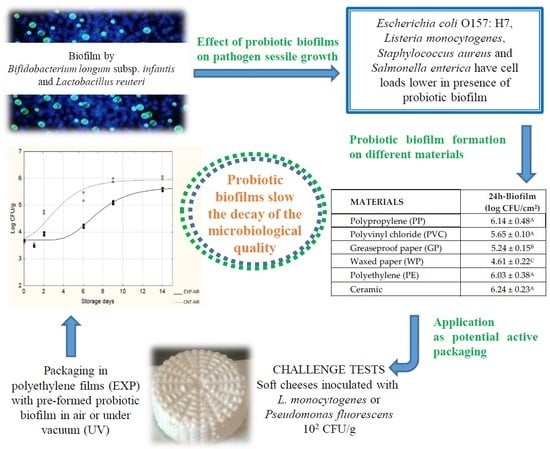
Evaluation of the Potential of Biofilm Formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as Competitive Biocontrol Agents Against Pathogenic and Food Spoilage Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Effect of Probiotic Biofilms on Pathogen Sessile Growth
2.1.1. Surfaces and Microorganisms
2.1.2. Experiment
2.2. Application as Potential Active Packaging
2.2.1. Probiotic Biofilm Formation on Different Materials
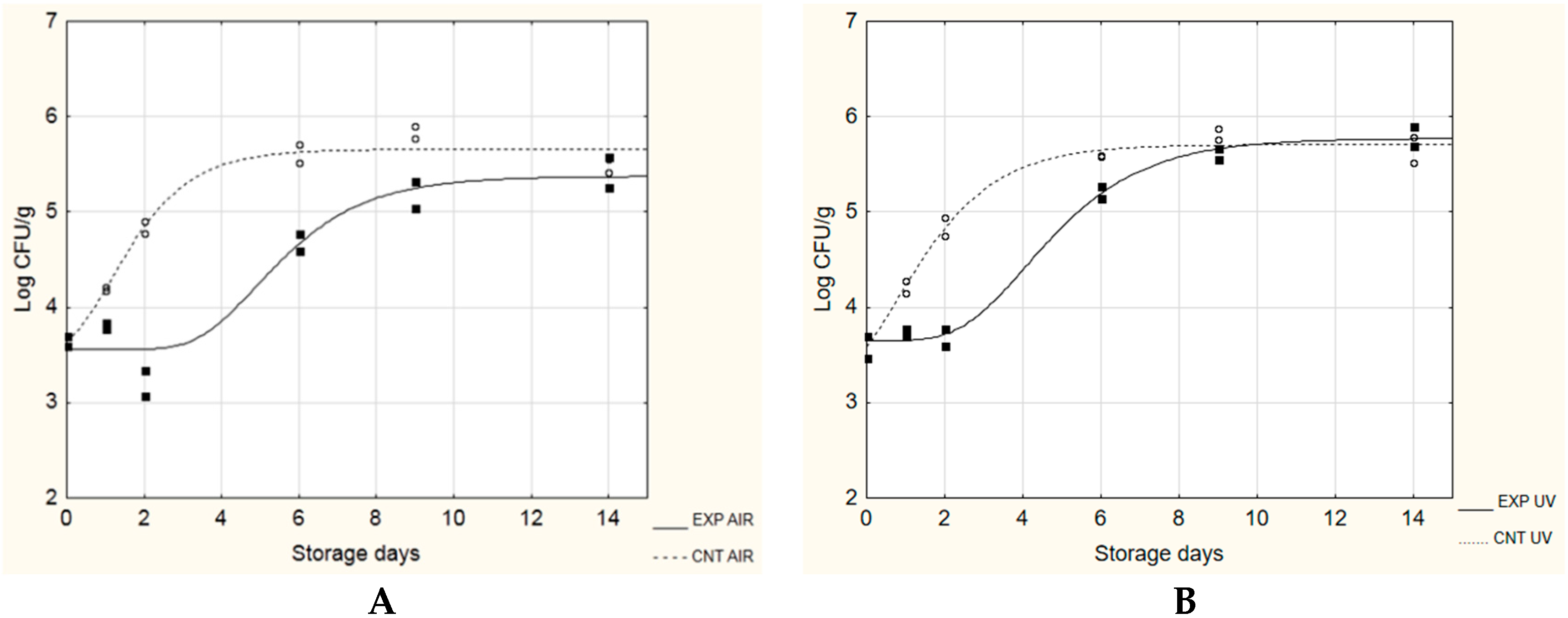
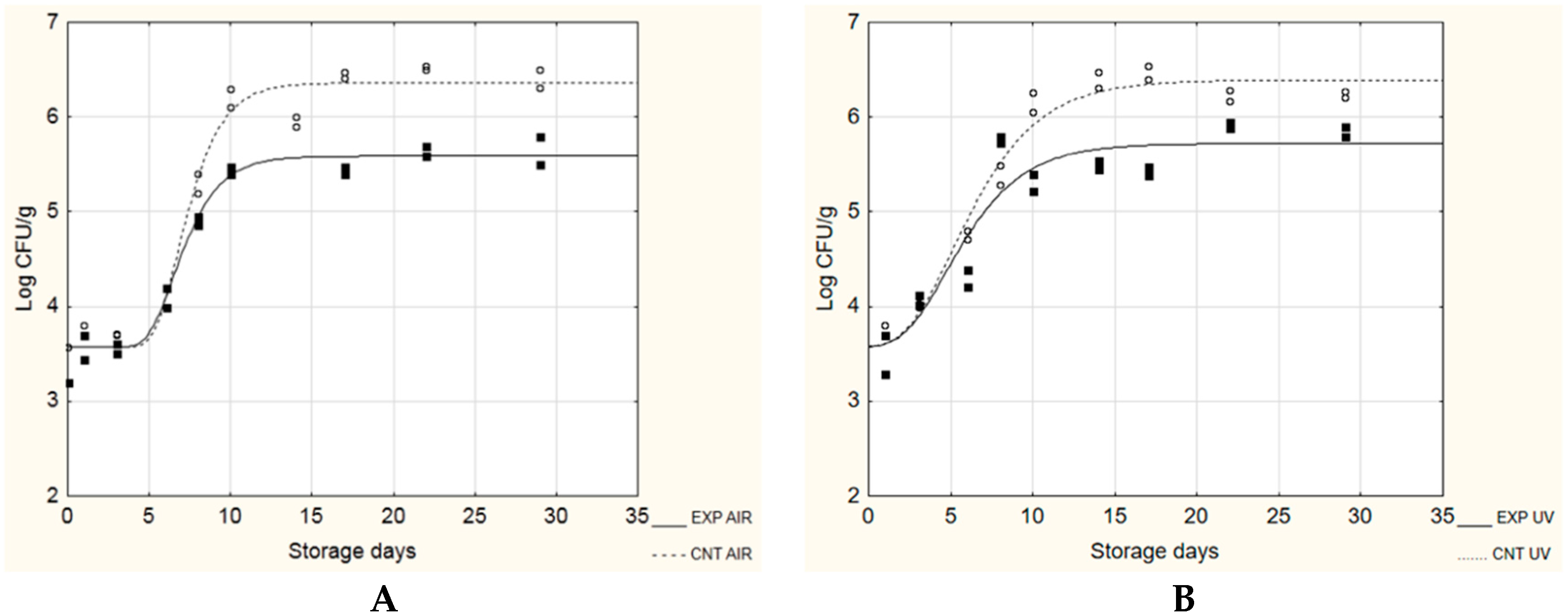
2.2.2. Challenge Tests
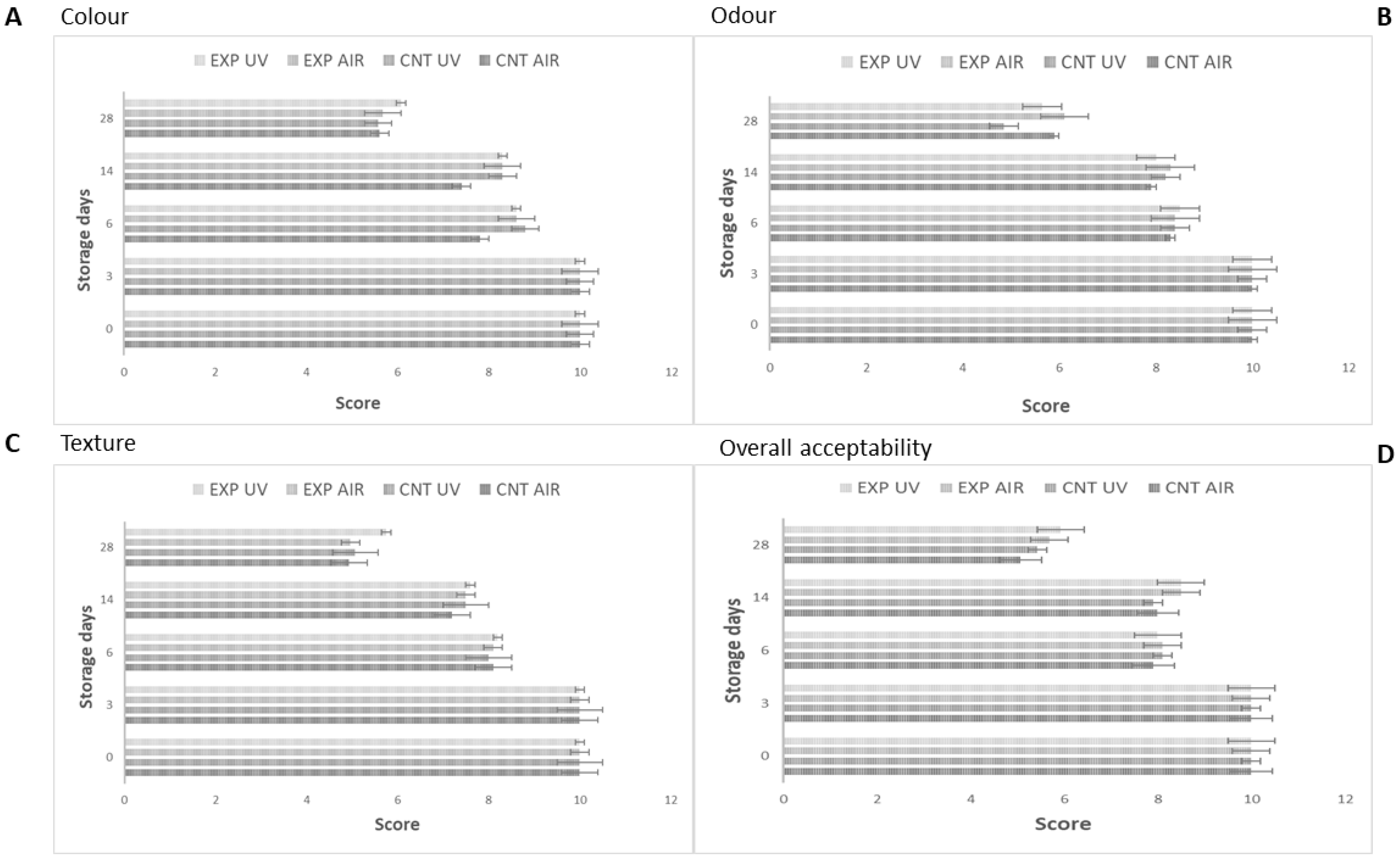
2.2.3. Microbiological, Chemico-Physical and Sensorial Analyses
2.2.4. Statistical Analyses
3. Results and Discussion
3.1. Effect of Probiotic Biofilms on Pathogen Sessile Growth
3.2. Application as Potential Active Packaging
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Ouwehand, A.C.; Salminen, S.; Isolauri, E. Probiotics: An overview of beneficial effects. Antonie Van Leeuwenhoek 2002, 82, 279–289. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepato. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Charalampopoulos, D.; Rastall, R.A. Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; p. 1262. [Google Scholar]
- Corbo, M.R.; Speranza, B.; Sinigaglia, M. Microbial biofilms in the food industry: only negative implications? In Biofilms: Formation, Development and Properties; Bailey, W.C., Ed.; Nova Science Publishers: New York, NY, USA, 2009; pp. 585–594. [Google Scholar]
- Carducci, A.; Verani, M.; Lombardi, R.; Casini, B.; Privitera, G. Environmental survey to assess viral contamination of air and surfaces in hospital settings. J. Hosp. Infect. 2011, 77, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Eschenbach, D.A.; Patton, D.L.; Hooton, T.M.; Meier, A.S.; Stapleton, A.; Aura, J.; Agnew, K. Effects of vaginal intercourse with and without a condom on vaginal flora and vaginal epithelium. J. Infect. Dis. 2001, 183, 913–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuotto, C.; Barbanti, F.; Mastrantonio, P.; Donelli, G. Lactobacillus brevis CD2 inhibits Prevotella melaninogenica biofilm. Oral. Dis. 2014, 20, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lv, Z.; Su, J.; Wang, J.; Yan, D.; Wei, J.; Pei, S. Consistent Condom Use Increases the Colonization of Lactobacillus crispatus in the Vagina. PLoS ONE 2013, 8, e70716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leccese Terraf, M.C.; Juarez Tomas, M.S.; Nader-Macıas, M.E.F.; Silva, C. Screening of biofilm formation by beneficial vaginal lactobacilli and influence of culture media components. J. Appl. Microbiol. 2012, 113, 1517–1529. [Google Scholar] [CrossRef] [PubMed]
- Salo, S.; Ehavald, H.; Raaska, L.; Vokk, R.; Wirtanen, G. Microbial surveys in Estonian dairies. LWT Food Sci. Technol. 2006, 39, 460–471. [Google Scholar] [CrossRef]
- Sharma, M.; Anand, S.K. Biofilms evaluation as an essential component of HACCP for food/dairy processing industry e a case. Food Control 2002, 13, 469–477. [Google Scholar] [CrossRef]
- Waak, E.; Tham, W.; Danielsson-Tham, M.L. Prevalence and fingerprinting of Listeria monocytogenes strains isolated from raw whole milk in farm bulk tanks and in dairy plant receiving tanks. Appl. Env. Microbiol. 2002, 68, 3366–3370. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Corbo, M.R. The impact of biofilms on food spoilage. In The Microbiological Quality of Food: Foodborne Spoilers; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Elsevier: Oxford, UK, 2017; pp. 259–282. [Google Scholar]
- Guillier, L.; Stahl, V.; Hezard, B.; Notz, E.; Briandet, R. Modelling the competitive growth between Listeria monocytogenes and biofilm microflora of smear cheese wooden shelves. Int. J. Food Microbiol. 2008, 128, 51–57. [Google Scholar] [CrossRef]
- Guerrieri, E.; de Niederhäusern, S.; Messi, P.; Sabia, C.; Iseppi, R.; Anacarso, I.; Bondi, M. Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control 2009, 20, 861–865. [Google Scholar] [CrossRef]
- Speranza, B.; Sinigaglia, M.; Corbo, M.R. Non starter lactic acid bacteria biofilms: A means to control the growth of Listeria monocytogenes in soft cheese. Food Control 2009, 20, 1063–1067. [Google Scholar] [CrossRef]
- Vandini, A.; Temmerman, R.; Frabetti, A.; Caselli, E.; Antonioli, P.; Balboni, P.G.; Platano, D.; Branchini, A.; Mazzacane, S. Hard Surface Biocontrol in Hospitals Using Microbial-Based Cleaning Products. PLoS ONE 2014, 9, e108598. [Google Scholar] [CrossRef] [Green Version]
- Speranza, B.; Liso, A.; Corbo, M.R. Use of design of experiments to optimize the production of microbial probiotic biofilms. PeerJ 2018, 6, e4826. [Google Scholar] [CrossRef]
- Method for Producing Microbial Probiotic Biofilms and Uses thereof WO2017203440. Available online: https://patents.google.com/patent/WO2017203440A1/en (accessed on 25 November 2019).
- Gudiña, E.J.; Teixeira, J.A.; Rodrigues, L.R. Isolation and functional characterization of a biosurfactant produced by Lactobacillus paracasei. Colloid Surf. B 2010, 76, 298–304. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.E.; Versalovic, J. Probiotic Lactobacillus reuteri biofilms produce antimicrobial and anti-inflammatory factors. BMC Microbiol 2009, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Biosurfactants: potential applications in medicine. J. Antimicrob. Chemoth. 2006, 57, 609–618. [Google Scholar] [CrossRef]
- Rodrigues, L.; Banat, I.M.; Teixeira, J.; Oliveira, R. Strategies for the prevention of microbial biofilm formation on silicone rubber voice prostheses. J. Biomed Mater Res. B 2007, 81, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; van der Mei, H.C.; Banat, I.M.; Teixeira, J.; Oliveira, R. Inhibition of microbial adhesion to silicone rubber treated with biosurfactant from Streptococcus thermophilus A. FEMS Immunol. Med. Microbiol. 2006, 46, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.; van der Mei, H.C.; Teixeira, J.; Oliveira, R. Influence of biosurfactants from probiotic bacteria on formation of biofilms on voice prostheses. Appl. Env. Microbiol. 2004, 70, 4408–4410. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.R.; Banat, I.M.; van der Mei, H.C.; Teixeira, J.A.; Oliveira, R. Interference in adhesion of bacteria and yeasts isolated from explanted voice prostheses to silicone rubber by rhamnolipid biosurfactants. J. Appl. Microbiol. 2006, 100, 470–480. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, L.R.; Teixeira, J.A.; van der Mei, H.C.; Oliveira, R. Physicochemical and functional characterization of a biosurfactant produced by Lactococcus lactis 53. Colloid Surf. B 2006, 49, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Gómez, N.C.; Ramiro, J.M.P.; Quecan, B.X.V.; de Melo Franco, B.D.G. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157: H7 biofilms formation. Front. Microbiol. 2016, 7, 863. [Google Scholar] [CrossRef] [Green Version]
- Mandakhalikar, K.D.; Rahmat, J.N.; Chiong, E.; Neoh, K.G.; Shen, L.; Tambyah, P.A. Extraction and quantification of biofilm bacteria: Method optimized for urinary catheters. Sci. Rep. 2018, 8, 8069. [Google Scholar] [CrossRef]
- Zwietering, M.H.; Jogenburger, I.; Rombouts, F.M.; Van’t Riet, K. Modelling of the bacterial growth curve. Appl. Environ. Microbiol. 1990, 56, 1975–1981. [Google Scholar] [CrossRef] [Green Version]
- Castillejo Rodrìguez, A.M.; Barco Alcalà, E.; Garcia Gimeno, R.M.; Zurera Cosano, G. Growth modelling of Listeria monocytogenes in packaged fresh green asparagus. Food Microbiol. 2000, 17, 421–427. [Google Scholar] [CrossRef]
- Riva, M.; Franzetti, L.; Galli, A. Microbiological quality and shelf life modeling of ready-to-eat cicorino. J. Food Prot. 2001, 64, 228–234. [Google Scholar] [CrossRef]
- Abdelhamid, A.G.; Esaam, A.; Hazaa, M.M. Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli. Saudi Pharm. J. 2018, 26, 603–607. [Google Scholar] [CrossRef]
- Kaboosi, H. Antibacterial effects of probiotics isolated from yoghurts against some common bacterial pathogens. Afr. J. Microbiol. Res. 2011, 5, 4363–4367. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. Antipathogenic activity of probiotics against Salmonella Typhimurium and Clostridium difficile in anaerobic batch culture systems: is it due to synergies in probiotic mixtures or the specificity of single strains? Anaerobe 2013, 24, 60–65. [Google Scholar] [CrossRef]
- Barbosa, M.S.; Todorov, S.D.; Jurkiewicz, C.H.; Franco, B.D.G.M. Bacteriocin production by Lactobacillus curvatus MBSa2 entrapped in calcium alginate during ripening of salami for control of Listeria monocytogenes. Food Control 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Gálvez, A.; Abriouel, H.; Ben Omar, N. Bacteriocin-based strategies for food biopreservation. Int. J. Food Microbiol. 2007, 120, 51–70. [Google Scholar] [CrossRef]
- Srey, S.; Jahid, I.K.; Ha, S. Biofilm formation in food industries: a food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- De Buyser, M.L.; Dufour, B.; Maire, M.; Lafarge, V. Implication of milk and milk products in food-borne diseases in France and different industrialized countries. Int. J. Food Microbiol. 2001, 67, 1–17. [Google Scholar] [CrossRef]
- Martin, N.H.; Murphy, S.C.; Ralyea, R.; Boor, K.J. When cheese gets the blues: Pseudomonas fluorescens as the causative agent of cheese spoilage. J. Dairy Sci. 2011, 94, 3176–3183. [Google Scholar] [CrossRef]
- Corbo, M.R.; Altieri, C.; D’Amato, D.; Campaniello, D.; Del Nobile, M.A.; Sinigaglia, M. Effect of temperature on shelf life and microbial population of lightly processed cactus pear fruit. Postharvest Biol. Technol. 2004, 31, 93–104. [Google Scholar] [CrossRef]
- Mariani, C.; Oulahal, N.; Chamba, J.F.; Dubois-Brissonnet, F.; Notz, E.; Briandet, R. Inhibition of Listeria monocytogenes by resident biofilms present on wooden shelves used for cheese ripening. Food Control 2011, 22, 1357–1362. [Google Scholar] [CrossRef]
- Léonard, L.; Degraeve, P.; Gharsallaoui, A.; Saurel, R.; Oulahal, N. Design of biopolymeric matrices entrapping bioprotective lactic acid bacteria to control Listeria monocytogenes growth: Comparison of alginate and alginate-caseinate matrices entrapping Lactococcus lactis subsp. lactis cells. Food Control 2014, 37, 200–209. [Google Scholar] [CrossRef]
- Schöbitz, R.; González, C.; Villarreal, K.; Horzella, M.; Nahuelquín, Y.; Fuentes, R. A biocontroller to eliminate Listeria monocytogenes from the food processing environment. Food Control 2014, 36, 217–223. [Google Scholar] [CrossRef]
| Strains | Source | Optimal Media and Growth Conditions |
|---|---|---|
| Listeria monocytogenes * | Culture Collection of the Laboratory of Predictive Microbiology, SAFE, University of Foggia | Listeria selective agar base (Oxoid) plus Listeria selective supplement-Oxoid formulation, incubated at 37 °C for 48 h |
| Escherichia coli O157:H7 | CECT 4267 | Sorbitol MacConkey Agar (Oxoid), incubated at 37 °C for 24 h |
| Staphylococcus aureus | ATCC 25923 | Baird-Parker Agar Base (Oxoid) plus Egg Yolk Tellurite Emulsion, incubated at 37 °C for 24 h |
| Salmonella enterica | ATCC 35664 | Chromatic Salmonella Agar (Liofilchem, Roseto degli Abruzzi, Teramo, Italy), incubated at 37 °C for 24 h |
| L. monocytogenes | |||
| Time (h) | CNT | ACT | ** Biofilm Efficacy |
| 0 | 4.21 ± 0.01 A,* | 3.46 ± 0.12 B | 0.75 ± 0.17 a,*** |
| 4 | 4.82 ± 0.16 A | 3.39 ± 0.20 B | 1.43 ± 0.28 b |
| 24 | 4.83 ± 0.13 A | 4.10 ± 0.10 B | 0.73 ± 0.14 a |
| 30 | 5.18 ± 0.25 A | 4.31 ± 0.11 B | 0.87 ± 0.16 a |
| 48 | 4.91 ± 0.01 A | 4.23 ± 0.10 B | 0.68 ± 0.14 a |
| E. coli O157:H7 | |||
| Time (h) | CNT | ACT | Biofilm Efficacy |
| 0 | 5.49 ± 0.01 A | 5.20 ± 0.25 A | 0.29 ± 0.35 a |
| 4 | 5.43 ± 0.14 A | 4.19 ± 0.22 B | 1.24 ± 0.31 b |
| 24 | 5.56 ± 0.41 A | 4.10 ± 0.01 B | 1.46 ± 0.01 b |
| 30 | 6.13 ± 0.30 A | 3.82 ± 0.01 B | 2.31 ± 0.01 c |
| 48 | 6.00 ± 0.25 A | 3.80 ± 0.20 B | 2.20 ± 0.28 c |
| St. aureus | |||
| Time (h) | CNT | ACT | Biofilm Efficacy |
| 0 | 5.07 ± 0.20 A | 4.88 ± 0.01 A | 0.19 ± 0.01 a,b |
| 4 | 5.02 ± 0.03 A | 4.70 ± 0.33 A | 0.32 ± 0.47 b,c |
| 24 | 5.57 ± 0.14 A | 4.89 ± 0.19 B | 0.68 ± 0.27 b,c |
| 30 | 5.16 ± 0.01 A | 3.86 ± 0.22 B | 1.30 ± 0.31 c,d |
| 48 | 5.16 ± 0.30 A | 3.71 ± 0.05 B | 1.45 ± 0.07 d |
| Salmonella enterica | |||
| Time (h) | CNT | ACT | Biofilm Efficacy |
| 0 | 5.94 ± 0.10 A | 4.37± 0.10 B | 1.57 ± 0.14 a |
| 4 | 5.38 ± 0.10 A | 4.47 ± 0.16 B | 0.91 ± 0.23 b |
| 24 | 5.53 ± 0.15 A | 4.54 ± 0.13 B | 0.99 ± 0.18 b |
| 30 | 5.35 ± 0.23 A | 4.88 ± 0.05 B | 0.47 ± 0.07 c |
| 48 | 4.98 ± 0.30 A | 4.77 ± 0.00 B | 0.21 ± 0.00 c |
| Materials | Cellular Probiotic Load in Sessile Form (log CFU/cm2) | ||
|---|---|---|---|
| 2 h | 24 h | 96 h | |
| Polypropylene (PP) | 6.64 ± 0.00 A | 6.14 ± 0.48 A | 5.88 ± 0.23 A |
| Polyvinyl chloride (PVC) | 6.54 ± 0.14 A | 5.65 ± 0.10 A | 5.87 ± 0.30 A |
| Greaseproof paper (GP) | 5.77 ± 0.23 B | 5.24 ± 0.15 B | 5.25 ± 0.06 B |
| Waxed paper (WP) | No adhesion | 4.61 ± 0.22 C | 4.53 ± 0.13 C |
| Polyethylene (PE) | 6.94 ± 0.00 A | 6.03 ± 0.38 A | 6.54 ± 0.14 D |
| Ceramic | 6.86 ± 0.20 A | 6.24 ± 0.23 A | 6.54 ± 0.14 D |
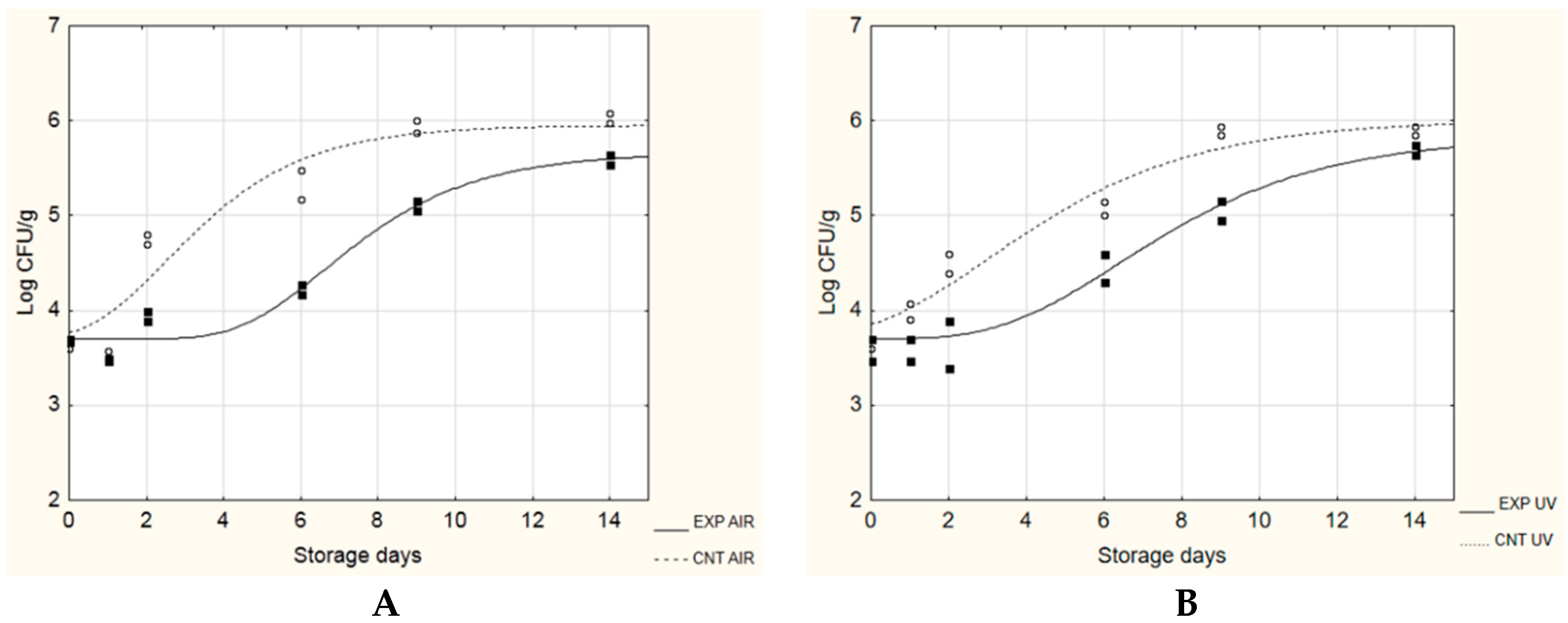
| L. monocytogenes | ||||
|---|---|---|---|---|
| A + No [Log CFU/g] | µmax [Log(CFU/g)/day] | λ [day] | TRS * [day] | |
| CNT AIR | 5.66 ± 0.31 A | 0.69 ± 0.14 A | 0.04 ± 0.67 A | 2.88 |
| EXP AIR | 5.37 ± 0.20 A | 0.43 ± 0.19 A | 3.37 ± 1.06 B | 4.62 |
| CNT UV | 5.94 ± 0.85 A | 0.68 ± 0.09 A | 0.00 ± 0.00 A | 2.95 |
| EXP UV | 5.39 ± 0.11 A | 0.47 ± 0.09 A | 2.40 ± 0.72 B | 4.30 |
| Ps. fluorescens | ||||
| A + No [Log CFU/g] | µmax [Log(CFU/g)/day] | λ [day] | ST ** [day] | |
| CNT AIR | 5.95 ± 0.33 A | 0.41 ± 0.23 A | 0.54 ± 0.61 A | 2.54 |
| EXP AIR | 5.66 ± 0.18 A | 0.33± 0.09 A | 4.40 ± 0.74 B | 6.59 |
| CNT UV | 6.00 ± 0.19 A | 0.28 ± 0.05 A | 0.00 ± 0.50 A | 3.03 |
| EXP UV | 5.84 ± 0.25 A | 0.26 ± 0.05 A | 3.33 ± 0.76 B | 6.35 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Speranza, B.; Liso, A.; Russo, V.; Corbo, M.R. Evaluation of the Potential of Biofilm Formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as Competitive Biocontrol Agents Against Pathogenic and Food Spoilage Bacteria. Microorganisms 2020, 8, 177. https://doi.org/10.3390/microorganisms8020177
Speranza B, Liso A, Russo V, Corbo MR. Evaluation of the Potential of Biofilm Formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as Competitive Biocontrol Agents Against Pathogenic and Food Spoilage Bacteria. Microorganisms. 2020; 8(2):177. https://doi.org/10.3390/microorganisms8020177
Chicago/Turabian StyleSperanza, Barbara, Arcangelo Liso, Vincenzo Russo, and Maria Rosaria Corbo. 2020. "Evaluation of the Potential of Biofilm Formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as Competitive Biocontrol Agents Against Pathogenic and Food Spoilage Bacteria" Microorganisms 8, no. 2: 177. https://doi.org/10.3390/microorganisms8020177
APA StyleSperanza, B., Liso, A., Russo, V., & Corbo, M. R. (2020). Evaluation of the Potential of Biofilm Formation of Bifidobacterium longum subsp. infantis and Lactobacillus reuteri as Competitive Biocontrol Agents Against Pathogenic and Food Spoilage Bacteria. Microorganisms, 8(2), 177. https://doi.org/10.3390/microorganisms8020177