Effect of a Profound Feedstock Change on the Structure and Performance of Biogas Microbiomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reactor Operation
- the reactor formerly fed with sugar beet silage was now given maize silage (SB-M1),
- the reactor formerly fed with maize silage was now given sugar beet silage (M-SB1),
- the reactor formerly fed with sugar beet silage and ammonium carbonate was now given maize silage and ammonium carbonate (SB-M2),
- the reactor formerly fed with maize silage with ammonium carbonate was now given sugar beet silage and ammonium carbonate (M-SB2),
- the reactor formerly fed with 25% sugar beet silage and 75% animal manure was now given 25% maize silage with 75% animal manure (SB-M3), and
- the reactor formerly with 25% maize silage and 75% animal manure was now given 25% sugar beet silage with 75% animal manure (M-SB3).
2.2. Chemical Analyses
2.3. Molecular Biological Analyses
2.4. Bioinformatics and Statistical Analysis
3. Results and Discussion
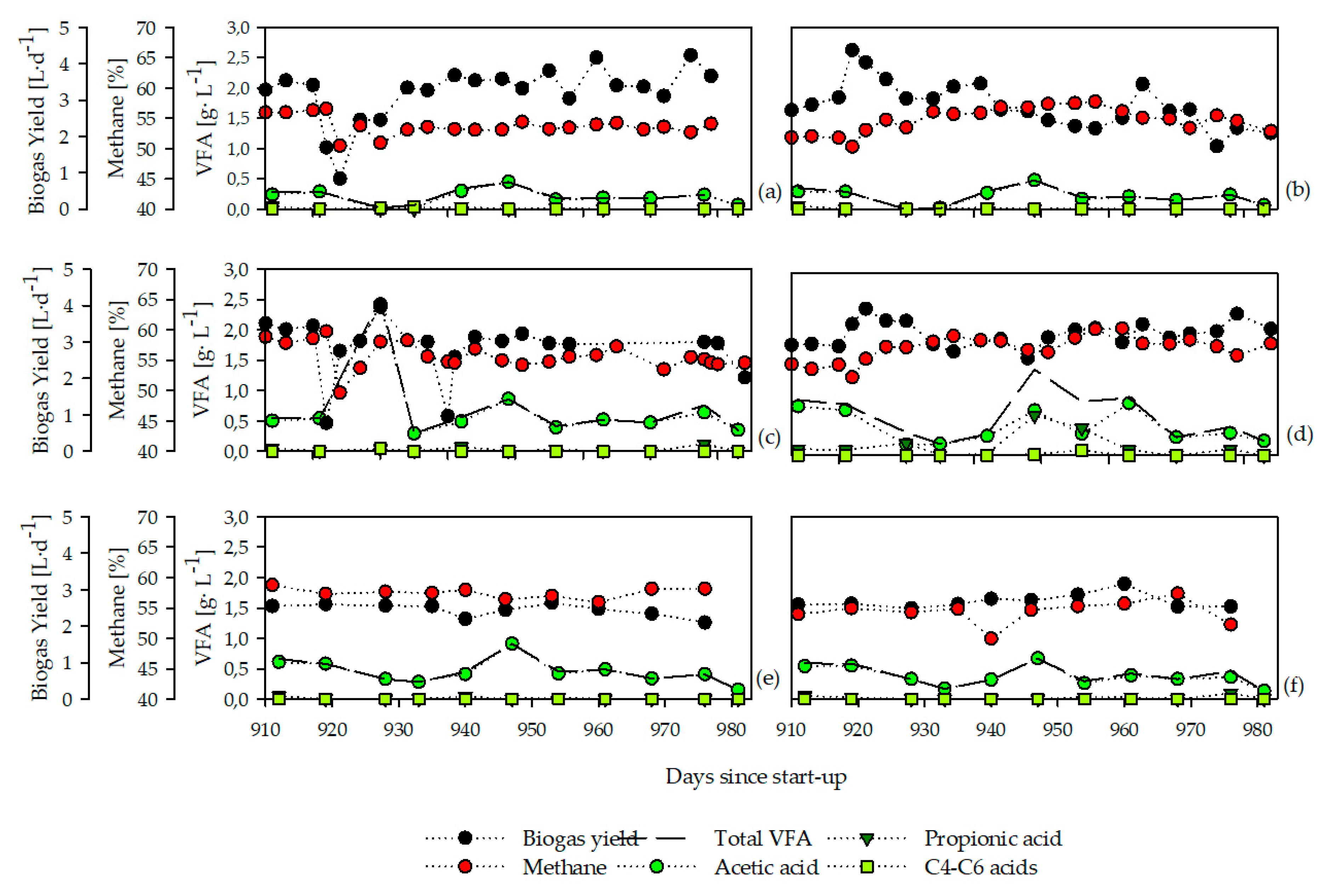
3.1. Chemical and Operational Parameters
3.2. Bacterial Community - General Overview
3.3. Bacterial Community at Low TAN Concentration
3.4. Bacterial Community at High TAN Concentration
3.5. Archaeal Community
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Theuerl, S.; Herrmann, C.; Heiermann, M.; Grundmann, P.; Landwehr, N.; Kreidenweis, U.; Prochnow, A.; Theuerl, S.; Herrmann, C.; Heiermann, M.; et al. The Future Agricultural Biogas Plant in Germany: A Vision. Energies 2019, 12, 396. [Google Scholar]
- Daniel-Gromke, J.; Rensberg, N.; Denysenko, V.; Stinner, W.; Schmalfuß, T.; Scheftelowitz, M.; Nelles, M.; Liebetrau, J. Current Developments in Production and Utilization of Biogas and Biomethane in Germany. Chem. Ing. Tech. 2018, 90, 17–35. [Google Scholar] [CrossRef]
- Statistisches Bundesamt. Statistisches Jahrbuch 2003 für die Bundesrepublik Deutschland; DigiZeitschriften: Göttingen, Germany, 2003. [Google Scholar]
- Statistisches Bundesamt. Statistisches Jahrbuch Deutschland und Internationales. 2014. Available online: https://www.destatis.de/GPStatistik/receive/DEAusgabe_ausgabe_00000535;jsessionid=6D423EAA819A1CEC33671FE7AC851546 (accessed on 24 January 2020).
- Herbes, C.; Jirka, E.; Braun, J.P.; Pukall, K. The Social Discourse on the “Maize Cap” before and after the 2012 Amendment of the German Renewable Energies Act (EEG). GAIA Ecol. Perspect. Sci. Soc. 2014, 23, 100–109. [Google Scholar]
- Balussou, D.; McKenna, R.; Möst, D.; Fichtner, W. A model-based analysis of the future capacity expansion for German biogas plants under different legal frameworks. Renew. Sustain. Energy Rev. 2018, 96, 119–131. [Google Scholar]
- Theuerl, S.; Klang, J.; Prochnow, A. Process Disturbances in Agricultural Biogas Production—Causes, Mechanisms and Effects on the Biogas Microbiome: A Review. Energies 2019, 12, 365. [Google Scholar] [CrossRef] [Green Version]
- Angelidaki, I.; Karakashev, D.; Batstone, D.J.; Plugge, C.M.; Stams, A.J.M. Biomethanation and its potential. Methods Enzymol. 2011, 494, 327–351. [Google Scholar] [PubMed]
- Schnürer, A. Biogas Production: Microbiology and Technology. Anaerobes Biotechnol. 2016, 10, 195–234. [Google Scholar]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2010, 85, 849–860. [Google Scholar]
- Calusinska, M.; Goux, X.; Fossépré, M.; Muller, E.E.L.L.; Wilmes, P.; Delfosse, P. A year of monitoring 20 mesophilic full-scale bioreactors reveals the existence of stable but different core microbiomes in bio-waste and wastewater anaerobic digestion systems. Biotechnol. Biofuels 2018, 11, 196. [Google Scholar] [CrossRef]
- De Vrieze, J.; Ijaz, U.Z.; Saunders, A.M.; Theuerl, S. Terminal restriction fragment length polymorphism is an “old school” reliable technique for swift microbial community screening in anaerobic digestion. Sci. Rep. 2018, 8, 16818. [Google Scholar]
- Klang, J.; Theuerl, S.; Szewzyk, U.; Huth, M.; Tölle, R.; Klocke, M. Dynamic variation of the microbial community structure during the long-time mono-fermentation of maize and sugar beet silage. Microb. Biotechnol. 2015, 8, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Klang, J.; Szewzyk, U.; Bock, D.; Theuerl, S. Nexus between the microbial diversity level and the stress tolerance within the biogas process. Anaerobe 2019, 56, 8–16. [Google Scholar] [CrossRef]
- Alsouleman, K.; Linke, B.; Klang, J.; Klocke, M.; Krakat, N.; Theuerl, S. Reorganisation of a mesophilic biogas microbiome as response to a stepwise increase of ammonium nitrogen induced by poultry manure supply. Bioresour. Technol. 2016, 208, 200–204. [Google Scholar] [CrossRef]
- Pap, B.; Maróti, G.; Mar?ti, G.; Maróti, G. Diversity of Microbial Communities in Biogas Reactors. Curr. Biochem. Eng. 2016, 3, 177–187. [Google Scholar] [CrossRef]
- Han, S.; Liu, Y.; Zhang, S.; Luo, G. Reactor performances and microbial communities of biogas reactors: effects of inoculum sources. Appl. Microbiol. Biotechnol. 2016, 100, 987–995. [Google Scholar] [CrossRef]
- De Francisci, D.; Kougias, P.G.; Treu, L.; Campanaro, S.; Angelidaki, I. Microbial diversity and dynamicity of biogas reactors due to radical changes of feedstock composition. Bioresour. Technol. 2015, 176, 56–64. [Google Scholar] [CrossRef]
- Theuerl, S.; Klang, J.; Heiermann, M.; De Vrieze, J. Marker microbiome clusters are determined by operational parameters and specific key taxa combinations in anaerobic digestion. Bioresour. Technol. 2018, 263, 128–135. [Google Scholar] [CrossRef]
- Westerholm, M.; Moestedt, J.; Schnürer, A. Biogas production through syntrophic acetate oxidation and deliberate operating strategies for improved digester performance. Appl. Energy 2016, 179, 124–135. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef]
- De Vrieze, J.; Hennebel, T.; Boon, N.; Verstraete, W. Methanosarcina: The rediscovered methanogen for heavy duty biomethanation. Bioresour. Technol. 2012, 112, 1–9. [Google Scholar] [CrossRef]
- Elhussein, A.; Weiland, P. Optimierung der Monovergärung von Nachwachsenden Rohstoffen durch die Zugabe von Spurenelementen. Bornimer Agrartech. Ber. 2009, 68, 69–78. [Google Scholar]
- Schönberg, M.; Linke, B. The influence of the temperature regime on the formation of methane in a two-phase anaerobic digestion process. Eng. Life Sci. 2012, 12, 279–286. [Google Scholar] [CrossRef]
- Rademacher, A.; Nolte, C.; Schönberg, M.; Klocke, M. Temperature increases from 55 to 75 °C in a two-phase biogas reactor result in fundamental alterations within the bacterial and archaeal community structure. Appl. Microbiol. Biotechnol. 2012, 96, 565–576. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64, 346–351. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glöckner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzéby, J.; Amann, R.; Rosselló-Móra, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing. 2018. Available online: https://www.r-project.org/foundation/ (accessed on 24 January 2020).
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R package version 2.5-2. Cran R 2019, 1, 2. [Google Scholar]
- Wittebolle, L.; Marzorati, M.; Clement, L.; Balloi, A.; Daffonchio, D.; Heylen, K.; De Vos, P.; Verstraete, W.; Boon, N. Initial community evenness favours functionality under selective stress. Nature 2009, 458, 623–626. [Google Scholar]
- Read, S.; Marzorati, M.; Guimarães, B.C.M.; Boon, N. Microbial Resource Management revisited: Successful parameters and new concepts. Appl. Microbiol. Biotechnol. 2011, 90, 861–871. [Google Scholar]
- Zeileis, A. ineq: Measuring Inequality, Concentration, and Poverty. R Package Version 0.2-13. 2014. Available online: https://cran.r-project.org/web/packages/ineq/ineq.pdf (accessed on 24 January 2020).
- Clarke, K.R. Non-parametic multivariate analyses of changes in community structure. Aust. J. Ecol. 1993, 18, 117–143. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 325–349. [Google Scholar]
- Dufrêne, M.; Legendre, P. Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecol. Monogr. 1997, 67, 345–366. [Google Scholar]
- De Cáceres, M. How to Use the Indicspecies Package (ver. 1.7.8). 2019. Available online: https://cran.r-project.org/web/packages/indicspecies/indicspecies.pdf (accessed on 24 January 2020).
- Leng, L.; Yang, P.; Singh, S.; Zhuang, H.; Xu, L.; Chen, W.H.; Dolfing, J.; Li, D.; Zhang, Y.; Zeng, H.; et al. A review on the bioenergetics of anaerobic microbial metabolism close to the thermodynamic limits and its implications for digestion applications. Bioresour. Technol. 2018, 247, 1095–1106. [Google Scholar]
- Morris, B.E.L.; Henneberger, R.; Huber, H.; Moissl-Eichinger, C. Microbial syntrophy: Interaction for the common good. FEMS Microbiol. Rev. 2013, 37, 384–406. [Google Scholar]
- McInerney, M.J.; Sieber, J.R.; Gunsalus, R.P. Syntrophy in anaerobic global carbon cycles. Curr. Opin. Biotechnol. 2009, 20, 623–632. [Google Scholar]
- De Vrieze, J.; Raport, L.; Roume, H.; Vilchez-Vargas, R.; Jáuregui, R.; Pieper, D.H.; Boon, N. The full-scale anaerobic digestion microbiome is represented by specific marker populations. Water Res. 2016, 104, 101–110. [Google Scholar]
- Lee, J.; Shin, S.G.; Han, G.; Koo, T.; Hwang, S. Bacteria and archaea communities in full-scale thermophilic and mesophilic anaerobic digesters treating food wastewater: Key process parameters and microbial indicators of process instability. Bioresour. Technol. 2017, 245, 689–697. [Google Scholar]
- Sun, L.; Müller, B.; Schnürer, A. Biogas production from wheat straw: Community structure of cellulose-degrading bacteria. Energy. Sustain. Soc. 2013, 3, 15. [Google Scholar]
- Ziganshin, A.M.; Liebetrau, J.; Pröter, J.; Kleinsteuber, S. Microbial community structure and dynamics during anaerobic digestion of various agricultural waste materials. Appl. Microbiol. Biotechnol. 2013, 97, 5161–5174. [Google Scholar]
- Ziganshin, A.M.; Ziganshina, E.E.; Kleinsteuber, S.; Nikolausz, M. Comparative analysis of methanogenic communities in different laboratory-scale anaerobic digesters. Archaea 2016, 2016, 3401272. [Google Scholar]
- Campanaro, S.; Treu, L.; Rodriguez-R, L.M.; Kovalovszki, A.; Ziels, R.M.; Maus, I.; Zhu, X.; Kougias, P.G.; Basile, A.; Luo, G.; et al. The anaerobic digestion microbiome: A collection of 1600 metagenome-assembled genomes shows high species diversity related to methane production. bioRxiv 2019, 680553. [Google Scholar] [CrossRef]
- Marzorati, M.; Wittebolle, L.; Boon, N.; Daffonchio, D.; Verstraete, W. How to get more out of molecular fingerprints: Practical tools for microbial ecology. Environ. Microbiol. 2008, 10, 1571–1581. [Google Scholar] [CrossRef]
- Werner, J.J.; Knights, D.; Garcia, M.L.; Scalfone, N.B.; Smith, S.; Yarasheski, K.; Cummings, T.A.; Beers, A.R.; Knight, R.; Angenent, L.T. Bacterial community structures are unique and resilient in full-scale bioenergy systems. Proc. Natl. Acad. Sci. USA 2011, 108, 4158–4163. [Google Scholar] [CrossRef] [Green Version]
- Goux, X.; Calusinska, M.; Lemaigre, S.; Marynowska, M.; Klocke, M.; Udelhoven, T.; Benizri, E.; Delfosse, P. Microbial community dynamics in replicate anaerobic digesters exposed sequentially to increasing organic loading rate, acidosis, and process recovery. Biotechnol. Biofuels 2015, 8, 122. [Google Scholar] [CrossRef] [Green Version]
- De Vrieze, J.; Saunders, A.M.; He, Y.; Fang, J.; Nielsen, P.H.; Verstraete, W.; Boon, N. Ammonia and temperature determine potential clustering in the anaerobic digestion microbiome. Water Res. 2015, 75, 312–323. [Google Scholar] [CrossRef]
- Hanreich, A.; Schimpf, U.; Zakrzewski, M.; Schlüter, A.; Benndorf, D.; Heyer, R.; Rapp, E.; Pühler, A.; Reichl, U.; Klocke, M. Metagenome and metaproteome analyses of microbial communities in mesophilic biogas-producing anaerobic batch fermentations indicate concerted plant carbohydrate degradation. Syst. Appl. Microbiol. 2013, 36, 330–338. [Google Scholar] [CrossRef]
- Westerholm, M.; Isaksson, S.; Karlsson Lindsjö, O.; Schnürer, A. Microbial community adaptability to altered temperature conditions determines the potential for process optimisation in biogas production. Appl. Energy 2018, 226, 838–848. [Google Scholar] [CrossRef]
- Kovács, E.; Wirth, R.; Maróti, G.; Bagi, Z.; Nagy, K.; Minárovits, J.; Rákhely, G.; Kovács, K.L. Augmented biogas production from protein-rich substrates and associated metagenomic changes. Bioresour. Technol. 2015, 178, 254–261. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.S.; Mahmood, S.; Adeolu, M. A phylogenomic and molecular signature based approach for characterization of the phylum Spirochaetes and its major clades: proposal for a taxonomic revision of the phylum. Front. Microbiol. 2013, 4, 217. [Google Scholar]
- Zhu, Z.; Li, W.; Zhang, R.; Thorin, E.; Khalid, H.; Chen, C.; Liu, G. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar]
- Shrestha, S.; Fonoll, X.; Khanal, S.K.; Raskin, L. Biological strategies for enhanced hydrolysis of lignocellulosic biomass during anaerobic digestion: Current status and future perspectives. Bioresour. Technol. 2017, 245, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, G.; van Lier, J.B.; Plugge, C.M.; Azman, S.; Khadem, A.F. Presence and Role of Anaerobic Hydrolytic Microbes in Conversion of Lignocellulosic Biomass for Biogas Production. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2523–2564. [Google Scholar]
- Hania, W.B.; Postec, A.; Aüllo, T.; Ranchou-Peyruse, A.; Erauso, G.; Brochier-Armanet, C.; Hamdi, M.; Ollivier, B.; Saint-Laurent, S.; Magot, M.; et al. Mesotoga sp. nov., a mesophilic member of the order Thermotogales, isolated from an underground gas storage aquifer. Int. J. Syst. Evol. Microbiol. 2013, 63, 3003–3008. [Google Scholar] [CrossRef] [PubMed]
- Nesbo, C.L.; Charchuk, R.; Pollo, S.M.J.; Budwill, K.; Kublanov, I.; Haverkamp, T.H.A.; Foght, J. Genomic insights into metabolism and phylogeography of the mesophilic Thermotogae genus Mesotoga. bioRxiv 2018, 322537. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, Y.; Wang, Y.; Chin, F.Y.L.; Zhang, T. Cellular adhesiveness and cellulolytic capacity in Anaerolineae revealed by omics-based genome interpretation. Biotechnol. Biofuels 2016, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- McInerney, M.J.; Rohlin, L.; Mouttaki, H.; Kim, U.; Krupp, R.S.; Rios-Hernandez, L.; Sieber, J.; Struchtemeyer, C.G.; Bhattacharyya, A.; Campbell, J.W.; et al. The genome of Syntrophus aciditrophicus: life at the thermodynamic limit of microbial growth. Proc. Natl. Acad. Sci. USA 2007, 104, 7600–7605. [Google Scholar] [CrossRef] [Green Version]
- Niu, Q.; Kubota, K.; Qiao, W.; Jing, Z.; Zhang, Y.; Yu-You, L. Effect of ammonia inhibition on microbial community dynamic and process functional resilience in mesophilic methane fermentation of chicken manure. J. Chem. Technol. Biotechnol. 2015, 90, 2161–2169. [Google Scholar] [CrossRef]
- Lv, Z.; Jiang, J.; Liebetrau, J.; Richnow, H.H.; Fischer, A.; Ács, N.; Nikolausz, M. Ammonium Chloride vs. Urea-Induced Ammonia Inhibition of the Biogas Process Assessed by Stable Isotope Analysis. Chem. Eng. Technol. 2018, 41, 671–679. [Google Scholar] [CrossRef]
- Baena, S.; Fardeau, M.; Labat, M.; Ollivier, B.; Thomas, P.; Garcia, J.; Patel, K. Aminobacterium colombiensegen. nov. sp. nov., an amino acid-degrading anaerobe isolated from anaerobic sludge. Anaerobe 1998, 4, 241–250. [Google Scholar] [CrossRef] [Green Version]
- Chertkov, O.; Sikorski, J.; Brambilla, E.; Lapidus, A.; Copeland, A.; del Rio, T.G.; Nolan, M.; Lucas, S.; Tice, H.; Cheng, J.F.; et al. Complete genome sequence of Aminobacterium colombiense type strain (ALA-1 T). Stand. Genomic Sci. 2010, 2, 280–289. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Regueiro, L.; Veiga, P.; Figueroa, M.; Alonso-Gutierrez, J.; Stams, A.J.M.; Lema, J.M.; Carballa, M. Relationship between microbial activity and microbial community structure in six full-scale anaerobic digesters. Microbiol. Res. 2012, 167, 581–589. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klang, J.; Szewzyk, U.; Bock, D.; Theuerl, S. Effect of a Profound Feedstock Change on the Structure and Performance of Biogas Microbiomes. Microorganisms 2020, 8, 169. https://doi.org/10.3390/microorganisms8020169
Klang J, Szewzyk U, Bock D, Theuerl S. Effect of a Profound Feedstock Change on the Structure and Performance of Biogas Microbiomes. Microorganisms. 2020; 8(2):169. https://doi.org/10.3390/microorganisms8020169
Chicago/Turabian StyleKlang, Johanna, Ulrich Szewzyk, Daniel Bock, and Susanne Theuerl. 2020. "Effect of a Profound Feedstock Change on the Structure and Performance of Biogas Microbiomes" Microorganisms 8, no. 2: 169. https://doi.org/10.3390/microorganisms8020169
APA StyleKlang, J., Szewzyk, U., Bock, D., & Theuerl, S. (2020). Effect of a Profound Feedstock Change on the Structure and Performance of Biogas Microbiomes. Microorganisms, 8(2), 169. https://doi.org/10.3390/microorganisms8020169




