Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Bacterial Strains
2.3. Effects of Compounds on Biofilm Viability in 96-Wells Microplates
2.4. Selection of The Best Biomass-Producer from the Clinical Strains
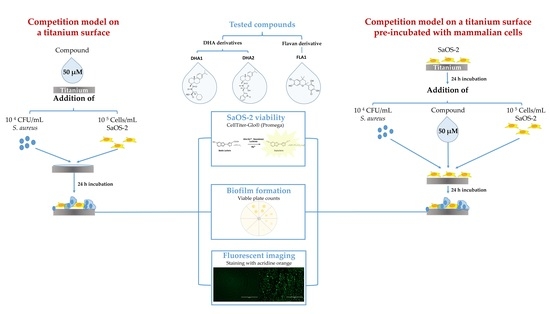
2.5. Competition Model on A Titanium Surface
2.5.1. Culture of Human Cells
2.5.2. Cytotoxicity of Compounds in 96-Well Plates
2.5.3. Culture of Staphylococci and Human Cells
2.6. Competition Model on a Titanium Surface Pre-Incubated with SaOS-2 Cells
2.7. Competition Model on a Titanium Surface Pre-Conditioned with Serum-Containing Media
2.8. Measurement of SaOS-2 Cells Viability
2.9. Bacterial Adherence and Biofilm Formation
2.10. Fluorescence Imaging
2.11. Statistical Analysis
3. Results
3.1. Effect on the Prevention and Killing of Biofilms Formed by S. aureus Clinical Strains in 96-well microplates
3.2. Effect on the prevention of S. aureus Adhesion in a Competitive Colonization Model on Titanium Coupons
3.3. Effect on the Prevention of S. aureus Adhesion in a Competitive Colonization Model on Titanium Coupons Pre-Incubated with SaOS-2 Cells
3.4. Effect on the Prevention of S. aureus Adhesion in a Competitive Colonization Model on Titanium Coupons Pre-Conditioned with Media Containing High Serum Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Alam, A.; Rani, M.; Ehtesham, N.Z.; Hasnain, S.E. Biofilms: Survival and defense strategy for pathogens. Int. J. Med Microbiol. Ijmm 2017, 307, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S.K.; Chowdhury, I.; Singh, R. Understanding the Mechanism of Bacterial Biofilms Resistance to Antimicrobial Agents. Open Microbiol. J. 2017, 11, 53–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paharik, A.E.; Horswill, A.R. The Staphylococcal Biofilm: Adhesins, Regulation, and Host Response. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95. [Google Scholar] [CrossRef] [PubMed]
- Jamsen, E.; Furnes, O.; Engesaeter, L.B.; Konttinen, Y.T.; Odgaard, A.; Stefansdottir, A.; Lidgren, L. Prevention of deep infection in joint replacement surgery A review. Acta Orthop. 2010, 81, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Gristina, A.G.; Naylor, P.T.; Myrvik, Q. The Race for the Surface: Microbes, Tissue Cells, and Biomaterials. In Molecular Mechanisms of Microbial Adhesion; Springer: New York, NY, USA, 1989; pp. 177–211. [Google Scholar]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Lidwell, O.M.; Lowbury, E.J.L.; Whyte, W.; Blowers, R.; Stanley, S.J.; Lowe, D. Airborne contamination of wounds in joint replacement operations: The relationship to sepsis rates. J. Hosp. Infect. 1983, 4, 111–131. [Google Scholar] [CrossRef]
- Subbiahdoss, G.; Kuijer, R.; Grijpma, D.W.; van der Mei, H.C.; Busscher, H.J. Microbial biofilm growth vs. tissue integration: “The race for the surface” experimentally studied. Acta Biomater. 2009, 5, 1399–1404. [Google Scholar] [CrossRef]
- Perez-Tanoira, R.; Aarnisalo, A.A.; Eklund, K.K.; Han, X.; Soininen, A.; Tiainen, V.M.; Esteban, J.; Kinnari, T.J. Prevention of Biomaterial Infection by Pre-Operative Incubation with Human Cells. Surg Infect (Larchmt) 2017, 18, 336–344. [Google Scholar] [CrossRef]
- Grice, E.A.; Segre, J.A. The skin microbiome. Nat. Rev. Microbiol. 2011, 9, 244–253. [Google Scholar] [CrossRef]
- Campoccia, D.; Baldassarri, L.; Pirini, V.; Ravaioli, S.; Montanaro, L.; Arciola, C.R. Molecular epidemiology of Staphylococcus aureus from implant orthopaedic infections: Ribotypes, agr polymorphism, leukocidal toxins and antibiotic resistance. Biomaterials 2008, 29, 4108–4116. [Google Scholar] [CrossRef]
- Jaskiewicz, M.; Janczura, A.; Nowicka, J.; Kamysz, W. Methods Used for the Eradication of Staphylococcal Biofilms. Antibiotics 2019, 8, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small molecule control of bacterial biofilms. Org. Biomol. Chem. 2012, 10, 7457–7474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huigens, R.W., 3rd. The Path to New Halogenated Quinolines With Enhanced Activities Against Staphylococcus epidermidis. Microbiol. Insights 2018, 11, 1178636118808532. [Google Scholar] [CrossRef] [PubMed]
- Kłodzińska, S.N.; Wan, F.; Jumaa, H.; Sternberg, C.; Rades, T.; Nielsen, H.M. Utilizing nanoparticles for improving anti-biofilm effects of azithromycin: A head-to-head comparison of modified hyaluronic acid nanogels and coated poly (lactic-co-glycolic acid) nanoparticles. J. Colloid Interface Sci. 2019, 555, 595–606. [Google Scholar] [CrossRef]
- Fallarero, A.; Skogman, M.; Kujala, J.; Rajaratnam, M.; Moreira, V.M.; Yli-Kauhaluoma, J.; Vuorela, P. (+)-Dehydroabietic acid, an abietane-type diterpene, inhibits Staphylococcus aureus biofilms in vitro. Int. J. Mol. Sci. 2013, 14, 12054–12072. [Google Scholar] [CrossRef] [Green Version]
- Manner, S.; Skogman, M.; Goeres, D.; Vuorela, P.; Fallarero, A. Systematic Exploration of Natural and Synthetic Flavonoids for the Inhibition of Staphylococcus aureus Biofilms. Int. J. Mol. Sci. 2013, 14, 19434–19451. [Google Scholar] [CrossRef] [Green Version]
- Manner, S.; Fallarero, A. Screening of Natural Product Derivatives Identifies Two Structurally Related Flavonoids as Potent Quorum Sensing Inhibitors against Gram-Negative Bacteria. Int. J. Mol. Sci. 2018, 19, 1346. [Google Scholar] [CrossRef] [Green Version]
- Manner, S.; Vahermo, M.; Skogman, M.E.; Krogerus, S.; Vuorela, P.M.; Yli-Kauhaluoma, J.; Fallarero, A.; Moreira, V.M. New derivatives of dehydroabietic acid target planktonic and biofilm bacteria in Staphylococcus aureus and effectively disrupt bacterial membrane integrity. Eur. J. Med. Chem. 2015, 102, 68–79. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R. D-amino acids trigger biofilm disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.; Pires, M.M. d-Amino acids do not inhibit biofilm formation in Staphylococcus aureus. PLoS ONE 2015, 10, e0117613. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boelens, J.J.; Dankert, J.; Murk, J.L.; Weening, J.J.; van der Poll, T.; Dingemans, K.P.; Koole, L.; Laman, J.D.; Zaat, S.A. Biomaterial-associated persistence of Staphylococcus epidermidis in pericatheter macrophages. J. Infect. Dis. 2000, 181, 1337–1349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczyk, P.; Junka, A.; Ziolkowski, G.; Smutnicka, D.; Bartoszewicz, M.; Chlebus, E. The ability of S.aureus to form biofilm on the Ti-6Al-7Nb scaffolds produced by Selective Laser Melting and subjected to the different types of surface modifications. Acta Bioeng. Biomech. 2013, 15, 69–76. [Google Scholar]
- Goodman, S.B.; Yao, Z.Y.; Keeney, M.; Yang, F. The future of biologic coatings for orthopaedic implants. Biomaterials 2013, 34, 3174–3183. [Google Scholar] [CrossRef] [Green Version]
- Raphel, J.; Holodniy, M.; Goodman, S.B.; Heilshorn, S.C. Multifunctional coatings to simultaneously promote osseointegration and prevent infection of orthopaedic implants. Biomaterials 2016, 84, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Chessa, D.; Ganau, G.; Spiga, L.; Bulla, A.; Mazzarello, V.; Campus, G.V.; Rubino, S. Staphylococcus aureus and Staphylococcus epidermidis Virulence Strains as Causative Agents of Persistent Infections in Breast Implants. PLoS ONE 2016, 11, e0146668. [Google Scholar] [CrossRef]
- Sabouni, F.; Mahmoudi, S.; Bahador, A.; Pourakbari, B.; Sadeghi, R.H.; Ashtiani, M.T.H.; Nikmanesh, B.; Mamishi, S. Virulence Factors of Staphylococcus aureus Isolates in an Iranian Referral Children’s Hospital. Osong Public Health Res Perspect 2014, 5, 96–100. [Google Scholar] [CrossRef] [Green Version]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.B.; Li, Y.Z.; Yu, K.; Yu, Z.; Wang, Y.; Jiang, Z.W.; Wang, H.M.; Yang, G.L. Effect of the Wnt signal-RANKL/OPG axis on the enhanced osteogenic integration of a lithium incorporated surface. Biomater. Sci. 2019, 7, 1101–1116. [Google Scholar] [CrossRef]
- Johnson, C.T.; Wroe, J.A.; Agarwal, R.; Martin, K.E.; Guldberg, R.E.; Donlan, R.M.; Westblade, L.F.; García, A.J. Hydrogel delivery of lysostaphin eliminates orthopedic implant infection by Staphylococcus aureus and supports fracture healing. Proc. Natl. Acad. Sci. USA 2018, 115, E4960–E4969. [Google Scholar] [CrossRef] [Green Version]
- Tran, P.A.; O’Brien-Simpson, N.; Palmer, J.A.; Bock, N.; Reynolds, E.C.; Webster, T.J.; Deva, A.; Morrison, W.A.; O’Connor, A.J. Selenium nanoparticles as anti-infective implant coatings for trauma orthopedics against methicillin-resistant Staphylococcus aureus and epidermidis: In vitro and in vivo assessment. Int. J. Nanomed. 2019, 14, 4613–4624. [Google Scholar] [CrossRef] [Green Version]
- Van Hengel, I.A.J.; Riool, M.; Fratila-Apachitei, L.E.; Witte-Bouma, J.; Farrell, E.; Zadpoor, A.A.; Zaat, S.A.J.; Apachitei, I. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Zhao, B.; van der Mei, H.C.; Rustema-Abbing, M.; Busscher, H.J.; Ren, Y. Osteoblast integration of dental implant materials after challenge by sub-gingival pathogens: A co-culture study in vitro. Int. J. Oral Sci. 2015, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Perez-Tanoira, R.; Han, X.; Soininen, A.; Aarnisalo, A.A.; Tiainen, V.M.; Eklund, K.K.; Esteban, J.; Kinnari, T.J. Competitive colonization of prosthetic surfaces by Staphylococcus aureus and human cells. J. Biomed. Mater. Res. Part A 2017, 105, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Esteban, J.; Gomez-Barrena, E.; Cordero, J.; Martin-de-Hijas, N.Z.; Kinnari, T.J.; Fernandez-Roblas, R. Evaluation of quantitative analysis of cultures from sonicated retrieved orthopedic implants in diagnosis of orthopedic infection. J. Clin. Microbiol. 2008, 46, 488–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J. Antibiot. 2012, 65, 453–459. [Google Scholar] [CrossRef] [Green Version]
- Kinnari, T.J.; Soininen, A.; Esteban, J.; Zamora, N.; Alakoski, E.; Kouri, V.P.; Lappalainen, R.; Konttinen, Y.T.; Gomez-Barrena, E.; Tiainen, V.M. Adhesion of staphylococcal and Caco-2 cells on diamond-like carbon polymer hybrid coating. J. Biomed. Mater. Res. Part A 2008, 86A, 760–768. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Agheli, H.; Sutherland, D.S.; Pirini, V.; Donati, M.E.; Arciola, C.R. Study of Staphylococcus aureus adhesion on a novel nanostructured surface by chemiluminometry. Int. J. Artif. Organs 2006, 29, 622–629. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Y.; Meng, L.; Chen, P.; Shi, T.; Liu, X.; Zheng, Y. Electrophoretic deposition of colloidal particles on Mg with cytocompatibility, antibacterial performance, and corrosion resistance. Acta Biomater. 2016, 45, 387–398. [Google Scholar] [CrossRef]
- Chu, L.; Yang, Y.; Yang, S.; Fan, Q.; Yu, Z.; Hu, X.L.; James, T.D.; He, X.P.; Tang, T. Preferential Colonization of Osteoblasts Over Co-cultured Bacteria on a Bifunctional Biomaterial Surface. Front. Microbiol. 2018, 9, 2219. [Google Scholar] [CrossRef]
- Foster, T.J.; Geoghegan, J.A.; Ganesh, V.K.; Hook, M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014, 12, 49–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerschmidt, S.; Rohde, M.; Preissner, K.T. Extracellular Matrix Interactions with Gram-Positive Pathogens. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Southwood, R.T.; Rice, J.L.; McDonald, P.J.; Hakendorf, P.H.; Rozenbilds, M.A. Infection in experimental hip arthroplasties. J. Bone Jt. Surg. Br. Vol. 1985, 67, 229–231. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic joint infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; van der Mei, H.C.; Kuijer, R.; Busscher, H.J.; Rochford, E.T. Mechanism of cell integration on biomaterial implant surfaces in the presence of bacterial contamination. J. Biomed. Mater. Res. Part A 2015, 103, 3590–3598. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.e.; Ao, H.; Zhou, J.; Tang, T.; Yue, B. Biofunctionalization of titanium with bacitracin immobilization shows potential for anti-bacteria, osteogenesis and reduction of macrophage inflammation. Colloids Surf. B Biointerfaces 2016, 145, 728–739. [Google Scholar] [CrossRef]
- Nie, B.; Ao, H.; Long, T.; Zhou, J.; Tang, T.; Yue, B. Immobilizing bacitracin on titanium for prophylaxis of infections and for improving osteoinductivity: An in vivo study. Colloids Surf. BBiointerfaces 2017, 150, 183–191. [Google Scholar] [CrossRef]
- Acharya, S.S.; Dimichele, D.M. Rare inherited disorders of fibrinogen. Haemophilia 2008, 14, 1151–1158. [Google Scholar] [CrossRef]
- Opperman, T.J.; Kwasny, S.M.; Williams, J.D.; Khan, A.R.; Peet, N.P.; Moir, D.T.; Bowlin, T.L. Aryl rhodanines specifically inhibit staphylococcal and enterococcal biofilm formation. Antimicrob. Agents Chemother. 2009, 53, 4357–4367. [Google Scholar] [CrossRef] [Green Version]
- Rao, S.; Hashemi Astaneh, S.; Villanueva, J.; Silva, F.; Takoudis, C.; Bijukumar, D.; Souza, J.C.M.; Mathew, M.T. Physicochemical and in-vitro biological analysis of bio-functionalized titanium samples in a protein-rich medium. J. Mech. Behav. Biomed. Mater. 2019, 96, 152–164. [Google Scholar] [CrossRef]
- Feng, B.; Weng, J.; Yang, B.C.; Chen, J.Y.; Zhao, J.Z.; He, L.; Qi, S.K.; Zhang, X.D. Surface characterization of titanium and adsorption of bovine serum albumin. Mater. Charact. 2002, 49, 129–137. [Google Scholar] [CrossRef]
- Anderson, L.; Anderson, N.G. High resolution two-dimensional electrophoresis of human plasma proteins. Proc. Natl. Acad. Sci. USA 1977, 74, 5421–5425. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reigada, I.; Pérez-Tanoira, R.; Patel, J.Z.; Savijoki, K.; Yli-Kauhaluoma, J.; Kinnari, T.J.; Fallarero, A. Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells. Microorganisms 2020, 8, 345. https://doi.org/10.3390/microorganisms8030345
Reigada I, Pérez-Tanoira R, Patel JZ, Savijoki K, Yli-Kauhaluoma J, Kinnari TJ, Fallarero A. Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells. Microorganisms. 2020; 8(3):345. https://doi.org/10.3390/microorganisms8030345
Chicago/Turabian StyleReigada, Inés, Ramón Pérez-Tanoira, Jayendra Z. Patel, Kirsi Savijoki, Jari Yli-Kauhaluoma, Teemu J. Kinnari, and Adyary Fallarero. 2020. "Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells" Microorganisms 8, no. 3: 345. https://doi.org/10.3390/microorganisms8030345
APA StyleReigada, I., Pérez-Tanoira, R., Patel, J. Z., Savijoki, K., Yli-Kauhaluoma, J., Kinnari, T. J., & Fallarero, A. (2020). Strategies to Prevent Biofilm Infections on Biomaterials: Effect of Novel Naturally-Derived Biofilm Inhibitors on a Competitive Colonization Model of Titanium by Staphylococcus aureus and SaOS-2 Cells. Microorganisms, 8(3), 345. https://doi.org/10.3390/microorganisms8030345