Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area, Sampled Sites, and E. coli Isolation
2.2. Antimicrobial Susceptibility Tests
2.3. Whole Genome Sequencing and Assembly
2.4. Whole Genome Sequence Analyses
3. Results
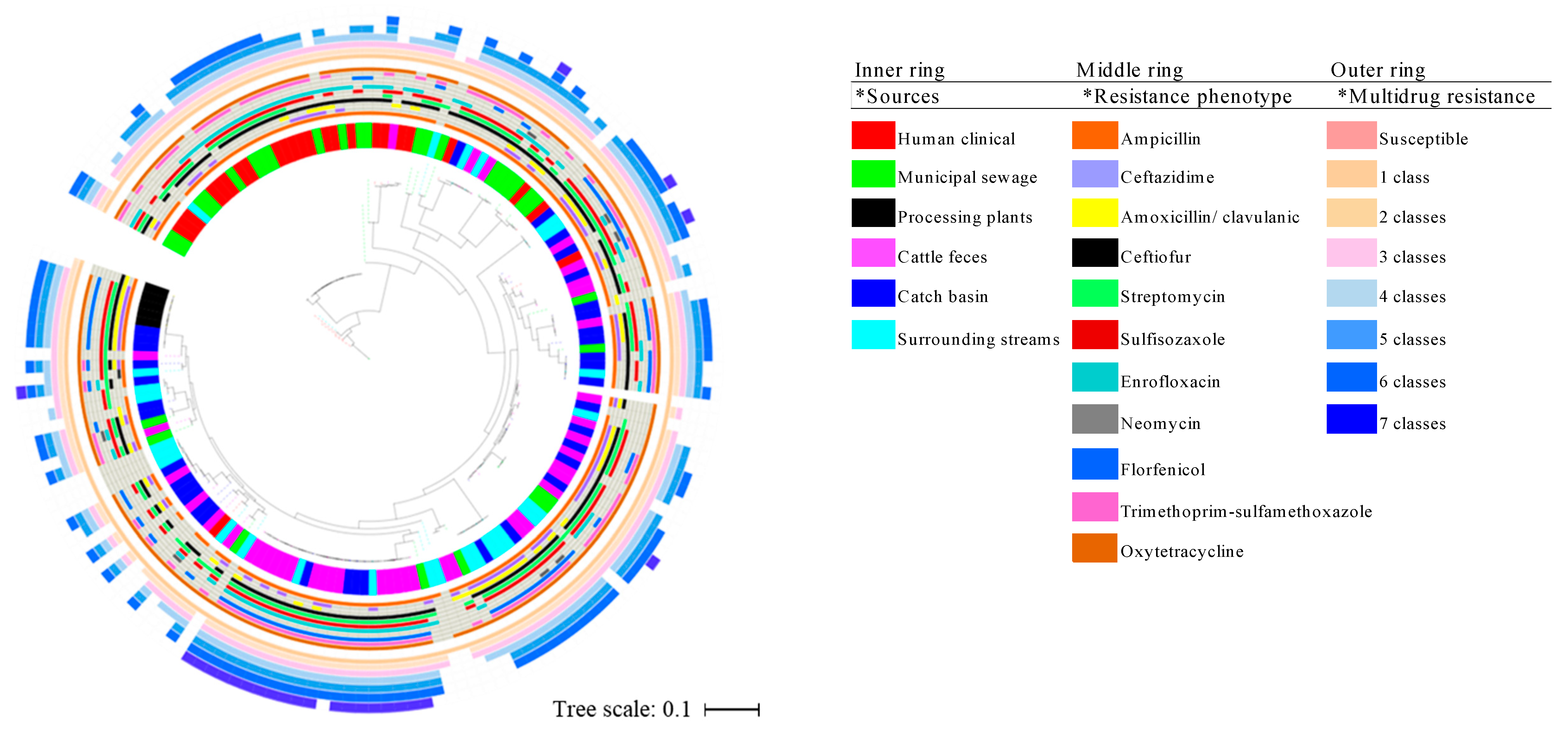
3.1. Occurrence of Phenotypic Antimicrobial Resistance
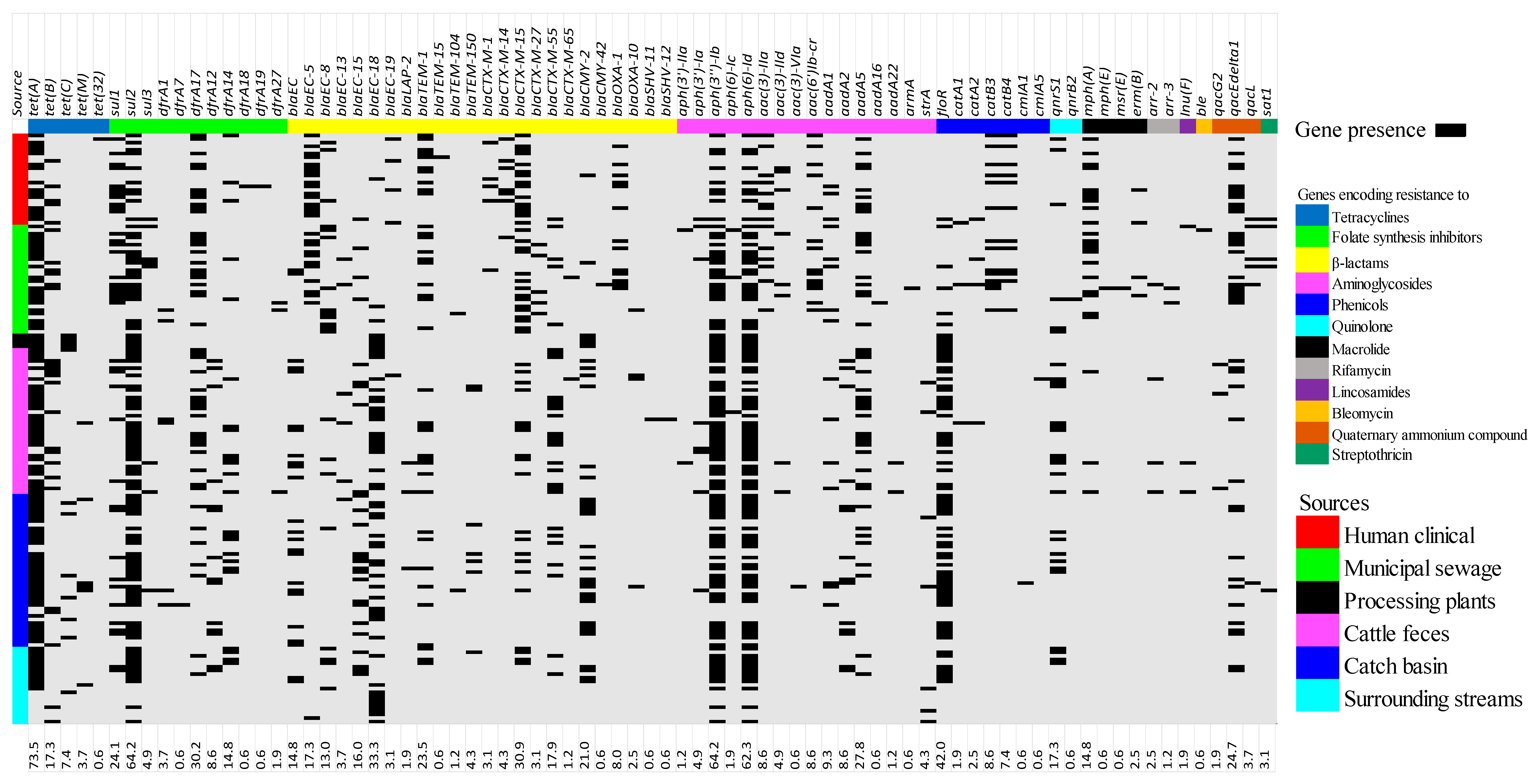
3.2. Antimicrobial Resistance Genes Prevalence
3.3. Pan-Genome Analysis and Compairson of AMR Determinants as Identified by WGS to the Occurrence of Phenotypic Reisistance
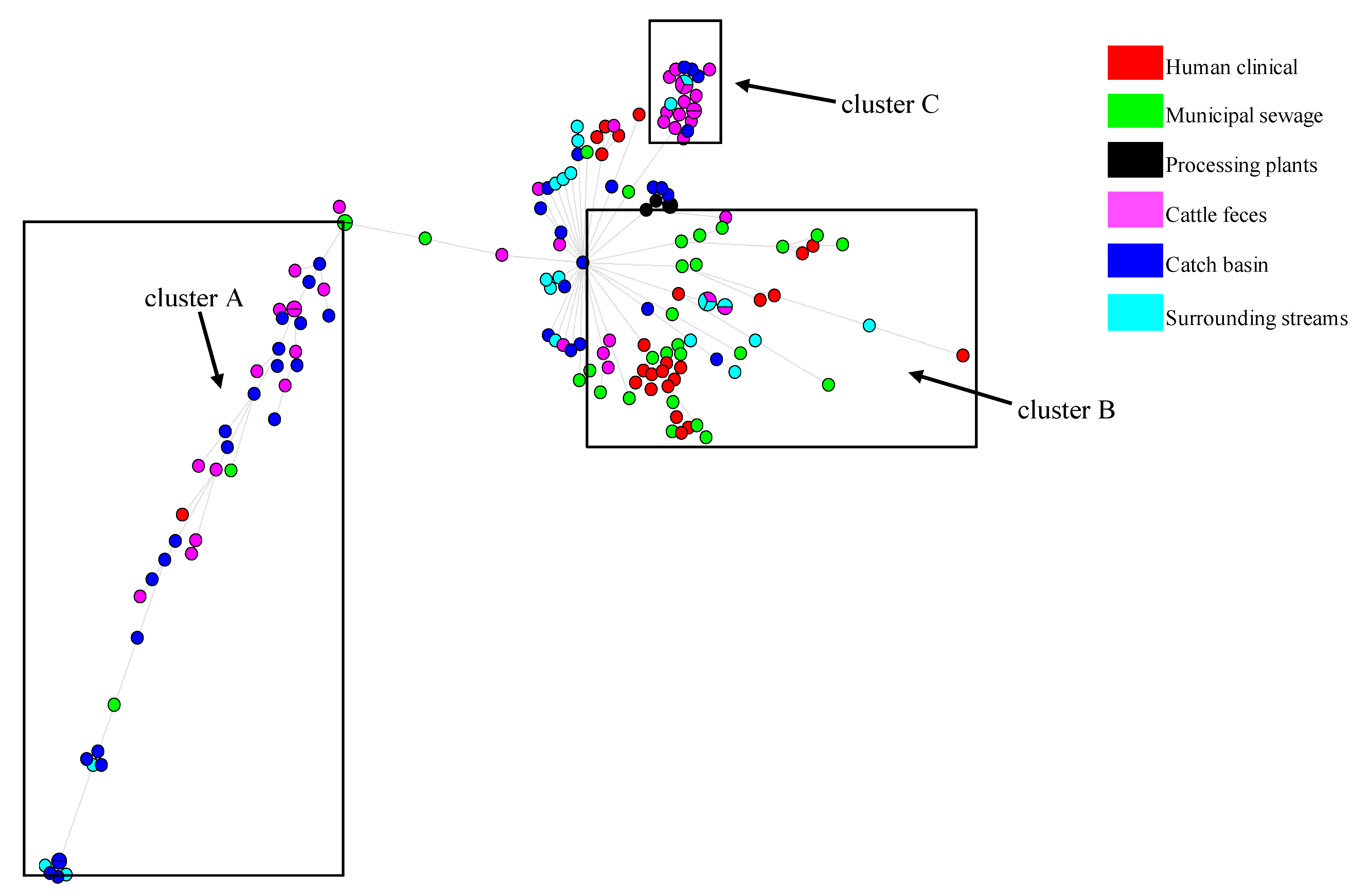
3.4. Phylogenetics, MLST Cluster, and Serogroups Analysis
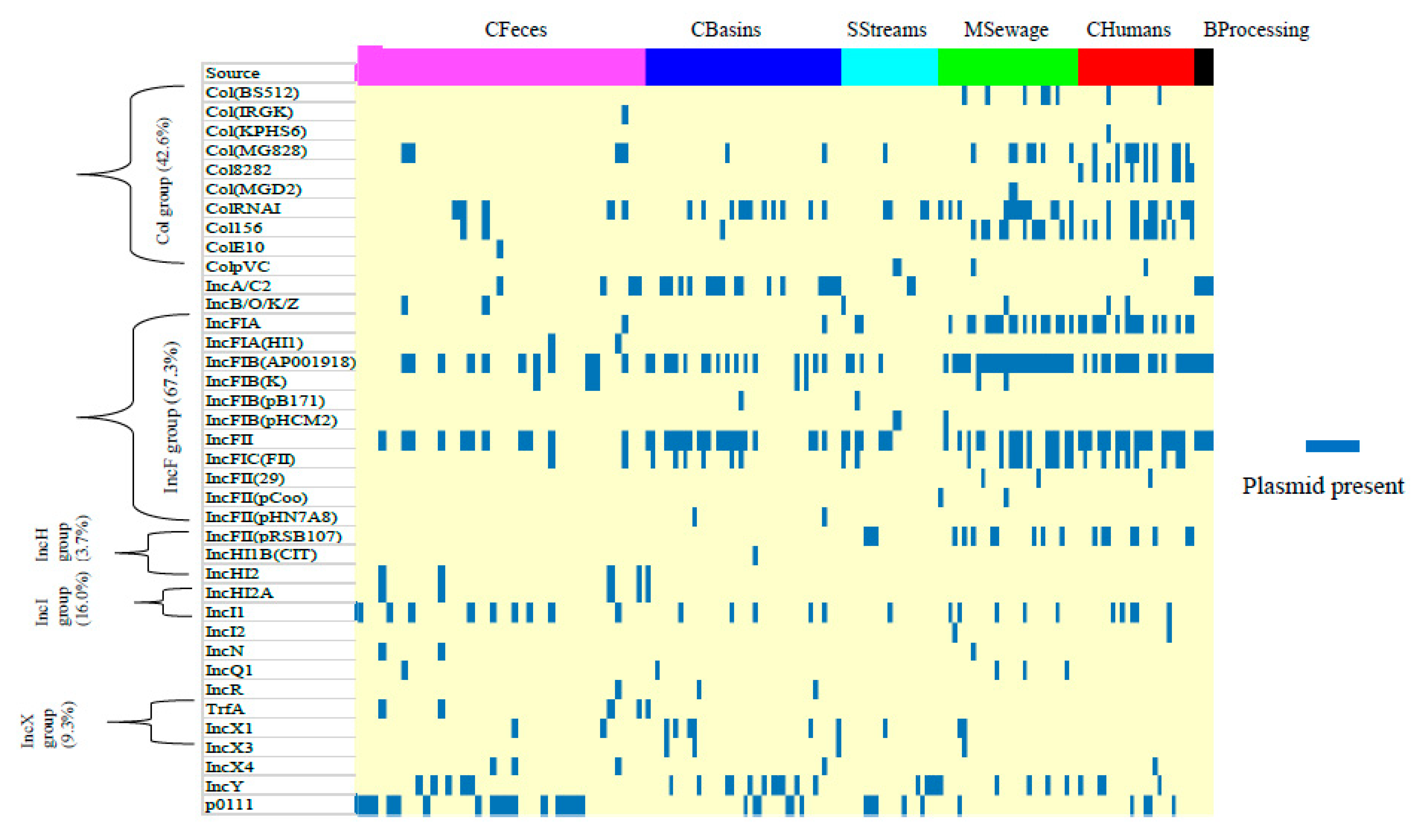
3.5. Prevalence of Plasmids and Integrative Conjugative Elements
3.6. Prevalence of Virulence Genes
4. Discussion
4.1. Antimicrobial Resistance Determinants
4.2. Genotype-Phenotype Antimicrobial Resistance Concordance
4.3. Insights from E. coli Phylogenetics, MLST and Serogroups
4.4. Mobile Elements: Plasmid and Integrative Conjugative Elements
4.5. Occurrence of Virulence Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| ARG | Antimicrobial resistance gene |
| BProcessing | Beef processing plant |
| CBasins | Catch basins |
| CFeces | Cattle feces |
| CHumans | Clinically ill humans |
| ECP | E. coli common pilus |
| ESBL | Extended spectrum beta-lactamase |
| ESBL-EC | Extended spectrum beta-lactamase E. coli |
| ExPEC | Extraintestinal Pathogenic Escherichia coli |
| HGT | Horizontal gene transfer |
| ICE | Integrative Conjugative Element |
| LEE | Locus for enterocyte effacement |
| MDR | Multidrug resistance |
| MGE | Mobile genetic element |
| MSewage | Municipal sewage treatment |
| QRDR | Quinolone resistance-determining region |
| SNPs | single nucleotide polymorphisms |
| SStreams | Surrounding streams |
| STEC | Shiga toxigenic E. coli |
| WGS | Whole genome sequence |
References
- Nicolas-Chanoine, M.H.; Bertrand, X.; Madec, J.Y. Escherichia coli ST131, an intriguing clonal group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, J.R.; Nicolas-Chanoine, M.H.; DebRoy, C.; Castanheira, M.; Robicsek, A.; Hansen, G.; Weissman, S.; Urban, C.; Platell, J.; Trott, D.; et al. Comparison of Escherichia coli ST131 pulsotypes, by epidemiologic traits, 1967–2009. Emerg. Infect. Dis. 2012, 18, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Ojer-Usoz, E.; González, D.; Vitas, A.I. Clonal diversity of ESBL-Producing Escherichia coli isolated from environmental, human and food samples. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehman, M.A.; Yin, X.; Lepp, D.; Laing, C.; Ziebell, K.; Talbot, G.; Topp, E.; Diarra, M.S. Genomic analysis of third generation cephalosporin resistant Escherichia coli from dairy cow manure. Vet. Sci. 2017, 4. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics, WHO Essential Medicines and Health Products Guidelines. 2017. Available online: https://www.who.int/medicines/publications/global-priority-list-antibiotic-resistant-bacteria/en/ (accessed on 27 February 2017).
- Hilty, M.; Betsch, B.Y.; Bögli-Stuber, K.; Heiniger, N.; Stadler, M.; Küffer, M.; Kronenberg, A.; Rohrer, C.; Aebi, S.; Endimiani, A.; et al. Transmission dynamics of extended-spectrum β-lactamase-producing Enterobacteriaceae in the tertiary care hospital and the household setting. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55, 967–975. [Google Scholar] [CrossRef]
- Ewers, C.; Bethe, A.; Stamm, I.; Grobbel, M.; Kopp, P.A.; Guerra, B.; Stubbe, M.; Doi, Y.; Zong, Z.; Kola, A.; et al. CTX-M-15-D-ST648 Escherichia coli from companion animals and horses: Another pandemic clone combining multiresistance and extraintestinal virulence? J. Antimicrob. Chemother. 2014, 69, 1224–1230. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Li, M.; Wu, L.; Song, Q.; Zhao, D.; Chen, Z.; Kang, M.; Xie, Y. Extended-spectrum β-lactamase-producing E. coli septicemia among rectal carriers in the ICU. Medicine 2018, 97, e12445. [Google Scholar] [CrossRef]
- Gudiol, C.; Royo-Cebrecos, C.; Tebe, C.; Abdala, E.; Akova, M.; Álvarez, R.; Maestro-de la Calle, G.; Cano, A.; Cervera, C.; Clemente, W.T.; et al. Clinical efficacy of β-lactam/β-lactamase inhibitor combinations for the treatment of bloodstream infection due to extended-spectrum β-lactamase-producing Enterobacteriaceae in haematological patients with neutropaenia: A study protocol for a retrospective observational study (BICAR). BMJ Open 2017, 7, e013268. [Google Scholar] [CrossRef] [Green Version]
- Malande, O.O.; Nuttall, J.; Pillay, V.; Bamford, C.; Eley, B. A ten-year review of ESBL and non-ESBL Escherichia coli bloodstream infections among children at a tertiary referral hospital in South Africa. PLoS ONE 2019, 14, e0222675. [Google Scholar] [CrossRef]
- Repessé, X.; Artiguenave, M.; Paktoris-Papine, S.; Espinasse, F.; Dinh, A.; Charron, C.; El Sayed, F.; Geri, G.; Vieillard-Baron, A. Epidemiology of extended-spectrum beta-lactamase-producing Enterobacteriaceae in an intensive care unit with no single rooms. Ann. Intensive Care 2017, 7, 73. [Google Scholar] [CrossRef]
- Roche, D.; Fléchard, M.; Lallier, N.; Répérant, M.; Brée, A.; Pascal, G.; Schouler, C.; Germon, P. ICEEc2, a new integrative and conjugative element belonging to the pKLC102/PAGI-2 family, identified in Escherichia coli strain BEN374. J. Bacteriol. 2010, 192, 5026–5036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Partridge, S.R.; Kwong, S.M.; Firth, N.; Jensen, S.O. Mobile genetic elements associated with antimicrobial resistance. Clin. Microbiol. Rev. 2018, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carattoli, A. Resistance plasmid families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Li, X.; Xie, Y.; Bi, D.; Sun, J.; Li, J.; Tai, C.; Deng, Z.; Ou, H.Y. ICEberg 2.0: An updated database of bacterial integrative and conjugative elements. Nucleic Acids Res. 2019, 47, D660–D665. [Google Scholar] [CrossRef]
- Bi, D.; Xu, Z.; Harrison, E.M.; Tai, C.; Wei, Y.; He, X.; Jia, S.; Deng, Z.; Rajakumar, K.; Ou, H.Y. ICEberg: A web-based resource for integrative and conjugative elements found in Bacteria. Nucleic Acids Res. 2012, 40, D621–D626. [Google Scholar] [CrossRef]
- Schmidt, J.W.; Griffin, D.; Kuehn, L.A.; Brichta-Harhay, D.M. Influence of therapeutic ceftiofur treatments of feedlot cattle on fecal and hide prevalences of commensal Escherichia coli resistant to expanded-spectrum cephalosporins, and molecular characterization of resistant isolates. Appl. Environ. Microbiol. 2013, 79, 2273–2283. [Google Scholar] [CrossRef] [Green Version]
- Aslam, M.; Diarra, M.S.; Service, C.; Rempel, H. Antimicrobial resistance genes in Escherichia coli isolates recovered from a commercial beef processing plant. J. Food Prot. 2009, 72, 1089–1093. [Google Scholar] [CrossRef]
- Kojima, A.; Ishii, Y.; Ishihara, K.; Esaki, H.; Asai, T.; Oda, C.; Tamura, Y.; Takahashi, T.; Yamaguchi, K. Extended-spectrum-β-lactamase-producing Escherichia coli strains isolated from farm animals from 1999 to 2002: Report from the Japanese Veterinary Antimicrobial Resistance Monitoring Program. Antimicrob. Agents Chemother. 2005, 49, 3533–3537. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, J.W.; Agga, G.E.; Bosilevac, J.M.; Brichta-Harhay, D.M.; Shackelford, S.D.; Wang, R.; Wheeler, T.L.; Arthur, T.M. Occurrence of antimicrobial-resistant Escherichia coli and Salmonella enterica in the beef cattle production and processing continuum. Appl. Environ. Microbiol. 2015, 81, 713–725. [Google Scholar] [CrossRef] [Green Version]
- Zaheer, R.; Cook, S.R.; Klima, C.L.; Stanford, K.; Alexander, T.; Topp, E.; Read, R.; McAllister, T.A. Effect of Subtherapeutic vs Therapeutic Administration of Macrolides on Antimicrobial Resistance in Mannheimia haemolytica and Enterococci isolated from Beef Cattle. Front. Microbiol. 2013, 4, 133. [Google Scholar] [CrossRef] [Green Version]
- van Belkum, A.; Dunne, W.M. Next-generation antimicrobial susceptibility testing. J. Clin. Microbiol. 2013, 51, 2018–2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quainoo, S.; Coolen, J.P.M.; van Hijum, S.A.F.T.; Huynen, M.A.; Melchers, W.J.G.; van Schaik, W.; Wertheim, H.F.L. Whole-Genome Sequencing of bacterial pathogens: The future of nosocomial outbreak analysis. Clin. Microbiol. Rev. 2017, 30, 1015–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDermott, P.F.; Tyson, G.H.; Kabera, C.; Chen, Y.; Li, C.; Folster, J.P.; Ayers, S.L.; Lam, C.; Tate, H.P.; Zhao, S. Whole-genome sequencing for detecting antimicrobial resistance in Nontyphoidal Salmonella. Antimicrob. Agents Chemother. 2016, 60, 5515–5520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ludden, C.; Raven, K.E.; Jamrozy, D.; Gouliouris, T.; Blane, B.; Coll, F.; de Goffau, M.; Naydenova, P.; Horner, C.; Hernandez-Garcia, J.; et al. One Health genomic surveillance of Escherichia coli demonstrates distinct lineages and mobile genetic elements in isolates from humans versus livestock. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence factors, prevalence and potential transmission of extraintestinal pathogenic Escherichia coli isolated from different sources: Recent reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusconi, B.; Sanjar, F.; Koenig, S.S.; Mammel, M.K.; Tarr, P.I.; Eppinger, M. Whole genome sequencing for genomics-guided investigations of Escherichia coli O157:H7 Outbreaks. Front. Microbiol. 2016, 7, 985. [Google Scholar] [CrossRef] [Green Version]
- Vincent, C.; Usongo, V.; Berry, C.; Tremblay, D.M.; Moineau, S.; Yousfi, K.; Doualla-Bell, F.; Fournier, E.; Nadon, C.; Goodridge, L.; et al. Comparison of advanced whole genome sequence-based methods to distinguish strains of Salmonella enterica serovar Heidelberg involved in foodborne outbreaks in Québec. Food Microbiol. 2018, 73, 99–110. [Google Scholar] [CrossRef]
- WHO. Integrated Surveillance of Antimicrobial Resistance in Foodborne Bacteria: Application of a One Health Approach: Guidance from the WHO Advisory Group on Integrated Surveillanec of Antimicrobial Resistance (AGISAR). 2017. Available online: https://www.who.int/foodsafety/publications/agisar_guidance2017/en/.
- Europrean Commission. A European One Health Action Plan Against Antimicrobial Resistance (AMR). 2017. Available online: https://ec.europa.eu/health/amr/antimicrobial-resistance_en/.
- Rousham, E.K.; Unicomb, L.; Islam, M.A. Human, animal and environmental contributors to antibiotic resistance in low-resource settings: Integrating behavioural, epidemiological and One Health approaches. Proc. Biol. Sci. 2018, 285. [Google Scholar] [CrossRef]
- Beukers, A.G.; Zaheer, R.; Cook, S.R.; Chaves, A.V.; Ward, M.P.; Tymensen, L.; Morley, P.S.; Hannon, S.; Booker, C.W.; Read, R.R.; et al. Comparison of antimicrobial resistance genes in feedlots and urban wastewater. Can. J. Vet. Res. 2018, 82, 24–38. [Google Scholar]
- Tymensen, L.; Booker, C.W.; Hannon, S.J.; Cook, S.R.; Jokinen, C.C.; Zaheer, R.; Read, R.; Boerlin, P.; McAllister, T.A. Plasmid distribution among Escherichia coli from livestock and associated wastewater: Unraveling factors that shape the presence of genes conferring Third-Generation Cephalosporin resistance. Environ. Sci. Technol. 2019, 53, 11666–11674. [Google Scholar] [CrossRef]
- Zaheer, R.; Cook, S.R.; Barbieri, R.; Goji, N.; Cameron, A.; Petkau, A.; Polo, R.O.; Tymensen, L.; Stamm, C.; Song, J.; et al. Surveillance of Enterococcus spp. reveals distinct species and antimicrobial resistance diversity across a One-Health continuum. Sci. Rep. 2020, 10, 3937. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O.; Jones, T. Microbiological sampling of carcasses by excision or swabbing. J. Food Prot. 2000, 63, 167–173. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Clinical and Laboratory Standards Istitute. Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fifth Informational Supplement; CLSI document M100S-26; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016; Volume 26. [Google Scholar]
- Desjardins, P.; Conklin, D. NanoDrop microvolume quantitation of nucleic acids. J. Vis. Exp. 2010, 2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, S.R. Quantitation of DNA and RNA with absorption and fluorescence Spectroscopy. Curr. Protoc. Immunol. 2017, 116, A.3L.1–A.3L.14. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Page, A.J.; Cummins, C.A.; Hunt, M.; Wong, V.K.; Reuter, S.; Holden, M.T.; Fookes, M.; Falush, D.; Keane, J.A.; Parkhill, J. Roary: Rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015, 31, 3691–3693. [Google Scholar] [CrossRef]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef]
- Babiker, A.; Mustapha, M.M.; Pacey, M.P.; Shutt, K.A.; Ezeonwuka, C.D.; Ohm, S.L.; Cooper, V.S.; Marsh, J.W.; Doi, Y.; Harrison, L.H. Use of online tools for antimicrobial resistance prediction by whole-genome sequencing in methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococci (VRE). J. Glob. Antimicrob. Resist. 2019, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- SAS-Institute. User’s Guide Version 5.0 SAS Institute Cary; SAS/STAT: Cary, NC, USA, 2015. [Google Scholar]
- Petkau, A.; Mabon, P.; Sieffert, C.; Knox, N.C.; Cabral, J.; Iskander, M.; Weedmark, K.; Zaheer, R.; Katz, L.S.; Nadon, C.; et al. SNVPhyl: A single nucleotide variant phylogenomics pipeline for microbial genomic epidemiology. Microb. Genom. 2017, 3, e000116. [Google Scholar] [CrossRef] [PubMed]
- Blattner, F.R.; Plunkett, G.; Bloch, C.A.; Perna, N.T.; Burland, V.; Riley, M.; Collado-Vides, J.; Glasner, J.D.; Rode, C.K.; Mayhew, G.F.; et al. The complete genome sequence of Escherichia coli K-12. Science 1997, 277, 1453–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016, 44, W242–W245. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-access bacterial population genomics: BIGSdb software, the PubMLST.org website and their applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [Green Version]
- Browne, A.S.; Biggs, P.J.; Wilkinson, D.A.; Cookson, A.L.; Midwinter, A.C.; Bloomfield, S.J.; Hranac, C.R.; Rogers, L.E.; Marshall, J.C.; Benschop, J.; et al. Use of genomics to investigate historical importation of Shiga toxin-producing Escherichia coli serogroup O26 and nontoxigenic variants into New Zealand. Emerg. Infect. Dis. 2019, 25, 489–500. [Google Scholar] [CrossRef] [Green Version]
- Ingle, D.J.; Valcanis, M.; Kuzevski, A.; Tauschek, M.; Inouye, M.; Stinear, T.; Levine, M.M.; Robins-Browne, R.M.; Holt, K.E. In silico serotyping of E. coli from short read data identifies limited novel O-loci but extensive diversity of O: H serotype combinations within and between pathogenic lineages. Microb. Genom. 2016, 2, e000064. [Google Scholar] [CrossRef]
- Petkau, A.; Stuart-Edwards, M.; Stothard, P.; Van Domselaar, G. Interactive microbial genome visualization with GView. Bioinformatics 2010, 26, 3125–3126. [Google Scholar] [CrossRef]
- Mbelle, N.M.; Feldman, C.; Osei Sekyere, J.; Maningi, N.E.; Modipane, L.; Essack, S.Y. The resistome, mobilome, virulome and phylogenomics of multidrug-resistant Escherichia coli clinical isolates from Pretoria, South Africa. Sci. Rep. 2019, 9, 16457. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of quinolone resistance in Escherichia coli and Salmonella: Recent developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef]
- Cameron, A.; McAllister, T.A. Antimicrobial usage and resistance in beef production. J. Anim. Sci. Biotechnol. 2016, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Bryan, A.; Shapir, N.; Sadowsky, M.J. Frequency and distribution of tetracycline resistance genes in genetically diverse, nonselected, and nonclinical Escherichia coli strains isolated from diverse human and animal sources. Appl. Environ. Microbiol. 2004, 70, 2503–2507. [Google Scholar] [CrossRef] [Green Version]
- Wilkerson, C.; Samadpour, M.; van Kirk, N.; Roberts, M.C. Antibiotic resistance and distribution of tetracycline resistance genes in Escherichia coli O157:H7 isolates from humans and bovines. Antimicrob. Agents Chemother. 2004, 48, 1066–1067. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Sun, H.; Bai, X.; Fu, S.; Fan, R.; Xiong, Y. Occurrence of multidrug-resistant and ESBL-producing atypical enteropathogenic Escherichia coli in China. Gut Pathog. 2018, 10, 8. [Google Scholar] [CrossRef] [Green Version]
- Noyes, N.R.; Yang, X.; Linke, L.M.; Magnuson, R.J.; Cook, S.R.; Zaheer, R.; Yang, H.; Woerner, D.R.; Geornaras, I.; McArt, J.A.; et al. Characterization of the resistome in manure, soil and wastewater from dairy and beef production systems. Sci. Rep. 2016, 6, 24645. [Google Scholar] [CrossRef]
- Poyart, C.; Celli, J.; Trieu-Cuot, P. Conjugative transposition of Tn916-related elements from Enterococcus faecalis to Escherichia coli and Pseudomonas fluorescens. Antimicrob. Agents Chemother. 1995, 39, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Gozi, K.S.; Froes, J.R.; Deus Ajude, L.P.T.; da Silva, C.R.; Baptista, R.S.; Peiró, J.R.; Marinho, M.; Mendes, L.C.N.; Nogueira, M.C.L.; Casella, T. Dissemination of multidrug-resistant commensal. Front. Microbiol. 2019, 10, 1394. [Google Scholar] [CrossRef]
- Seenama, C.; Thamlikitkul, V.; Ratthawongjirakul, P. Multilocus sequence typing and blaESBL characterization of extended-spectrum beta-lactamase-producing Escherichia coli isolated from healthy humans and swine in Northern Thailand. Infect. Drug Resist. 2019, 12, 2201–2214. [Google Scholar] [CrossRef] [Green Version]
- Frye, J.G.; Jackson, C.R. Genetic mechanisms of antimicrobial resistance identified in Salmonella enterica, Escherichia coli, and Enteroccocus spp. isolated from U.S. food animals. Front. Microbiol. 2013, 4, 135. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.D. Antibiotic resistance in the environment: A link to the clinic? Curr. Opin. Microbiol. 2010, 13, 589–594. [Google Scholar] [CrossRef]
- Reinthaler, F.F.; Galler, H.; Feierl, G.; Haas, D.; Leitner, E.; Mascher, F.; Melkes, A.; Posch, J.; Pertschy, B.; Winter, I.; et al. Resistance patterns of Escherichia coli isolated from sewage sludge in comparison with those isolated from human patients in 2000 and 2009. J. Water Health 2013, 11, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Pärnänen, K.M.M.; Narciso-da-Rocha, C.; Kneis, D.; Berendonk, T.U.; Cacace, D.; Do, T.T.; Elpers, C.; Fatta-Kassinos, D.; Henriques, I.; Jaeger, T.; et al. Antibiotic resistance in European wastewater treatment plants mirrors the pattern of clinical antibiotic resistance prevalence. Sci. Adv. 2019, 5, eaau9124. [Google Scholar] [CrossRef] [Green Version]
- Dortet, L.; Nordmann, P.; Poirel, L. Association of the emerging carbapenemase NDM-1 with a bleomycin resistance protein in Enterobacteriaceae and Acinetobacter baumannii. Antimicrob. Agents Chemother. 2012, 56, 1693–1697. [Google Scholar] [CrossRef] [Green Version]
- Slipski, C.J.; Zhanel, G.G.; Bay, D.C. Biocide selective TolC-independent efflux pumps in Enterobacteriaceae. J. Membr. Biol. 2018, 251, 15–33. [Google Scholar] [CrossRef]
- Gaze, W.H.; Abdouslam, N.; Hawkey, P.M.; Wellington, E.M. Incidence of class 1 integrons in a quaternary ammonium compound-polluted environment. Antimicrob. Agents Chemother. 2005, 49, 1802–1807. [Google Scholar] [CrossRef] [Green Version]
- Jaglic, Z.; Cervinkova, D. Genetic basis of resistance to quaternary ammonium compounds--the qac genes and their role: A review. Vet. Med. 2012, 57. [Google Scholar] [CrossRef] [Green Version]
- Health Quality Ontario. Portable ultraviolet light surface-disinfecting devices for prevention of hospital-acquired infections: A health technology assessment. Ont. Health Technol. Assess. Ser. 2018, 18, 1–73. [Google Scholar]
- Han, J.H.; Sullivan, N.; Leas, B.F.; Pegues, D.A.; Kaczmarek, J.L.; Umscheid, C.A. Cleaning hospital room surfaces to prevent health care–associated infections: A technical brief. Ann. Intern. Med. 2015, 163, 598–607. [Google Scholar] [CrossRef] [Green Version]
- Cormier, A.; Zhang, P.L.C.; Chalmers, G.; Weese, J.S.; Deckert, A.; Mulvey, M.; McAllister, T.; Boerlin, P. Diversity of CTX-M-positive Escherichia coli recovered from animals in Canada. Vet. Microbiol. 2019, 231, 71–75. [Google Scholar] [CrossRef]
- Pitout, J.D.; DeVinney, R. ST131: A multidrug-resistant clone primed for global domination. F1000Res 2017, 6. [Google Scholar] [CrossRef] [Green Version]
- Grönthal, T.; Österblad, M.; Eklund, M.; Jalava, J.; Nykäsenoja, S.; Pekkanen, K.; Rantala, M. Sharing more than friendship - transmission of NDM-5 ST167 and CTX-M-9 ST69 Escherichia coli between dogs and humans in a family, Finland, 2015. Euro Surveill. 2018, 23. [Google Scholar] [CrossRef]
- Dahms, C.; Hübner, N.O.; Kossow, A.; Mellmann, A.; Dittmann, K.; Kramer, A. Occurrence of ESBL-producing Escherichia coli in livestock and farm workers in Mecklenburg-Western Pomerania, Germany. PLoS ONE 2015, 10, e0143326. [Google Scholar] [CrossRef]
- Pouget, J.G.; Coutinho, F.J.; Reid-Smith, R.J.; Boerlin, P. Characterization of bla(SHV) genes on plasmids from Escherichia coli and Salmonella enterica isolates from Canadian food animals (2006–2007). Appl. Environ. Microbiol. 2013, 79, 3864–3866. [Google Scholar] [CrossRef] [Green Version]
- Carattoli, A.; Lovari, S.; Franco, A.; Cordaro, G.; Di Matteo, P.; Battisti, A. Extended-spectrum beta-lactamases in Escherichia coli isolated from dogs and cats in Rome, Italy, from 2001 to 2003. Antimicrob. Agents Chemother. 2005, 49, 833–835. [Google Scholar] [CrossRef] [Green Version]
- Teshager, T.; Domínguez, L.; Moreno, M.A.; Saénz, Y.; Torres, C.; Cardeñosa, S. Isolation of an SHV-12 beta-lactamase-producing Escherichia coli strain from a dog with recurrent urinary tract infections. Antimicrob. Agents Chemother. 2000, 44, 3483–3484. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Mi, Z.; Wang, C. A novel beta-lactamase gene, LAP-2, produced by an Enterobacter cloacae clinical isolate in China. J. Hosp. Infect. 2008, 70, 95–96. [Google Scholar] [CrossRef]
- Le, V.; Nhu, N.T.; Cerdeno-Tarraga, A.; Campbell, J.I.; Tuyen, H.T.; Nhu, T.o.H.; Tam, P.T.; Schultsz, C.; Thwaites, G.; Thomson, N.R.; et al. Genetic characterization of three qnrS1-harbouring multidrug-resistance plasmids and qnrS1-containing transposons circulating in Ho Chi Minh City, Vietnam. J. Med. Microbiol. 2015, 64, 869–878. [Google Scholar] [CrossRef]
- Park, Y.J.; Yu, J.K.; Kim, S.I.; Lee, K.; Arakawa, Y. Accumulation of plasmid-mediated fluoroquinolone resistance genes, qepA and qnrS1, in Enterobacter aerogenes co-producing RmtB and class A beta-lactamase LAP-1. Ann. Clin. Lab. Sci. 2009, 39, 55–59. [Google Scholar]
- Varughese, L.R.; Rajpoot, M.; Goyal, S.; Mehra, R.; Chhokar, V.; Beniwal, V. Analytical profiling of mutations in quinolone resistance determining region of gyrA gene among UPEC. PLoS ONE 2018, 13, e0190729. [Google Scholar] [CrossRef] [Green Version]
- Stoesser, N.; Batty, E.M.; Eyre, D.W.; Morgan, M.; Wyllie, D.H.; Del Ojo Elias, C.; Johnson, J.R.; Walker, A.S.; Peto, T.E.; Crook, D.W. Predicting antimicrobial susceptibilities for Escherichia coli and Klebsiella pneumoniae isolates using whole genomic sequence data. J. Antimicrob. Chemother. 2013, 68, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Tyson, G.H.; McDermott, P.F.; Li, C.; Chen, Y.; Tadesse, D.A.; Mukherjee, S.; Bodeis-Jones, S.; Kabera, C.; Gaines, S.A.; Loneragan, G.H.; et al. WGS accurately predicts antimicrobial resistance in Escherichia coli. J. Antimicrob. Chemother. 2015, 70, 2763–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, A.C.; Chait, R.; Kishony, R. Nonoptimal gene expression creates latent potential for antibiotic resistance. Mol. Biol. Evol. 2018, 35, 2669–2684. [Google Scholar] [CrossRef] [Green Version]
- Gouliouris, T.; Raven, K.E.; Ludden, C.; Blane, B.; Corander, J.; Horner, C.S.; Hernandez-Garcia, J.; Wood, P.; Hadjirin, N.F.; Radakovic, M.; et al. Genomic Surveillance of Enterococcus faecium Reveals Limited Sharing of Strains and Resistance Genes between Livestock and Humans in the United Kingdom. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Manges, A.R. Escherichia coli causing bloodstream and other extraintestinal infections: Tracking the next pandemic. Lancet Infect. Dis. 2019, 19, 1269–1270. [Google Scholar] [CrossRef] [Green Version]
- Salinas, L.; Cárdenas, P.; Johnson, T.J.; Vasco, K.; Graham, J.; Trueba, G. Diverse commensal Escherichia coli clones and plasmids disseminate antimicrobial resistance genes in domestic animals and children in a Semirural Community in Ecuador. mSphere 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Mulvey, M.R.; Susky, E.; McCracken, M.; Morck, D.W.; Read, R.R. Similar cefoxitin-resistance plasmids circulating in Escherichia coli from human and animal sources. Vet. Microbiol. 2009, 134, 279–287. [Google Scholar] [CrossRef]
- Peirano, G.; Richardson, D.; Nigrin, J.; McGeer, A.; Loo, V.; Toye, B.; Alfa, M.; Pienaar, C.; Kibsey, P.; Pitout, J.D. High prevalence of ST131 isolates producing CTX-M-15 and CTX-M-14 among extended-spectrum-beta-lactamase-producing Escherichia coli isolates from Canada. Antimicrob. Agents Chemother. 2010, 54, 1327–1330. [Google Scholar] [CrossRef] [Green Version]
- Pietsch, M.; Irrgang, A.; Roschanski, N.; Brenner Michael, G.; Hamprecht, A.; Rieber, H.; Käsbohrer, A.; Schwarz, S.; Rösler, U.; Kreienbrock, L.; et al. Whole genome analyses of CMY-2-producing Escherichia coli isolates from humans, animals and food in Germany. BMC Genom. 2018, 19, 601. [Google Scholar] [CrossRef]
- Mathers, A.J.; Peirano, G.; Pitout, J.D. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin. Microbiol. Rev. 2015, 28, 565–591. [Google Scholar] [CrossRef] [Green Version]
- Adator, E.H.; Cheng, M.; Holley, R.; McAllister, T.; Narvaez-Bravo, C. Ability of Shiga toxigenic Escherichia coli to survive within dry-surface biofilms and transfer to fresh lettuce. Int. J. Food Microbiol. 2018, 269, 52–59. [Google Scholar] [CrossRef]
- Wang, J.; Stanford, K.; McAllister, T.A.; Johnson, R.P.; Chen, J.; Hou, H.; Zhang, G.; Niu, Y.D. Biofilm formation, virulence gene profiles, and antimicrobial resistance of nine serogroups of non-O157 shiga toxin-producing Escherichia coli. Foodborne Pathog. Dis. 2016, 13, 316–324. [Google Scholar] [CrossRef]
- Cernicchiaro, N.; Cull, C.A.; Paddock, Z.D.; Shi, X.; Bai, J.; Nagaraja, T.G.; Renter, D.G. Prevalence of Shiga toxin-producing Escherichia coli and associated virulence genes in feces of commercial feedlot cattle. Foodborne Pathog. Dis. 2013, 10, 835–841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanford, K.; Johnson, R.P.; Alexander, T.W.; McAllister, T.A.; Reuter, T. Influence of season and feedlot location on prevalence and virulence factors of seven serogroups of Escherichia coli in feces of western-Canadian slaughter cattle. PLoS ONE 2016, 11, e0159866. [Google Scholar] [CrossRef]
- Aslantaş, Ö.; Yilmaz, E. Prevalence and molecular characterization of extended-spectrum β-lactamase (ESBL) and plasmidic AmpC β-lactamase (pAmpC) producing Escherichia coli in dogs. J. Vet. Med. Sci. 2017, 79, 1024–1030. [Google Scholar] [CrossRef] [Green Version]
- Sharp, H.; Valentin, L.; Fischer, J.; Guerra, B.; Appel, B.; Käsbohrer, A. Estimation of the transfer of ESBL-producing Escherichia coli to humans in Germany. Berl. Munch. Tierarztl. Wochenschr. 2014, 127, 464–477. [Google Scholar]
- Johnson, T.J.; Wannemuehler, Y.M.; Johnson, S.J.; Logue, C.M.; White, D.G.; Doetkott, C.; Nolan, L.K. Plasmid replicon typing of commensal and pathogenic Escherichia coli isolates. Appl. Environ. Microbiol. 2007, 73, 1976–1983. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Fakhr, M.K.; Nolan, L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 2005, 151, 2097–2110. [Google Scholar] [CrossRef] [Green Version]
- Obi, C.C.; Vayla, S.; de Gannes, V.; Berres, M.E.; Walker, J.; Pavelec, D.; Hyman, J.; Hickey, W.J. The Integrative Conjugative Element clc (ICEclc) of Pseudomonas aeruginosa JB2. Front. Microbiol. 2018, 9, 1532. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.M.; Grossman, A.D. Integrative and Conjugative Elements (ICEs): What They Do and How They Work. Annu. Rev. Genet. 2015, 49, 577–601. [Google Scholar] [CrossRef] [Green Version]
- Wozniak, R.A.; Fouts, D.E.; Spagnoletti, M.; Colombo, M.M.; Ceccarelli, D.; Garriss, G.; Déry, C.; Burrus, V.; Waldor, M.K. Comparative ICE genomics: Insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet. 2009, 5, e1000786. [Google Scholar] [CrossRef] [Green Version]
- Garnett, J.A.; Martínez-Santos, V.I.; Saldaña, Z.; Pape, T.; Hawthorne, W.; Chan, J.; Simpson, P.J.; Cota, E.; Puente, J.L.; Girón, J.A.; et al. Structural insights into the biogenesis and biofilm formation by the Escherichia coli common pilus. Proc. Natl. Acad. Sci. USA 2012, 109, 3950–3955. [Google Scholar] [CrossRef] [Green Version]
- Cameron, E.A.; Curtis, M.M.; Kumar, A.; Dunny, G.M.; Sperandio, V. Microbiota and pathogen proteases modulate type III secretion activity in enterohemorrhagic Escherichia coli. mBio 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Bihannic, M.; Ghanbarpour, R.; Auvray, F.; Cavalié, L.; Châtre, P.; Boury, M.; Brugère, H.; Madec, J.Y.; Oswald, E. Identification and detection of three new F17 fimbrial variants in Escherichia coli strains isolated from cattle. Vet. Res. 2014, 45, 76. [Google Scholar] [CrossRef] [Green Version]
- Puhar, A.; Sansonetti, P.J. Type III secretion system. Curr. Biol. 2014, 24, R784–R791. [Google Scholar] [CrossRef] [Green Version]


| Phenotype: Resistant | Phenotype: Susceptible | |||||||
|---|---|---|---|---|---|---|---|---|
| Gene Positive | Gene Negative | Gene Positive | Gene Negative | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
| Aminoglycoside | ||||||||
| Streptomycin | 111 | 4 | 16 | 31 | 96.5 | 66.0 | 87.4 | 88.6 |
| Neomycin | 8 | 0 | 96 | 58 | 100.0 | 37.7 | 7.7 | 100.0 |
| Beta-lactam/beta-lactam inhibitor | ||||||||
| Cephems | ||||||||
| Ceftazidime | 61 | 2 | 74 | 25 | 96.8 | 25.3 | 45.2 | 92.6 |
| Ceftiofor | 127 | 3 | 8 | 24 | 97.7 | 75.0 | 94.1 | 88.9 |
| Penicillin | ||||||||
| Ampicillin | 136 | 10 | 0 | 16 | 93.2 | 100.0 | 100.0 | 61.5 |
| Folate pathway inhibitors | ||||||||
| Trimethoprim/sulfamethoxazole | 93 | 0 | 29 | 40 | 100.0 | 58.0 | 76.2 | 100.0 |
| Sulfisoxazole | 112 | 5 | 6 | 39 | 95.7 | 86.7 | 94.9 | 88.6 |
| Phenicol | ||||||||
| Florfenicol | 67 | 3 | 16 | 76 | 95.7 | 82.6 | 80.7 | 96.2 |
| Quinolones | ||||||||
| Enrofloxacin | 70 | 0 | 91 | 1 | 100.0 | 1.1 | 43.5 | 100.0 |
| Tetracyclines | ||||||||
| Oxytetracycline | 144 | 5 | 2 | 11 | 95.7 | 84.6 | 98.6 | 68.8 |
| ICE name | Function | Family | Source Bacteria |
|---|---|---|---|
| ICEVchBan8 | toxin-antitoxin system | SXT/R391 | Vibrio cholerae MZO-3 |
| HAI2 | Unknown (-) | Unclassified | Erwinia carotovora subsp. atroseptica SCRI1043 |
| ICECroICC168–1 | Unknown (-) | Unclassified | Citrobacter rodentium ICC168 |
| ICE6441 | Unknown (-) | Unclassified | Pseudomonas aeruginosa FFUP PS CB5 |
| ICE6440 | Unknown (-) | Unclassified | Pseudomonas aeruginosa HSV3483 |
| AICEScatt35120 | Unknown (-) | Unclassified | Streptomyces cattleya DSM |
| ICEValHN437 | Unknown (-) | SXT/R391 | Vibrio alginolyticus HN437 |
| ICEValHN396 | Unknown (-) | SXT/R391 | Vibrio alginolyticus HN396 |
| ICEValA056–2 | Unknown (-) | SXT/R391 | Vibrio alginolyticus 103826 |
| ICEKpnHS11286–2 | Unknown (-) | Unclassified | Klebsiella pneumoniae HS11286 |
| Tn6098 | Unknown (-) | Unclassified | Lactococcus lactis subsp. lactis KF147 |
| YAPI | Pathogenicity | ICEYe1 | Yersinia pseudotuberculosis IP 31758 |
| YAPI | Pathogenicity | ICEYe2 | Yersinia pseudotuberculosis 32777 |
| ICESenTy2–1 | Unknown (-) | SPI-7 | Salmonella enterica subsp. enterica serovar Typhi str. Ty2 |
| PFGI-1 | Unknown (-) | PAPI-1 | Pseudomonas fluorescens Pf-5 |
| ICEDzeEch1591–1 | Unknown (-) | ICEKp1 | Dickeya zeae Ech1591 |
| ICESb2 | Unknown (-) | SPI-7 | Salmonella bongori 2022/77 |
| ICESb1 | Unknown (-) | SPI-7 | Salmonella bongori CEIM46082 |
| ICEYe1 | Unknown (-) | ICEYe1 | Yersinia enterocolitica Y69 |
| ICEEc2 | Unknown (-) | Unclassified | Escherichia coli BEN374 |
| ICEPaeLESB58–1 | Mercuric resistance | ICEclc | Pseudomonas aeruginosa LESB58 |
| ICEVflInd1 | Antibiotic resistance; toxin-antitoxin system | SXT/R391 | Vibrio fluvialis Ind1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adator, E.H.; Walker, M.; Narvaez-Bravo, C.; Zaheer, R.; Goji, N.; Cook, S.R.; Tymensen, L.; Hannon, S.J.; Church, D.; Booker, C.W.; et al. Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum. Microorganisms 2020, 8, 448. https://doi.org/10.3390/microorganisms8030448
Adator EH, Walker M, Narvaez-Bravo C, Zaheer R, Goji N, Cook SR, Tymensen L, Hannon SJ, Church D, Booker CW, et al. Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum. Microorganisms. 2020; 8(3):448. https://doi.org/10.3390/microorganisms8030448
Chicago/Turabian StyleAdator, Emelia H., Matthew Walker, Claudia Narvaez-Bravo, Rahat Zaheer, Noriko Goji, Shaun R. Cook, Lisa Tymensen, Sherry J. Hannon, Deirdre Church, Calvin W. Booker, and et al. 2020. "Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum" Microorganisms 8, no. 3: 448. https://doi.org/10.3390/microorganisms8030448
APA StyleAdator, E. H., Walker, M., Narvaez-Bravo, C., Zaheer, R., Goji, N., Cook, S. R., Tymensen, L., Hannon, S. J., Church, D., Booker, C. W., Amoako, K., Nadon, C. A., Read, R., & McAllister, T. A. (2020). Whole Genome Sequencing Differentiates Presumptive Extended Spectrum Beta-Lactamase Producing Escherichia coli along Segments of the One Health Continuum. Microorganisms, 8(3), 448. https://doi.org/10.3390/microorganisms8030448






