkiella cookevillensis’, two intranuclear bacteria of freshwater amoebae. Int. J. Syst. Evol. Microbiol. 2015, 66, 536–541. [Google Scholar] [CrossRef]
- Beliavskaia, A.Y.; Predeus, A.V.; Garushyants, S.K.; Logacheva, M.D.; Gong, J.; Zou, S.; Gelfand, M.S.; Rautian, M.S. New Intranuclear Symbiotic Bacteria from Macronucleus of Paramecium putrinum—“Candidatus Gortzia Yakutica”. Diversity 2020, 12, 198. [Google Scholar] [CrossRef]
- Bella, C.; Koehler, L.; Grosser, K.; Berendonk, T.U.; Petroni, G.; Schrallhammer, M. Fitness impact of obligate intranuclear bacterial symbionts depends on host growth phase. Front. Microbiol. 2016, 7, 2084. [Google Scholar] [CrossRef] [Green Version]
- Zielinski, F.U.; Pernthaler, A.; Duperron, S.; Raggi, L.; Giere, O.; Borowski, C.; Dubilier, N. Widespread occurrence of an intranuclear bacterial parasite in vent and seep bathymodiolin mussels. Environ. Microbiol. 2009, 11, 1150–1167. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, C. Fine structure of endonucleobiotic bacteria in the gill epithelium of Ruditapes decussatus. Mar. Biol. 1989, 100, 339–341. [Google Scholar] [CrossRef]
- Elston, R.A. An intranuclear pathogen [nuclear inclusion X (NIX)] associated with massive mortalities of the Pacific razor clam, Siliqua patula. J. Invertebr. Pathol. 1986, 47, 93–104. [Google Scholar] [CrossRef]
- Friedrich, A.B.; Merkert, H.; Fendert, T.; Hacker, J.; Proksch, P.; Hentschel, U. Microbial diversity in the marine sponge Aplysina cavernicola (formerly Verongia cavernicola) analyzed by fluorescence in situ hybridization (FISH). Mar. Biol. 1999, 134, 461–470. [Google Scholar] [CrossRef]
- Vadivelu, J.; Vellasamy, K.M.; Thimma, J.; Mariappan, V.; Kang, W.T.; Choh, L.C.; Shankar, E.M.; Wong, K.T. Survival and intra-nuclear trafficking of Burkholderia pseudomallei: Strategies of evasion from immune surveillance? PLoS Negl. Trop. Dis. 2017, 11, e0005241. [Google Scholar] [CrossRef] [PubMed]
- Urakami, H.; Tsuruhara, T.; Tamura, A. Intranuclear Rickettsia tsutsugamushi in cultured mouse fibroblasts (L Cells). Microbiol. Immunol. 1982, 26, 445–447. [Google Scholar] [CrossRef] [Green Version]
- Burgdorfer, W.; Anacker, R.L.; Bird, R.G.; Bertram, D.S. Intranuclear growth of Rickettsia rickettsii. J. Bac. 1968, 96, 1415–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgdorfer, W.; Brinton, L.P. Intranuclear growth of Rickettsia canada, a member of the Typhus group. Infect. Immun. 1970, 2, 112–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bierne, H. Nuclear microbiology—Bacterial assault on the nucleolus. EMBO Rep. 2013, 14, 663–664. [Google Scholar] [CrossRef] [PubMed]
- Berk, S.G.; Ting, R.S.; Turner, G.W.; Ashburn, R.J. Production of respirable vesicles containing live Legionella pneumophila cells by two Acanthamoeba spp. Appl. Environ. Microbiol. 1998, 64, 279–286. [Google Scholar] [CrossRef] [Green Version]
- Brandl, M.T.; Rosenthal, B.M.; Haxo, A.F.; Berk, S.G. Enhanced survival of Salmonella enterica in vesicles released by a soilborne Tetrahymena species. Appl. Environ. Microbiol. 2005, 71, 1562–1569. [Google Scholar] [CrossRef] [Green Version]
- Raghu Nadhanan, R.; Thomas, C.J. Colpoda secrete viable Listeria monocytogenes within faecal pellets. Environ. Microbiol. 2014, 16, 396–404. [Google Scholar] [CrossRef]
- Berk, S.G.; Faulkner, G.; Garduño, E.; Joy, M.C.; Ortiz-Jimenez, M.A.; Garduño, R.A. Packaging of Live Legionella pneumophila into Pellets Expelled by Tetrahymena spp. Does Not Require Bacterial Replication and Depends on a Dot/Icm-Mediated Survival Mechanism. Appl. Environ. Microbiol. 2008, 74, 2187–2199. [Google Scholar] [CrossRef] [Green Version]
- Bouyer, S.; Imbert, C.; Rodier, M.H.; Hechard, Y. Long-term survival of Legionella pneumophila associated with Acanthamoeba castellanii vesicles. Environ. Microbiol. 2007, 9, 1341–1344. [Google Scholar] [CrossRef]
- El-Etr, S.H.; Margolis, J.J.; Monack, D.; Robison, R.A.; Cohen, M.; Moore, E.; Rasley, A. Francisella tularensis type A strains cause the rapid encystment of Acanthamoeba castellanii and survive in amoebal cysts for three weeks postinfection. Appl. Environ. Microbiol. 2009, 75, 7488–7500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abd, H.; Saeed, A.; Weintraub, A.; Nair, G.B.; Sandstrom, G. Vibrio cholerae O1 strains are facultative intracellular bacteria, able to survive and multiply symbiotically inside the aquatic free-living amoeba Acanthamoeba castellanii. FEMS Microbiol. Ecol. 2007, 60, 33–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambrecht, E.; Bare, J.; Chavatte, N.; Bert, W.; Sabbe, K.; Houf, K. Protozoan Cysts Act as a Survival Niche and Protective Shelter for Foodborne Pathogenic Bacteria. Appl. Environ. Microbiol. 2015, 81, 5604–5612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez-Hidalgo, A.; Obregon-Henao, A.; Wheat, W.H.; Jackson, M.; Gonzalez-Juarrero, M. Mycobacterium bovis hosted by free-living-amoebae permits their long-term persistence survival outside of host mammalian cells and remain capable of transmitting disease to mice. Environ. Microbiol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Trigui, H.; Paquet, V.E.; Charette, S.J.; Faucher, S.P. Packaging of Campylobacter jejuni into multilamellar bodies by the ciliate Tetrahymena pyriformis. Appl. Environ. Microbiol. 2016, 82, 2783–2790. [Google Scholar] [CrossRef] [Green Version]
- Paquet, V.E.; Lessire, R.; Domergue, F.; Fouillen, L.; Filion, G.; Sedighi, A.; Charette, S.J. Lipid composition of multilamellar bodies secreted by Dictyostelium discoideum reveals their amoebal origin. Eukaryot. Cell. 2013, 12, 1326–1334. [Google Scholar] [CrossRef] [Green Version]
- Denoncourt, A.M.; Paquet, V.E.; Sedighi, A.; Charette, S.J. Identification of Proteins Associated with Multilamellar Bodies Produced by Dictyostelium discoideum. PLoS ONE 2016, 11, e0158270. [Google Scholar] [CrossRef]
- Chen, J.; de Felipe, K.S.; Clarke, M.; Lu, H.; Anderson, O.R.; Segal, G.; Shuman, H.A. Legionella Effectors That Promote Nonlytic Release from Protozoa. Science 2004, 303, 1358. [Google Scholar] [CrossRef] [Green Version]
- Corno, G.; Jurgens, K. Direct and indirect effects of protist predation on population size structure of a bacterial strain with high phenotypic plasticity. Appl Environ Microbiol 2006, 72, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.-P.; Chou, C.-F. Morphological plasticity of bacteria—Open questions. Biomicrofluidics 2016, 10, 031501. [Google Scholar] [CrossRef] [Green Version]
- Justice, S.S.; Hunstad, D.A.; Cegelski, L.; Hultgren, S.J. Morphological plasticity as a bacterial survival strategy. Nat. Rev. Microbiol. 2008, 6, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.W.; Höfle, M.G. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 2001, 35, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Jürgens, K.; Matz, C. Predation as a shaping force for the phenotypic and genotypic composition of planktonic bacteria. Antonie Van Leeuwenhoek 2002, 81, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Horvath, D.J., Jr.; Li, B.; Casper, T.; Partida-Sanchez, S.; Hunstad, D.A.; Hultgren, S.J.; Justice, S.S. Morphological plasticity promotes resistance to phagocyte killing of uropathogenic Escherichia coli. Microbes Infect. 2011, 13, 426–437. [Google Scholar] [CrossRef] [Green Version]
- Lamrabet, O.; Drancourt, M. Mycobacterium gilvum illustrates size-correlated relationships between mycobacteria and Acanthamoeba polyphaga. Appl. Environ. Microbiol. 2013, 79, 1606–1611. [Google Scholar] [CrossRef] [Green Version]
- Butler, R.E.; Cihlarova, V.; Stewart, G.R. Effective generation of reactive oxygen species in the mycobacterial phagosome requires K+ efflux from the bacterium. Cell. Microbiol. 2010, 12, 1186–1193. [Google Scholar] [CrossRef]
- Marks, L.R.; Davidson, B.A.; Knight, P.R.; Hakansson, A.P. Interkingdom Signaling Induces Streptococcus pneumoniae Biofilm Dispersion and Transition from Asymptomatic Colonization to Disease. mBio 2013, 4, e00438. [Google Scholar] [CrossRef] [Green Version]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef]
- Rendueles, O.; Ghigo, J.-M. Multi-species biofilms: How to avoid unfriendly neighbors. FEMS Microbiol. Rev. 2012, 36, 972–989. [Google Scholar] [CrossRef]
- Rodríguez-Zaragoza, S. Ecology of Free-Living Amoebae. Crit. Rev. Microbiol. 1994, 20, 225–241. [Google Scholar] [CrossRef]
- Weitere, M.; Bergfeld, T.; Rice, S.A.; Matz, C.; Kjelleberg, S. Grazing resistance of Pseudomonas aeruginosa biofilms depends on type of protective mechanism, developmental stage and protozoan feeding mode. Environ. Microbiol. 2005, 7, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Jousset, A.; Bonkowski, M. The model predator Acanthamoeba castellanii induces the production of 2,4, DAPG by the biocontrol strain Pseudomonas fluorescens Q2-87. Soil Biol. Biochem. 2010, 42, 1647–1649. [Google Scholar] [CrossRef]
- Souza, T.K.d.; Soares, S.S.; Benitez, L.B.; Rott, M.B. Interaction between Methicillin-Resistant Staphylococcus aureus (MRSA) and Acanthamoeba polyphaga. Curr. Microbiol. 2017, 74, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.F.; Hornàk, K.; Simek, K.; Pernthaler, J. Aggregate formation in a freshwater bacterial strain induced by growth state and conspecific chemical cues. Environ. Microbiol. 2010, 12, 2486–2495. [Google Scholar] [CrossRef]
- Corno, G.; Caravati, E.; Callieri, C.; Bertoni, R. Effects of predation pressure on bacterial abundance, diversity, and size-structure distribution in an oligotrophic system. J. Limnol. 2008, 67, 107–119. [Google Scholar] [CrossRef] [Green Version]
- Pernthaler, J.; Posch, T.; Simek, K.; Vrba, J.; Amann, R.; Psenner, R. Contrasting bacterial strategies to coexist with a flagellate predator in an experimental microbial assemblage. Appl. Environ. Microbiol. 1997, 63, 596–601. [Google Scholar] [CrossRef] [Green Version]
- Simek, K.; Pernthaler, J.; Weinbauer, M.G.; Hornák, K.; Dolan, J.R.; Nedoma, J.; Masín, M.; Amann, R. Changes in bacterial community composition and dynamics and viral mortality rates associated with enhanced flagellate grazing in a mesoeutrophic reservoir. Appl. Environ. Microbiol. 2001, 67, 2723–2733. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.W.; Moore, E.R.; Höfle, M.G. Bacterial filament formation, a defense mechanism against flagellate grazing, is growth rate controlled in bacteria of different phyla. Appl. Environ. Microbiol. 1999, 65, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Queck, S.-Y.; Weitere, M.; Moreno, A.M.; Rice, S.A.; Kjelleberg, S. The role of quorum sensing mediated developmental traits in the resistance of Serratia marcescens biofilms against protozoan grazing. Environ. Microbiol. 2006, 8, 1017–1025. [Google Scholar] [CrossRef]
- Salcher, M.M.; Hofer, J.; Horňák, K.; Jezbera, J.; Sonntag, B.; Vrba, J.; Simek, K.; Posch, T. Modulation of microbial predator-prey dynamics by phosphorus availability: Growth patterns and survival strategies of bacterial phylogenetic clades. FEMS Microbiol. Ecol. 2007, 60, 40–50. [Google Scholar] [CrossRef]
- Pohnert, G.; Steinke, M.; Tollrian, R. Chemical cues, defence metabolites and the shaping of pelagic interspecific interactions. Trends Ecol. Evol. 2007, 22, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Kusch, J. Behavioural and morphological changes in ciliates induced by the predator Amoeba proteus. Oecologia 1993, 96, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Gavriilidou, A.; Gutleben, J.; Versluis, D.; Forgiarini, F.; van Passel, M.W.J.; Ingham, C.J.; Smidt, H.; Sipkema, D. Comparative genomic analysis of Flavobacteriaceae: Insights into carbohydrate metabolism, gliding motility and secondary metabolite biosynthesis. BMC Genom. 2020, 21, 569. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.L.; Zhao, F.; Chen, X.L.; Zhang, X.Y.; Zhang, Y.Z.; Song, X.Y.; Sun, C.Y.; Yang, J. Promotion of Wound Healing and Prevention of Frostbite Injury in Rat Skin by Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Mar. Drugs 2020, 18, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.L.; Zhao, F.; Shi, M.; Zhang, X.Y.; Zhou, B.C.; Zhang, Y.Z.; Chen, X.L. Characterization and Biotechnological Potential Analysis of a New Exopolysaccharide from the Arctic Marine Bacterium Polaribacter sp. SM1127. Sci. Rep. 2015, 5, 18435. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guillonneau, R.; Baraquet, C.; Molmeret, M. Marine Bacteria Display Different Escape Mechanisms When Facing Their Protozoan Predators. Microorganisms 2020, 8, 1982. https://doi.org/10.3390/microorganisms8121982
Guillonneau R, Baraquet C, Molmeret M. Marine Bacteria Display Different Escape Mechanisms When Facing Their Protozoan Predators. Microorganisms. 2020; 8(12):1982. https://doi.org/10.3390/microorganisms8121982
Chicago/Turabian StyleGuillonneau, Richard, Claudine Baraquet, and Maëlle Molmeret. 2020. "Marine Bacteria Display Different Escape Mechanisms When Facing Their Protozoan Predators" Microorganisms 8, no. 12: 1982. https://doi.org/10.3390/microorganisms8121982
APA StyleGuillonneau, R., Baraquet, C., & Molmeret, M. (2020). Marine Bacteria Display Different Escape Mechanisms When Facing Their Protozoan Predators. Microorganisms, 8(12), 1982. https://doi.org/10.3390/microorganisms8121982



