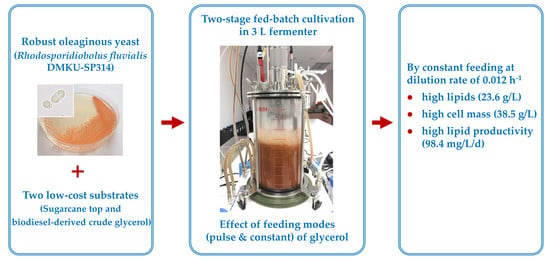
Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain and Growth Conditions
2.2. Raw Materials
2.3. Preparation of Sugarcane Top Hydrolysate (STH)
2.4. Effect of Crude Glycerol Concentration
2.5. Batch Cultivation in a 3 L Fermenter
2.6. Two-Stage Fed-Batch Cultivation in a 3 L Fermenter
2.7. Analytical Methods
2.8. Statistical Analysis
3. Results and Discussion
3.1. Effect of Crude Glycerol Concentration on Growth and Lipid Accumulation
3.2. Batch Cultivation in a 3 L Fermenter
3.3. Two-Stage Fed-Batch Cultivation in a 3 L Fermenter
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, Q.; Du, W.; Liu, D. Perspectives of microbial oils for biodiesel production. Appl. Microbiol. Biotechnol. 2008, 80, 749–756. [Google Scholar] [CrossRef]
- Boviatsi, E.; Papadaki, A.; Efthymiou, M.; Nychas, G.E.; Papanikolaou, S.; da Silva, J.A.C.; Freire, D.M.G.; Koutinas, A. Valorisation of sugarcane molasses for the production of microbial lipids via fermentation of two Rhodosporidium strains for enzymatic synthesis of polyol esters. J. Chem. Technol. Biotechnol. 2020, 95, 402–407. [Google Scholar] [CrossRef]
- Papadaki, A.; Mallouchos, A.; Efthymiou, M.; Gardeli, C.; Kopsahelis, N.; Aguieiras, E.C.G.; Freire, D.M.G.; Papanikolaou, S.; Koutinas, A.A. Production of wax esters via microbial oil synthesis from food industry waste and by-product streams. Bioresour. Technol. 2017, 245, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Papadaki, A.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Kopsahelis, N.; Freire, D.M.G.; Mandala, I.; Koutinas, A.A. Development of microbial oil wax-based oleogel with potential application in food formulations. Food Bioprocess Tech. 2019, 12, 899–909. [Google Scholar] [CrossRef]
- Wei, Y.; Siewers, V.; Nielsen, J. Cocoa butter-like lipid production ability of non-oleaginous and oleaginous yeasts under nitrogen-limited culture conditions. Appl. Microbiol. Biotechnol. 2017, 101, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.R.; Kaur, R.; Yellapu, S.K.; Zhang, X.; Tyagi, R.D. Biodiesel Production from Oleaginous Microorganisms with Wastes as Raw Materials. In Biomass, Biofuels, Biochemicals, Biofuels: Alternative Feedstocks and Conversion Processes for the Production of Liquid and Gaseous Biofuels, 2nd ed.; Pandey, A., Larroche, C., Dussap, C., Gnansounou, E., Khanal, S.K., Ricke, S., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 661–690. [Google Scholar]
- Cho, H.; Park, J. Biodiesel production by various oleaginous microorganisms from organic wastes. Bioresour. Technol. 2018, 256, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.A.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Efficient oleaginous yeasts for lipid production from lignocellulosic sugars and effects of lignocellulose degradation compounds on growth and lipid production. Process Biochem. 2017, 53, 44–60. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J. Simultaneous production of lipids and carotenoids by the red yeast Rhodotorula from waste glycerol fraction and potato wastewater. Appl. Biochem. Biotechnol. 2019, 189, 589–607. [Google Scholar] [CrossRef]
- Patel, A.; Arora, N.; Mehtani, J.; Pruthi, V.; Pruthi, P.A. Assessment of fuel properties on the basis of fatty acid profiles of oleaginous yeast for potential biodiesel production. Renew. Sust. Energy Rev. 2017, 77, 604–616. [Google Scholar] [CrossRef]
- Xu, J.; Du, W.; Zhao, X.; Zhang, G.; Liu, D. Microbial oil production from various carbon sources and its use for biodiesel preparation. Biofuels Bioprod. Bioref. 2013, 7, 65–77. [Google Scholar] [CrossRef]
- Poontawee, R.; Yongmanitchai, W.; Limtong, S. Lipid production from a mixture of sugarcane top hydrolysate and biodiesel-derived crude glycerol by the oleaginous red yeast, Rhodosporidiobolus fluvialis. Process Biochem. 2018, 66, 150–161. [Google Scholar] [CrossRef]
- Lim, H.C.; Shin, H.S. Fed-Batch Cultures: Principles and Applications of Semi-Batch Bioreactors. In Cambridge Series in Chemical Engineering; Cambridge University Press: Cambridge, UK, 2013; pp. 1–10. [Google Scholar]
- Yamanè, T.; Shimizu, S. Fed-batch techniques in microbial processes. In Bioprocess Parameter Control, 1st ed.; Fiechter, A., Ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 1984; pp. 147–194. [Google Scholar]
- Chang, Y.; Chang, K.; Hsu, C.; Chuang, L.; Chen, C.; Huang, F.; Jang, H. A comparative study on batch and fed-batch cultures of oleaginous yeast Cryptococcus sp. in glucose-based media and corncob hydrolysate for microbial oil production. Fuel 2013, 105, 711–717. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, X.; Zhu, X.; Li, Y.; Xu, H.; Zhao, B.; Chen, L.; Zhang, X. Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenergy 2011, 35, 1906–1911. [Google Scholar] [CrossRef]
- Meesters, P.A.E.P.; Huijberts, G.N.M.; Eggink, G. High-cell-density cultivation of the lipid accumulating yeast Cryptococcus curvatus using glycerol as a carbon source. Appl. Microbiol. Biotechnol. 1996, 45, 575–579. [Google Scholar] [CrossRef]
- Kitcha, S.; Cheirsilp, B. Enhancing lipid production from crude glycerol by newly isolated oleaginous yeasts: Strain selection, process optimization, and fed-batch strategy. Bioenerg Res. 2013, 6, 300–310. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.; Bai, F. High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb. Technol. 2007, 41, 312–317. [Google Scholar] [CrossRef]
- Polburee, P.; Yongmanitchai, W.; Honda, K.; Ohashi, T.; Yoshida, T.; Fujiyama, K.; Limtong, S. Lipid production from biodiesel-derived crude glycerol by Rhodosporidium fluviale DMKU-RK253 using temperature shift with high cell density. Biochem. Eng. J. 2016, 112, 208–218. [Google Scholar] [CrossRef]
- Yang, X.; Jin, G.; Gong, Z.; Shen, H.; Bai, F.; Zhao, Z.K. Recycling biodiesel-derived glycerol by the oleaginous yeast Rhodosporidium toruloides Y4 through the two-stage lipid production process. Biochem. Eng. J. 2014, 91, 86–91. [Google Scholar] [CrossRef]
- Lin, J.; Li, S.; Sun, M.; Zhang, C.; Yang, W.; Zhang, Z.; Li, X.; Li, S. Microbial lipid production by oleaginous yeast in D-xylose solution using a two-stage culture mode. RSC Adv. 2014, 4, 34944–34949. [Google Scholar] [CrossRef]
- Fei, Q.; Chang, H.N.; Shang, L.; Choi, J. Exploring low-cost carbon sources for microbial lipids production by fed-batch cultivation of Cryptococcus albidus. Biotechnol. Bioprocess Eng. 2011, 16, 482–487. [Google Scholar] [CrossRef]
- Anschau, A.; Xavier, M.C.A.; Hernalsteens, S.; Franco, T.T. Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour. Technol. 2014, 157, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Sathiyamoorthi, E.; Dikshit, P.K.; Kumar, P.; Kim, B.S. Co-fermentation of agricultural and industrial waste by Naganishia albida for microbial lipid production in fed-batch fermentation. J. Chem. Technol. Biotechnol. 2019. [Google Scholar] [CrossRef]
- Lorenz, E.; Runge, D.; Marbà-Ardébol, A.; Schmacht, M.; Stahl, U.; Senz, M. Systematic development of a two-stage fed-batch process for lipid accumulation in Rhodotorula glutinis. J. Biotechnol. 2017, 246, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Liu, Y.X.; Chang, J. The effects of feeding criteria on the growth of oleaginous yeast—Rhodotorula glutinis in a pilot-scale airlift bioreactor. J. Taiwan Inst. Chem. Eng. 2015, 49, 67–71. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Liu, H.; Zhang, J. Enhanced lipid production with undetoxified corncob hydrolysate by Rhodotorula glutinis using a high cell density culture strategy. Bioresour. Technol. 2015, 180, 32–39. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Holub, B.J.; Skeaff, C.M. Nutritional regulation of cellular phosphatidylinositol. Methods Enzymol. 1987, 141, 234–244. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Holdsworth, J.E.; Ratledge, C. Lipid turnover in oleaginous yeast. J. Gen. Microbiol. 1988, 134, 339–346. [Google Scholar] [CrossRef][Green Version]
- Saenge, C.; Cheirsilp, B.; Suksaroge, T.T.; Bourtoom, T. Potential use of oleaginous red yeast Rhodotorula glutinis for the bioconversion of crude glycerol from biodiesel plant to lipids and carotenoids. Process Biochem. 2011, 46, 210–218. [Google Scholar] [CrossRef]
- Rakicka, M.; Lazar, Z.; Dulermo, T.; Fickers, P.; Nicaud, J.M. Lipid production by the oleaginous yeast Yarrowia lipolytica using industrial by-products under different culture conditions. Biotechnol. Biofuels 2015, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Ma, Y.; Wang, Q.; Zhang, M.; Wang, J.; Liu, Y. Effect of crude glycerol impurities on lipid preparation by Rhodosporidium toruloides yeast 32489. Bioresour. Technol. 2016, 218, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Karamerou, E.E.; Webb, C. Cultivation modes for microbial oil production using oleaginous yeasts—A review. Biochem. Eng. J. 2019, 151, 107322. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, C.; Wu, S.; Shen, H.; Zhao, Z.K. Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J. Ind. Microbiol. Biotechnol. 2011, 38, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Signori, L.; Ami, D.; Posteri, R.; Giuzzi, A.; Mereghetti, P.; Porro, D.; Branduardi, P. Assessing an effective feeding strategy to optimize crude glycerol utilization as sustainable carbon source for lipid accumulation in oleaginous yeasts. Microb. Cell Fact. 2016, 15, 75. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Hall, M.J.; Ratledge, C. Lipid accumulation in an oleaginous yeast (Candida 107) growing on glucose in single-stage continuous culture. Appl. Environ. Microbiol. 1977, 33, 231–239. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Wu, D.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. Economical lipid production from Trichosporon oleaginosus via dissolved oxygen adjustment and crude glycerol addition. Bioresour. Technol. 2019, 273, 288–296. [Google Scholar] [CrossRef]
- Karamerou, E.E.; Theodoropoulos, C.; Webb, C. Evaluating feeding strategies for microbial oil production from glycerol by Rhodotorula glutinis. Eng. Life Sci. 2017, 17, 314–324. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Wang, W.; Du, W.; Liu, D. Microbial conversion of biodiesel byproduct glycerol to triacylglycerols by oleaginous yeast Rhodosporidium toruloides and the individual effect of some impurities on lipid production. Biochem. Eng. J. 2012, 65, 30–36. [Google Scholar] [CrossRef]







| Fatty Acid | Fatty Acid (% of Total Fatty Acid) at Different Crude Glycerol Concentration (g/L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 110 | 120 | |
| Myristic acid (14:0) | nd | nd | 0.5 ± 0.9 ab | 1.4 ± 0.1 a | 1.3 ± 0.0 a | 0.9 ± 0.8 ab | nd | nd | nd | nd |
| Palmitic acid (16:0) | 32.4 ± 0.0 a | 32.9 ± 0.4 a | 31.9 ± 0.9 a | 29.7 ± 0.9 b | 30.5 ± 0.8 b | 27.4 ± 1.4 c | 28.4 ± 0.3 bc | 28.7 ± 0.7 bc | 28.4 ± 0.0 bc | 27.4 ± 0.1 c |
| Palmitoleic acid (16:1) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| Stearic acid (18:0) | 10.0 ± 0.8 f | 10.9 ± 0.2 e | 11.9 ± 0.5 d | 11.9 ± 0.3 d | 13.2 ± 0.1 c | 13.0 ± 0.6 c | 14.0 ± 0.2 b | 14.8 ± 0.4 a | 15.2 ± 0.1 a | 15.1 ± 0.2 a |
| Oleic acid (18:1) | 34.7 ± 0.4 a | 34.9 ± 0.2 a | 35.0 ± 0.3 a | 34.8 ± 0.4 a | 34.2 ± 0.2 a | 34.3 ± 1.8 a | 35.9 ± 0.5 a | 36.1 ± 0.2 a | 35.6 ± 0.1 a | 34.9 ± 0.6 a |
| Linoleic acid (18:2) | 18.2 ± 0.3 a | 17.6 ± 0.2 a | 16.9 ± 0.5 a | 18.1 ± 1.6 a | 15.9 ± 1.2 a | 18.1 ± 2.3 a | 17.4 ± 1.0 a | 16.5 ± 0.7 a | 16.9 ± 0.1 a | 17.5 ± 0.7 a |
| γ-linolenic acid (18:3) | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| α-linolenic acid (18:3) | 4.7 ± 0.0 ab | 3.7 ± 0.2 b | 3.8 ± 0.2 b | 4.2 ± 0.1 ab | 4.8 ± 0.6 ab | 6.4 ± 2.4 a | 4.3 ± 0.9 ab | 4.0 ± 0.3 ab | 4.0 ± 0.1 ab | 5.1 ± 1.3 ab |
| Saturated fatty acids (SFAs) | 42.4 ± 0.7 ab | 43.8 ± 0.6 ab | 44.4 ± 0.5 a | 42.9 ± 1.2 ab | 44.6 ± 0.7 a | 41.3 ± 2.6 b | 42.4 ± 0.6 ab | 43.5 ± 1.1 ab | 43.6 ± 0.1 ab | 42.6 ± 0.1 ab |
| Unsaturated fatty acids (UFAs) | 57.6 ± 0.7 ab | 56.2 ± 0.6 ab | 55.6 ± 0.5 b | 57.1 ± 1.2 ab | 55.4 ± 0.7 b | 58.7 ± 2.6 a | 57.6 ± 0.6 ab | 56.5 ± 1.1 ab | 56.4 ± 0.1 ab | 57.4 ± 0.1 ab |
| Response | Batch Cultivation | Two-Stage Fed-Batch Cultivation | ||||||
|---|---|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | ||
| Initial working volume (L) | 2.0 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 | 1.2 |
| Final working volume (L) | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Feeding starting time point (h) | - | 48 | 24 | 72 | 48 | 48 | 48 | 48 |
| Mode of feeding | - | Pulse | Pulse | Pulse | Constant | Constant | Constant | Constant |
| Feed rate (mL/h) | - | - | - | - | 9.6 | 16.0 | 26.4 | 9.6 |
| Dilution rate (h−1) | - | - | - | - | 0.012 | 0.020 | 0.033 | 0.012 |
| Concentration of crude glycerol in reservoir (g/L) | - | 147.5 | 147.5 | 147.5 | 147.5 | 147.5 | 147.5 | 175 |
| Total concentration of crude glycerol in the process (g/L) | - | 59 | 59 | 59 | 59 | 59 | 59 | 70 |
| Final pH medium | 7.46 c | 7.58 b | 7.67 a | 7.68 a | 6.99 d | 7.36 c | 7.61 b | 7.00 d |
| Cell mass concentration (g/L) | 30.3 d | 33.2 c | 31.0 d | 26.3 g | 38.5 a | 34.2 b | 28.8 e | 27.4 f |
| Lipid concentration (g/L) | 19.1 bc | 18.3 cd | 17.3 de | 13.3 f | 23.6 a | 19.9 b | 16.0 e | 16.6 e |
| Lipid content (% of dry cell mass) | 63.1 a | 54.9 d | 54.8 d | 53.4 d | 61.4 ab | 55.9 cd | 41.6 e | 59.1 bc |
| Cultivation time (h) | 240 | 240 | 240 | 240 | 240 | 240 | 240 | 240 |
| Maximum specific growth rate; µmax (h−1) | 0.104 a | 0.085 de | 0.085 e | 0.087 cd | 0.092 b | 0.092 b | 0.090 bc | 0.083 e |
| Cell mass productivity; QX (mg/L/h) | 126.2 d | 138.5 c | 129.1 d | 109.7 g | 160.4 a | 142.4 b | 120.0 e | 114.2 f |
| Lipid productivity; QL (mg/L/h) | 79.6 bc | 76.2 cd | 71.9 de | 55.5 f | 98.4 a | 83.1 b | 66.5 e | 69.3 e |
| Total reducing sugar consumed (g/L) | 20.0 c | 21.1 bc | 19.9 c | 23.6 ab | 20.5 c | 17.2 d | 18.9 cd | 25.4 a |
| Crude glycerol consumed (g/L) | 63.6 a | 60.8 b | 56.0 c | 59.6 b | 51.1 e | 53.0 d | 53.2 d | 37.7 f |
| Cell mass yield (g/g) a | 0.36 e | 0.41 cd | 0.41 cd | 0.32 f | 0.54 a | 0.49 b | 0.40 d | 0.44 c |
| Lipid yield (g/g) b | 0.23 c | 0.22 c | 0.23 c | 0.16 d | 0.33 a | 0.28 b | 0.22 c | 0.26 b |
| Yeast Strain | Volume | Substrate | Cultivation Mode | Cultivation Time (h) | Cell Mass (g/L) | Lipid (g/L) | Cell Mass Productivity (g/L/h) | Lipid Productivity (mg/L/h) | Cell Mass Yield (g/g) | Lipid Yield (g/g) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rhodosporidiobolus fluvialis DMKU-SP314 | Fermenter (3 L) | STH and crude glycerol | Two-stage fed-batch (CF) | 240 | 38.5 | 23.6 | 0.160 | 98.4 | 0.54 | 0.33 | This study |
| Two-stage fed-batch (PF) | 240 | 33.2 | 18.3 | 0.139 | 76.2 | 0.41 | 0.22 | ||||
| Cryptococcus sp. SM5S05 | Flask (500 mL) | Glucose | Fed-batch (PF) | 144 | 11.4 | 7.3 | - | 51 | - | 0.64 | [16] |
| Corncob hydrolysate | Fed-batch (PF) | 144 | 10.8 | 6.6 | - | 46 | - | 0.61 | |||
| Naganishia albida KCTC 17541 | Fermenter (1 L) | Onion waste and crude glycerol | Fed-batch (IF) | 168 | 21.1 | 7.19 | - | 42.8 | - | 0.236 | [26] |
| Rhodotorula glutinis CICC 31596 | Fermenter (2 L) | Pure glycerol | Fed-batch (PF) | 168 | 23 | 9.38 | - | 60 | - | 0.059 | [42] |
| Fed-batch (CF) | 168 | 30.63 | 16.28 | - | 100 | - | 0.087 | ||||
| Cryptococcus albidus var. albidus ATCC 10672 | Fermenter (2.5 L) | Volatile fatty acids | Fed-batch (IF) | 192 | - | 14.5 | - | - | - | - | [24] |
| Lipomyces starkeyi DSM 70296 | Fermenter (3 L) | Glucose:xylose (30:70) | Fed-batch (PF) | 138 | 82.4 | 38.6 | 0.597 | 280 | 0.322 | 0.151 | [25] |
| Rhodotorula glutinis Rh-00301 | Fermenter (5 L) | Sucrose | Two-stage fed-batch (CF) | 80 | 106 | 63 | - | 84 | - | 0.18 | [27] |
| Trichosporonoides spathulata JU4-57 | Fermenter (5 L) | Crude glycerol | One-stage fed-batch (IF) | 144 | 17.3 | 7.25 | - | - | - | - | [19] |
| Two-stage fed-batch (IF) | 132 | 13.8 | 7.78 | - | - | - | - | ||||
| Rhodosporidium toruloides Y4 | Fermenter (15 L) | Glucose | Fed-batch (IF) | 146.7 | 89.0 | 52.2 | - | 36 | - | 0.20 | [20] |
| Fed-batch (CF) | 138.5 | 127.5 | 78.8 | - | 57 | - | 0.23 | ||||
| Rhodotorula glutinis BCRC21418 | Fermenter (50 L) | Crude glycerol | Fed-batch (PF) | 120 | 46.4 | - | - | - | - | 0.622 | [28] |
| Fed-batch (CF) | 96 | 44.5 | - | - | - | - | 0.621 | ||||
| Fed-batch (EF) | 84 | 39.2 | - | - | - | - | 0.433 |
| Fatty Acid | Fatty Acid (% of Total Fatty Acid) | |||||||
|---|---|---|---|---|---|---|---|---|
| Batch Cultivation | Two-Stage Fed-Batch Cultivation | |||||||
| 1st | 2nd | 3rd | 4th | 5th | 6th | 7th | ||
| Myristic acid (14:0) | 1.0 ± 0.1 b | 1.1 ± 0.0 ab | 1.2 ± 0.0 ab | 1.6 ± 0.4 a | 1.6 ± 0.7 a | 1.1 ± 0.0 ab | 1.2 ± 0.1 ab | 1.1 ± 0.0 ab |
| Palmitic acid (16:0) | 29.2 ± 2.6 bc | 31.8 ± 0.4 a | 31.8 ± 0.6 a | 28.2 ± 0.3 c | 29.5 ± 0.5 bc | 31.1 ± 0.8 ab | 31.2 ± 1.6 ab | 29.7 ± 0.5 bc |
| Palmitoleic acid (16:1) | 0.7 ± 0.1 b | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.7 ± 0.1 b | 0.7 ± 0.0 b | 0.6 ± 0.0 b | 1.1 ± 0.5 a | 0.9 ± 0.1 ab |
| Stearic acid (18:0) | 13.2 ± 0.2 a | 13.2 ± 0.2 a | 14.3 ± 0.8 a | 14.5 ± 0.3 a | 13.3 ± 0.0 a | 14.4 ± 0.5 a | 13.7 ± 0.5 a | 14.2 ± 1.2 a |
| Oleic acid (18:1) | 34.9 ± 0.3 a | 35.2 ± 0.2 a | 35.7 ± 0.3 a | 35.0 ± 0.4 a | 35.1 ± 0.3 a | 35.1 ± 1.0 a | 32.7 ± 0.5 b | 33.6 ± 0.2 b |
| Linoleic acid (18:2) | 17.3 ± 1.2 a | 15.2 ± 0.4 c | 13.5 ± 0.1 d | 15.9 ± 0.3 bc | 16.8 ± 0.0 ab | 14.9 ± 0.4 c | 16.5 ± 1.1 ab | 17.1 ± 0.8 ab |
| γ-linolenic acid (18:3) | nd | nd | nd | nd | nd | nd | nd | nd |
| α-linolenic acid (18:3) | 3.7 ± 0.9 ab | 2.9 ± 0.1 c | 2.9 ± 0.1 c | 4.2 ± 0.1 a | 3.1 ± 0.1 bc | 2.8 ± 0.4 c | 3.6 ± 0.1 ab | 3.5 ± 0.2 b |
| Saturated fatty acids (SFAs) | 43.4 ± 2.5 d | 46.1 ± 0.7 abc | 47.3 ± 0.2 a | 44.2 ± 0.2 cd | 44.4 ± 0.2 cd | 46.6 ± 1.2 ab | 46.1 ± 1.2 abc | 44.9 ± 0.8 bcd |
| Unsaturated fatty acids (UFAs) | 56.6 ± 2.5 a | 53.9 ± 0.7 bcd | 52.7 ± 0.2 d | 55.8 ± 0.2 ab | 55.6 ± 0.2 ab | 53.4 ± 1.2 cd | 53.9 ± 1.2 bcd | 55.1 ± 0.8 abc |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poontawee, R.; Limtong, S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms 2020, 8, 151. https://doi.org/10.3390/microorganisms8020151
Poontawee R, Limtong S. Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms. 2020; 8(2):151. https://doi.org/10.3390/microorganisms8020151
Chicago/Turabian StylePoontawee, Rujiralai, and Savitree Limtong. 2020. "Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis" Microorganisms 8, no. 2: 151. https://doi.org/10.3390/microorganisms8020151
APA StylePoontawee, R., & Limtong, S. (2020). Feeding Strategies of Two-Stage Fed-Batch Cultivation Processes for Microbial Lipid Production from Sugarcane Top Hydrolysate and Crude Glycerol by the Oleaginous Red Yeast Rhodosporidiobolus fluvialis. Microorganisms, 8(2), 151. https://doi.org/10.3390/microorganisms8020151