A Potent Burkholderia Endophyte against Boxwood Blight Caused by Calonectria pseudonaviculata
Abstract
1. Introduction
2. Materials and Methods
2.1. Cps Isolate and Conidia Production
2.2. Plant Growth Conditions and Biosafety Measures for All in-Planta Inoculation Studies Buxus Sempervirens
2.3. Isolation and Selection of the Bacterial Endophyte from Boxwood Leaves with Unusual Response to Cps Inoculation
2.4. SSG Cell Suspension and Cell-Free Supernatant Preparation
2.5. SSG Effect on Cps Conidia Survival and Germination
2.6. Effect of SSG on Boxwood Blight
2.7. Effect of SSG Concentration on Boxwood Blight
2.8. Effect of SSG Treatment Lead Time on Boxwood Blight
2.9. Effect of Post-Inoculation SSG Treatment on Boxwood Blight
2.10. SSG Effect on Potential of Diseased Leaves as a Source of Inoculum
2.11. SSG Species Identity and Differentiation from Epidemic Strains of Burkholderia cepacia
2.12. Data Analysis
3. Results
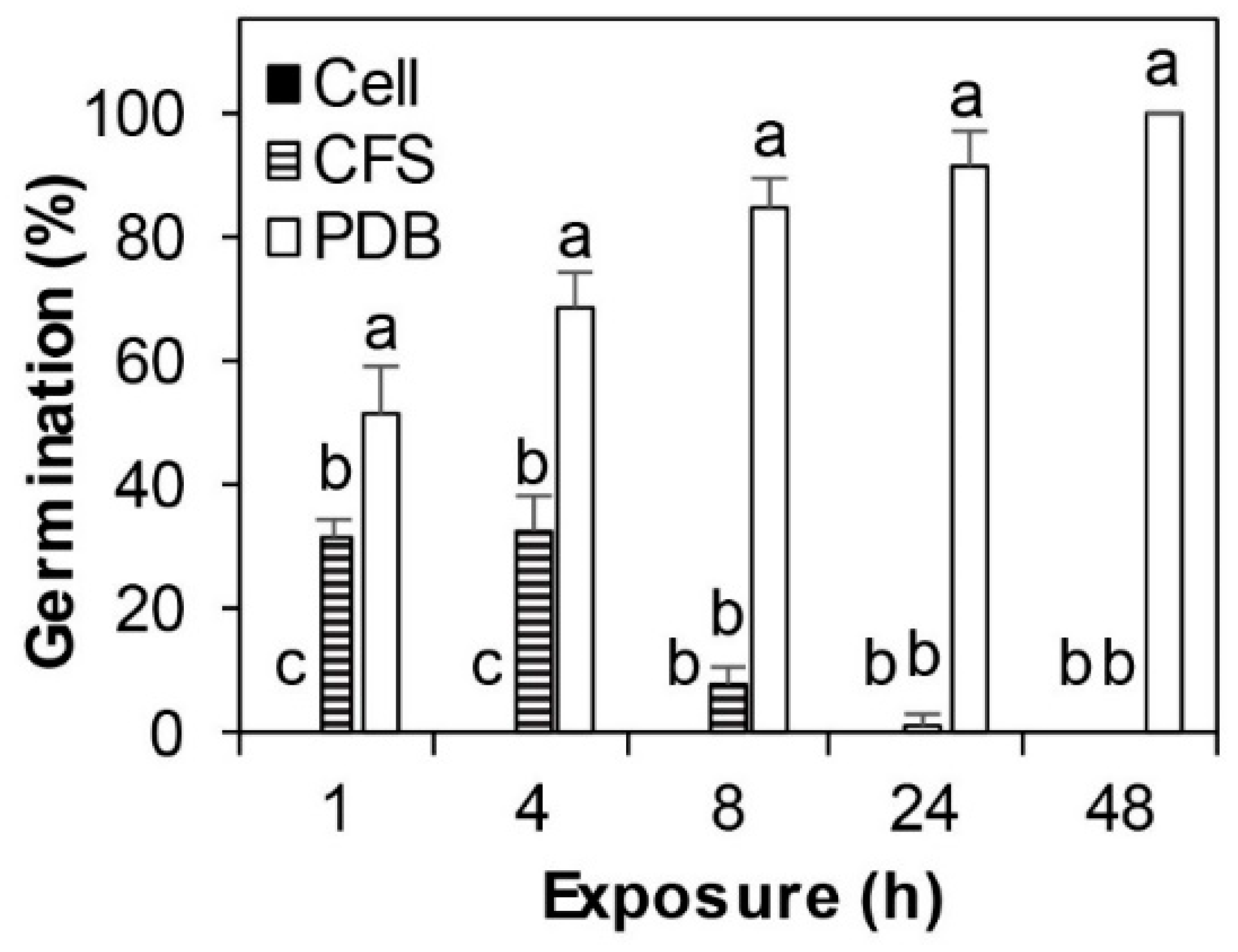
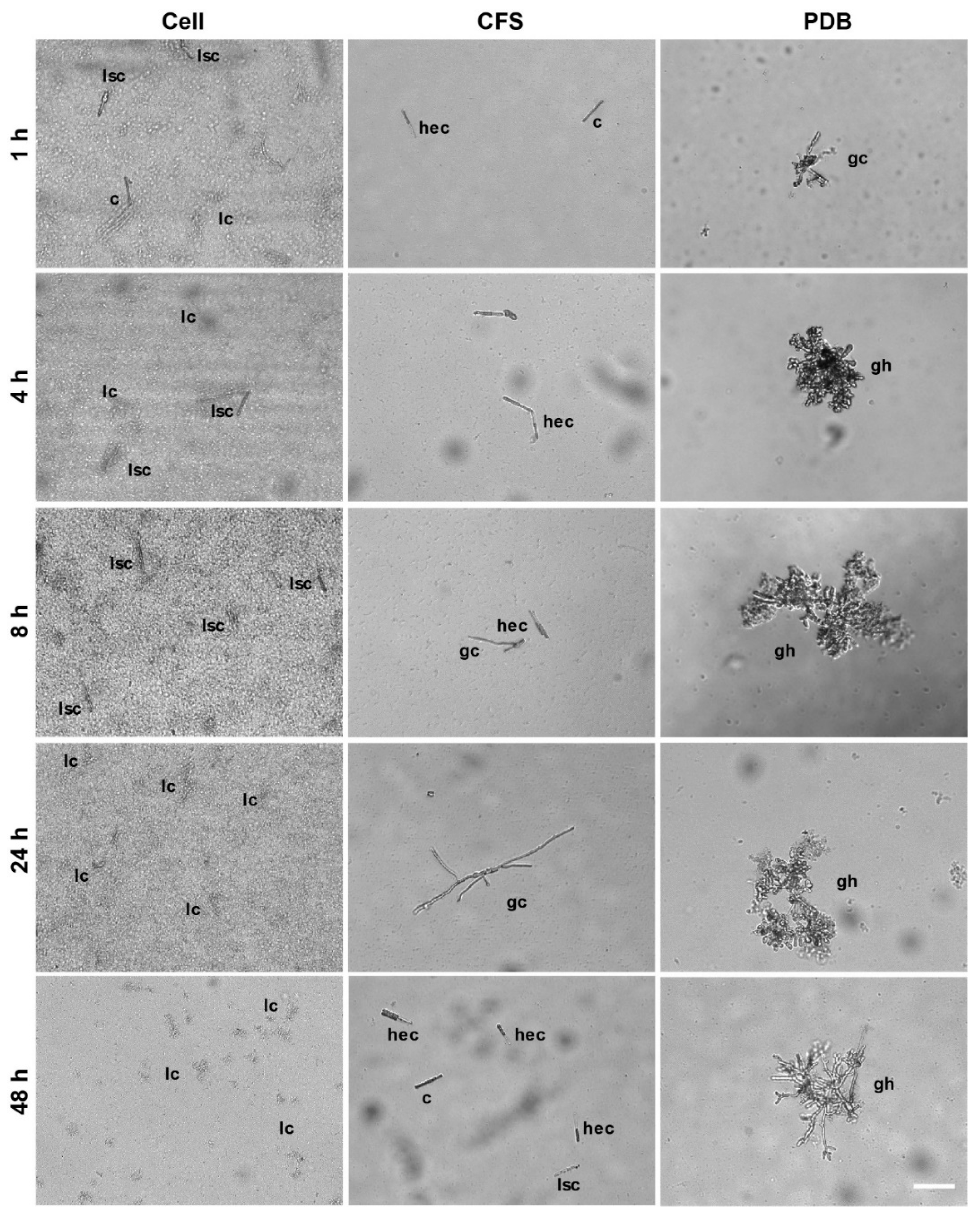
3.1. SSG Effect on Cps Conidia Survival and Germination
3.2. Effect of SSG on Boxwood Blight
3.3. Effect of SSG Concentration on Boxwood Blight
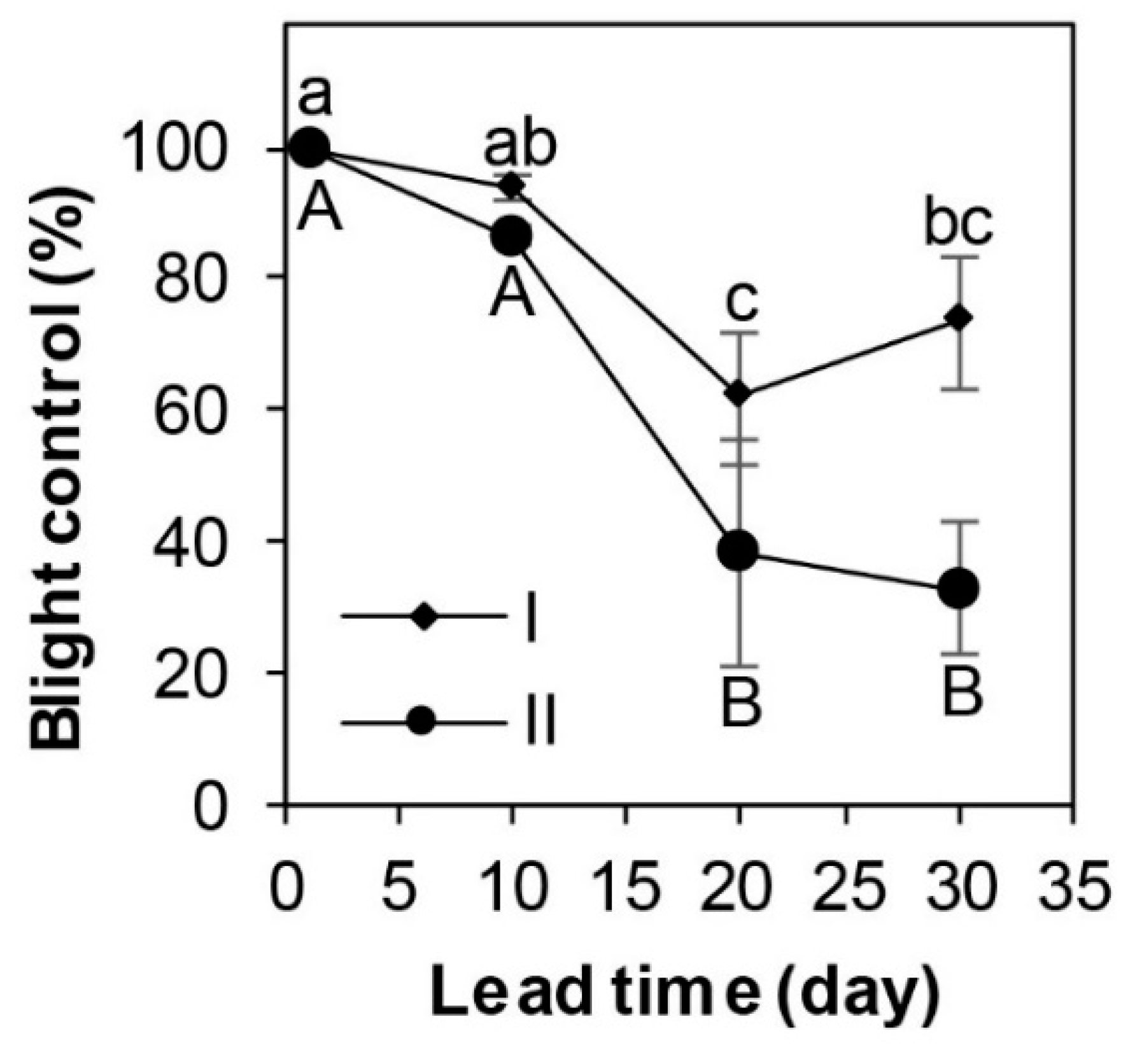
3.4. Effect of SSG Treatment Lead Time on Boxwood Blight
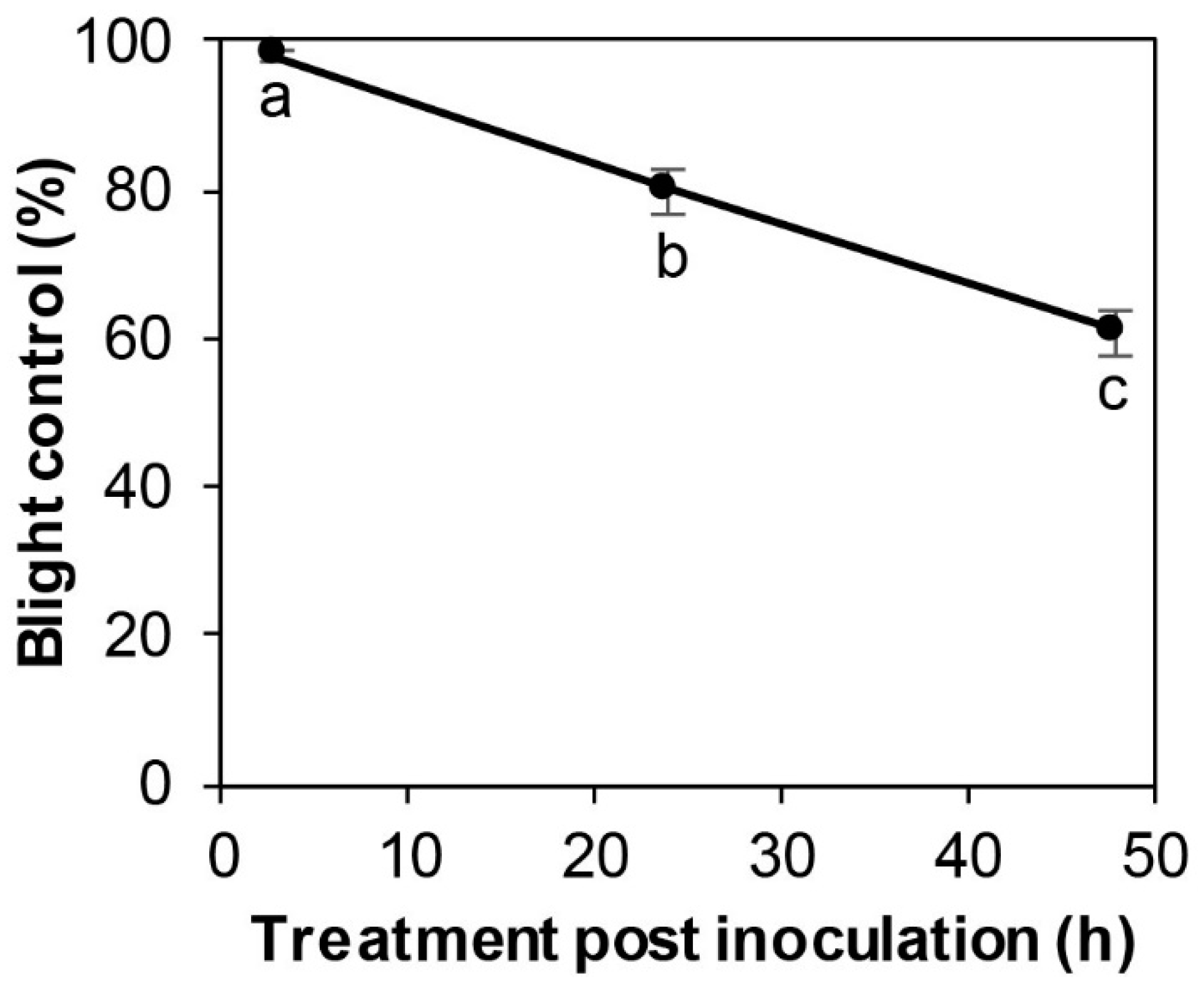
3.5. Effect of Post-Inoculation SSG Treatment on Boxwood Blight
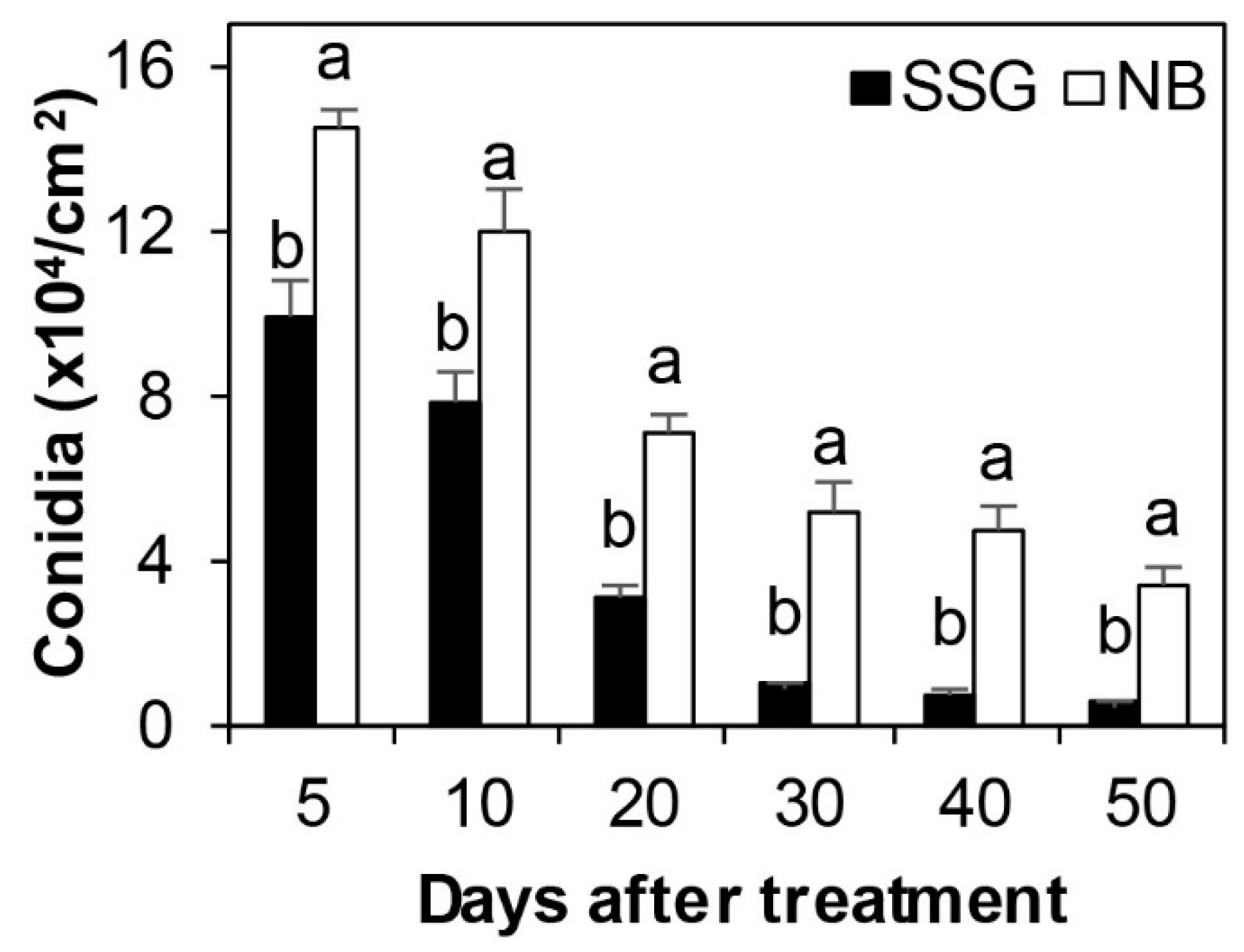
3.6. SSG Effect on Potential of Diseased Leaves as a Source of Inoculum
3.7. SSG Species Identity and Differentiation from Epidemic Strains of Burkholderia cepacia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Daughtrey, M.L. Boxwood blight: Threat to ornamentals. Annu. Rev. Phytopathol. 2019, 57, 189–209. [Google Scholar] [CrossRef]
- Hong, C.X. Fighting plant pathogens together. Science 2019, 365, 229. [Google Scholar] [PubMed]
- Ridley, G. New plant fungus found in Auckland box hedges (Buxus). For. Health News 1998, 77, 1–2. [Google Scholar]
- Henricot, B.; Sierra, A.P.; Prior, C. A new blight disease on Buxus in the UK caused by the fungus Cylind. Plant Pathol. 2000, 49, 805. [Google Scholar] [CrossRef]
- Ivors, K.L.; Lacey, L.W.; Milks, D.C.; Douglas, S.M.; Inman, M.K.; Marra, R.E.; LaMondia, J.A. First report of boxwood blight caused by Cylindrocladium pseudonaviculatum in the United States. Plant Dis. 2012, 96, 1070. [Google Scholar] [CrossRef]
- Hong, C.X. Saving American gardens from boxwood blight. Boxwood Bull. J. Am. Boxwood Soc. 2019, 58, 4–10. [Google Scholar]
- Batdorf, L.R. Boxwood Handbook—A Practical Guide to Knowing and Growing Boxwood, 3rd ed.; The American Boxwood Society: Boyce, VA, USA, 2005; p. 123. [Google Scholar]
- Saunders, P. Boxwood Guide, 5th ed.; The Saunders Brothers Family: Piney River, VA, USA, 2017; p. 95. [Google Scholar]
- Kong, P.; Hong, C.X. Host responses and impact on the boxwood blight pathogen, Calonectria pseudonaviculata. Planta 2019, 249, 831–838. [Google Scholar] [CrossRef]
- Henricot, B.; Gorton, C.; Denton, G.; Denton, J. Studies on the control of Cylindrocladium buxicola using fungicides and host resistance. Plant Dis. 2008, 92, 1273–1279. [Google Scholar] [CrossRef]
- Vitale, A.; Crous, P.W.; Lombard, L.; Polizzi, G. Calonectria diseases on ornamental plants in Europe and the Mediterranean basin: An overview. J. Plant Pathol. 2013, 95, 463–476. [Google Scholar]
- Kong, P.; Likins, T.M.; Hong, C.X. First report of Pachysandra terminalis leaf spot caused by Calonectria pseudonaviculata in Virginia. Plant Dis. 2017, 101, 509. [Google Scholar] [CrossRef]
- LaMondia, J.A. Pachysandra species and cultivar susceptibility to the boxwood blight pathogen, Calonectria pseudonaviculata. Plant Health Progr. 2017. [Google Scholar] [CrossRef]
- LaMondia, J.A.; Li, D.W. Calonectria pseudonaviculata can cause leaf spot and stem blight of Pachysandra procumbens. Plant Health Progr. 2013. [Google Scholar] [CrossRef]
- LaMondia, J.A.; Li, D.W.; Marra, R.E.; Douglas, S.M. First report of Cylindrocladium pseudonaviculatum causing leaf spot of Pachysandra terminalis. Plant Dis. 2012, 96, 1069. [Google Scholar] [CrossRef] [PubMed]
- Kong, P.; Likins, T.M.; Hong, C.X. First report of blight of Sarcococca hookeriana var. humilis by Calonectria pseudonaviculata in Virginia. Plant Dis. 2017, 101, 247. [Google Scholar]
- Malapi-Wight, M.; Salgado-Salazar, C.; Demers, J.E.; Clement, D.L.; Rane, K.K.; Crouch, J.A. Sarcococca blight: Use of whole-genome sequencing for fungal plant disease diagnosis. Plant Dis. 2016, 100, 1093–1100. [Google Scholar] [CrossRef]
- Ryan, C.; Williams-Woodward, J.; Zhang, D.L. Susceptibility of Sarcococca taxa to boxwood blight by Calonectria pseudonaviculata. Proc. South. Nurs. Assoc. Res. Conf. 2018, 62, 64–67. [Google Scholar]
- Richardson, P.A.; Daughtrey, M.L.; Hong, C.X. Indications of susceptibility to Calonectria pseudonaviculata in som common groundcovers and boxwood companion plants. Plant Dis. 2019. [Google Scholar] [CrossRef]
- Henricot, B. Box blight rampages onwards. Plantsman 2006, 5, 153–157. [Google Scholar]
- Ganci, M.L. Investigation of Host Resistance in Buxus Species to the Fungal Plant Pathogen Calonectria pseudonaviculata (=Cylindrocladium buxicola), the Causal Agent of Boxwood Blight and Determination of Overwinter Pathogen Survival. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2014. [Google Scholar]
- Gehesquière, B. Cylindrocladium buxicola (syn. Calonectria pseudonaviculata) on Buxus: Molecular Characterization, Epidemiology, Host Resistance and Fungicide Control. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2014. [Google Scholar]
- LaMondia, J.A.; Shishkoff, N. Susceptibility of boxwood accessions from the National Boxwood Collection to boxwood blight and the potential for differences between Calonectria pseudonaviculata and C. henricotiae. HortScience 2017, 52, 873–879. [Google Scholar] [CrossRef]
- Shishkoff, N.; Daughtrey, M.L.; Aker, S.; Olson, R.T. Evaluating boxwood susceptibility to Calonectria pseudonaviculata using cuttings from the national boxwood collection. Plant Health Progr. 2015, 16, 11–15. [Google Scholar] [CrossRef]
- Ganci, M.L.; Benson, D.M.; Ivors, K.L. Susceptibility of commercial boxwood cultivars to Cylindrocladium buxicola, the causal agent of box blight (ABstr.). Phytopathology 2013, 103, 47. [Google Scholar]
- Cinquerrui, A.; Polizzi, G.; Aiello, D.; Vitale, A. Integrated management for the reduction of Calonectria infections in ornamental nurseries. Plant Dis. 2017, 101, 165–169. [Google Scholar] [CrossRef] [PubMed]
- LaMondia, J.A. Management of Calonectria pseudonaviculata in boxwood with fungicides and less susceptible host species and varieties. Plant Dis. 2015, 99, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Chlorothalonil Fungicide Banned in European Union. 2019. Available online: https://vegetablegrowersnews.com/news/chlorothalonil-fungicide-banned-in-european-union/ (accessed on 22 February 2020).
- Likins, M.T.; Kong, P.; Avenot, H.F.; Marine, S.C.; Baudoin, A.; Hong, C.X. Preventing soil inoculum of Calonectria pseudonaviculata from splashing onto healthy boxwood foliage by mulching. Plant Dis. 2019, 103, 357–363. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C.X. Evaluation of biofungicides for control of boxwood blight on boxwood, 2017. Plant Dis. Manag. Rep. 2017, 11, OT023. [Google Scholar]
- Kong, P.; Hong, C.X. Biocontrol of boxwood blight by Trichoderma koningiopsis Mb2. Crop Prot. 2017, 98, 124–127. [Google Scholar] [CrossRef]
- Yang, X.; Hong, C.X. Biological control of boxwood blight by Pseudomonas protegens recovered from recycling irrigation systems. Biol. Control 2018, 124, 68–73. [Google Scholar] [CrossRef]
- Kong, P. Evaluation of novel endophyte Pseudomonas lactis strains for control of boxwood blight. J. Environ. Hortic. 2019, 37, 39–43. [Google Scholar]
- Schulz, B.; Römmert, A.-K.; Dammann, U.; Aust, H.-J.; Strack, D. The endophyte-host interaction: A balanced antagonism? Mycol. Res. 1999, 103, 1275–1283. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Nejad, P.; Johnson, P.A. Endophytic bacteria induce growth promotion and wilt disease suppression in oilseed rape and tomato. Biol. Control 2000, 18, 208–215. [Google Scholar] [CrossRef]
- Díaz Herrera, S.; Grossi, C.; Zawoznik, M.; Groppa, M.D. Wheat seeds harbour bacterial endophytes with potential as plant growth promoters and biocontrol agents of Fusarium graminearum. Microbiol. Res. 2016, 186, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Eljounaidi, K.; Lee, S.K.; Bae, H. Bacterial endophytes as potential biocontrol agents of vascular wilt diseases—Review and future prospects. Biol. Control 2016, 103, 62–68. [Google Scholar] [CrossRef]
- Omomowo, O.I.; Babalola, O.O. Bacterial and fungal endophytes: Tiny giants with immense beneficial potential for plant growth and sustainable agricultural productivity. Microorganisms 2019, 7, 481. [Google Scholar] [CrossRef] [PubMed]
- Nübel, U.; Engelen, B.; Felske, A.; Snaidr, J.; Wieshuber, A.; Amann, R.I.; Ludwig, W.; Backhaus, H. Sequence heterogeneties of gens encoding 16S rRNAs in Paenibacillus polymyxa detected by temperature gradient gel electrophoresis. J. Bacteriol. 1996, 178, 5636–5643. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Bischof, J.; Byrne, S.K.; Radomski, C.; Davies, J.E.; Av-Gay, Y.; Vandamme, P. DNA-Based Diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 2000, 38, 3165–3173. [Google Scholar] [CrossRef]
- Mahenthiralingam, E.; Simpson, D.A.; Speert, D.P. Identification and characterization of a novel DNA marker associated with epidemic Burkholderia cepacia strains recovered from patients with cystic fibrosis. J. Clin. Microbiol. 1997, 35, 808–816. [Google Scholar] [CrossRef]
- Gonzalez, C.F.; Pettit, E.A.; Valadez, V.A.; Provin, E.M. Mobilization, cloning, and sequence determination of a plasmid-encoded polygalacturonase from a phytopathogenic Burkholderia (Pseudomonas) cepacia. Mol. Plant-Microbe Interact. 1997, 10, 840–851. [Google Scholar] [CrossRef]
- Gonzalez, C.F.; Vidaver, A.K. Bacteriocin, plasmid and pectolytic diversity in Pseudommnas cepacia of clinical and plant origin. J. Gen. Microbiol. 1979, 110, 161–170. [Google Scholar] [CrossRef]
- Henry, D.A.; Campbell, M.E.; LiPuma, J.J.; Speert, D.P. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 1997, 35, 614–619. [Google Scholar] [CrossRef]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Baudoin, A.; Avenot, H.F.; Edwards, T.; Diallo, Y.; Lucernoni, C. Evaluation of fungicides for control of boxwood blight, 2014. Plant Dis. Manag. Rep. 2015, 9, OT006. [Google Scholar]
- Henricot, B.; Wedgwood, E. Evaluation of foliar fungicide sprays for the control of boxwood blight, caused by the fungus Cylindrocladium buxicola. Plant Health Progr. 2013. [Google Scholar] [CrossRef]
- Ivors, K.L.; Lacey, L.W.; Ganci, M. Evaluation of fungicides for the prevention of boxwood blight, 2012. Plant Dis. Manag. Rep. 2013, 7, OT014. [Google Scholar]
- Healy, S.E. Biology and Management of Box Blight Caused by Cylindrocladium buxicola. Master’s Thesis, The University of Guelph, Toronto, ON, Canada, 2014. [Google Scholar]
- Depoorter, E.; Bull, M.J.; Peeters, C.; Coenye, T.; Vandamme, P.; Mahenthiralingam, E. Burkholderia: An update on taxonomy and biotechnological potential as antibiotic producers. Appl. Microbiol. Biotechnol. 2016, 100, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Rosenblueth, M.; Martinez-Romero, E. Bacterial endophytes and their interactions with hosts. Mol. Plant-Microbe Interact. 2006, 19, 827–837. [Google Scholar] [CrossRef]
- Joy, A.E.; Parke, J.L. Biocontrol of Alternaria leaf blight on American ginseng by Burkholderia cepacia AMMD. In Proceedings of the Challenge of the 21st Century; Simon Fraser University: Burnaby, BC, Canada, 1994; pp. 93–100. [Google Scholar]
- Parke, J.L.; Gurian-Sherman, D. Diversity of the burkholderia cepacia complex and implications for risk assessment of biological control strains. Annu. Rev. Phytopathol. 2001, 39, 225–258. [Google Scholar] [CrossRef]
- Bunt, C.R.; Price, S.; Hampton, J.; Setelting, S. Coated solid substract microbe formulations: Pseudomonas spp. and zeolite. In Microbial-Based Biopesticides: Methods and Protocoles, Methods in Molecular Biology; Glare, T.R., Moran-Diez, M.E., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1477, pp. 49–57. [Google Scholar]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, P.; Hong, C. A Potent Burkholderia Endophyte against Boxwood Blight Caused by Calonectria pseudonaviculata. Microorganisms 2020, 8, 310. https://doi.org/10.3390/microorganisms8020310
Kong P, Hong C. A Potent Burkholderia Endophyte against Boxwood Blight Caused by Calonectria pseudonaviculata. Microorganisms. 2020; 8(2):310. https://doi.org/10.3390/microorganisms8020310
Chicago/Turabian StyleKong, Ping, and Chuanxue Hong. 2020. "A Potent Burkholderia Endophyte against Boxwood Blight Caused by Calonectria pseudonaviculata" Microorganisms 8, no. 2: 310. https://doi.org/10.3390/microorganisms8020310
APA StyleKong, P., & Hong, C. (2020). A Potent Burkholderia Endophyte against Boxwood Blight Caused by Calonectria pseudonaviculata. Microorganisms, 8(2), 310. https://doi.org/10.3390/microorganisms8020310





