Effect of Supplemental Protease on Growth Performance and Excreta Microbiome of Broiler Chicks
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Treatments
2.3. Ammonia Analysis
2.4. Short Chain Fatty Acid Analysis
2.5. DNA Extraction and Sequencing
2.6. 16.S rRNA Gene Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Microbial Fermentation Byproducts: Ammonia and Short Chain Fatty Acids
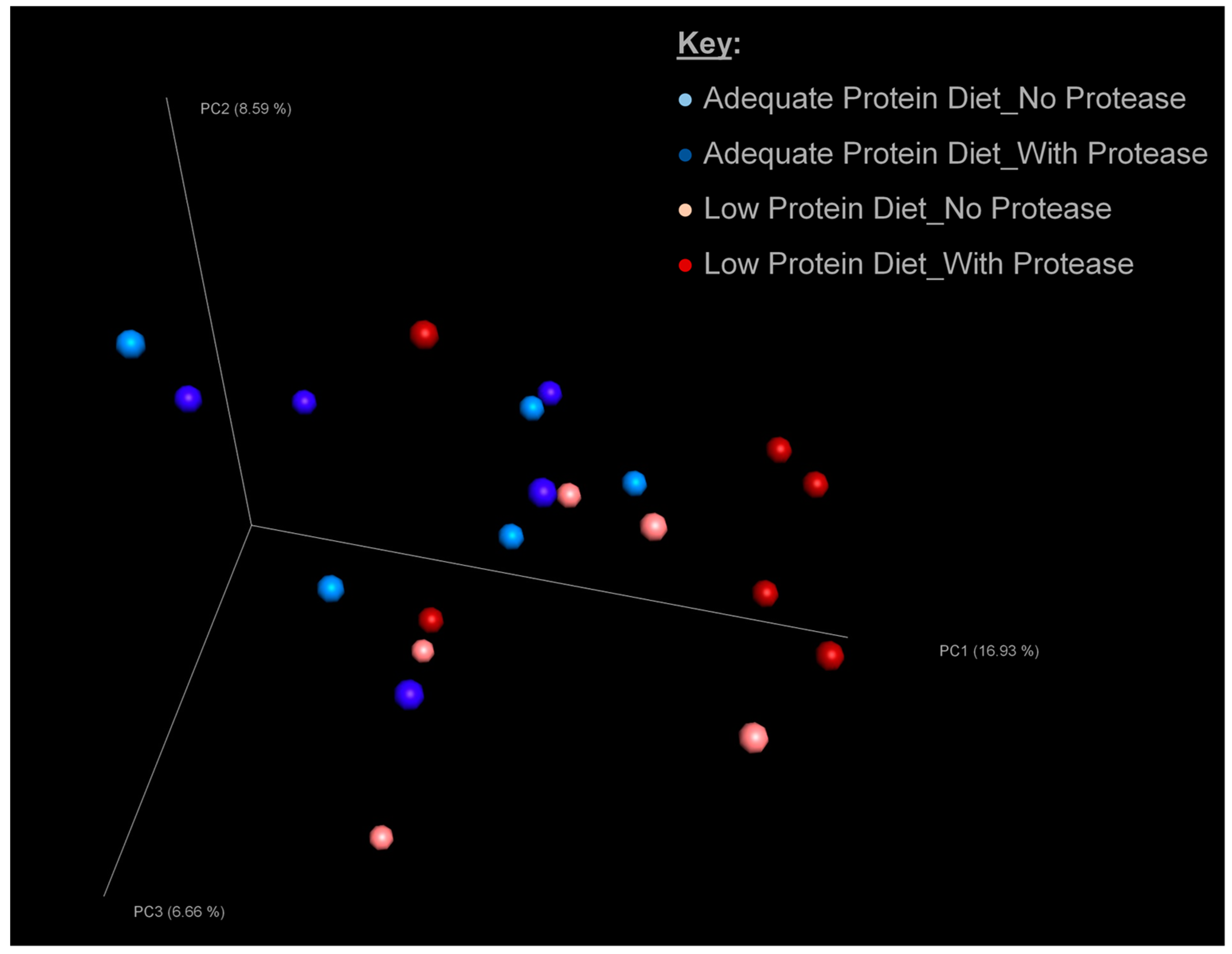
3.3. Microbial Diversity of Excreta Microbiota
3.4. Microbial Composition of Excreta Microbiota
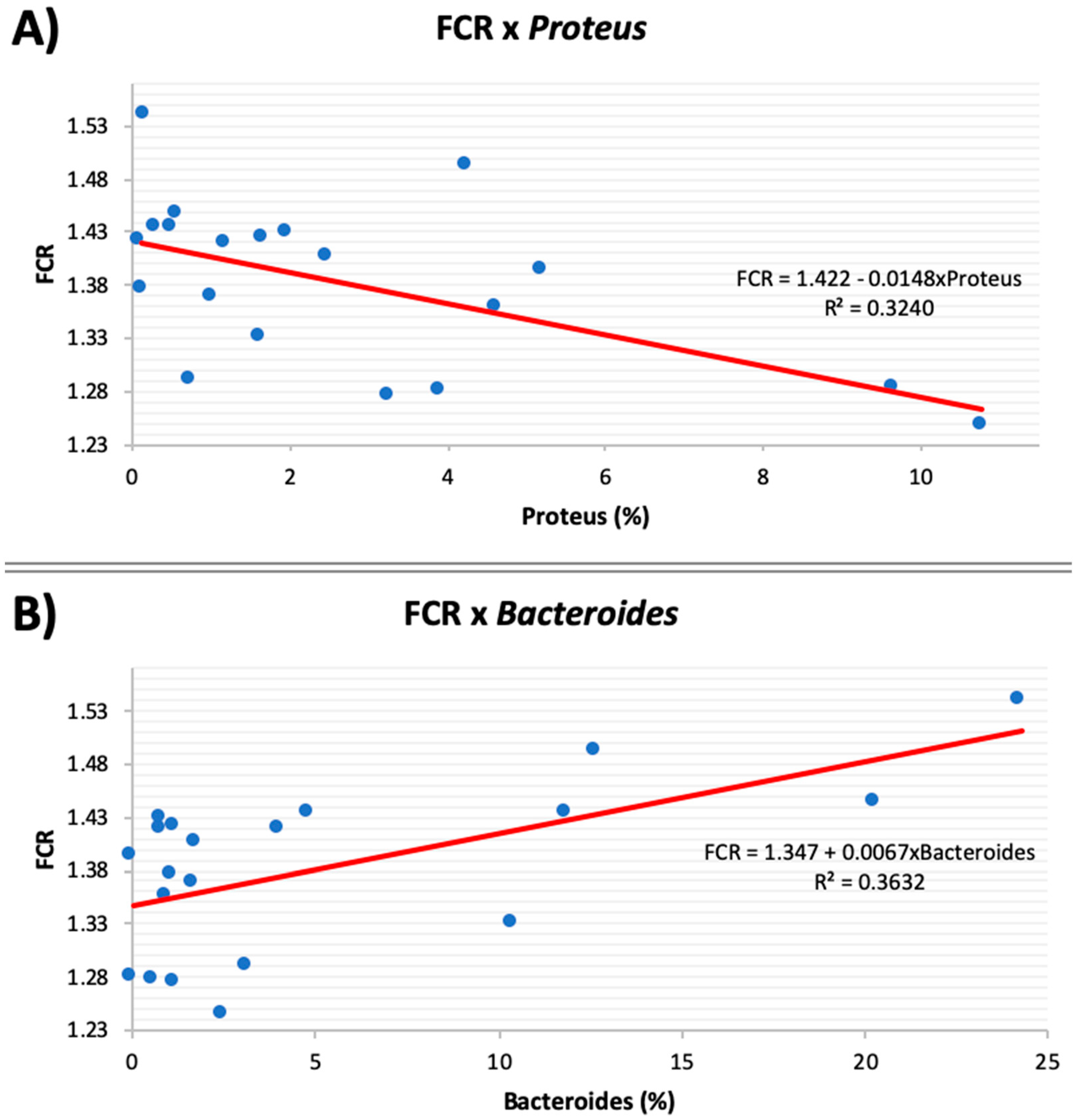
3.5. Microbial Correlation with Feed Efficiency
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Acamovic, T. Commercial application of enzyme technology for poultry production. Worlds Poult. Sci. J. 2001, 57, 225–242. [Google Scholar] [CrossRef]
- Barletta, A. Introduction: Current Market and Expected Developments. In Enzymes in Farm Animal Nutrition, 2nd ed.; Bedford, M.R., Partridge, G.G., Eds.; CAB International: Oxfordshire, UK, 2010; pp. 1–11. [Google Scholar]
- Adeola, O.; Cowieson, A.J. Board-Invited Review: Opportunities and challenges in using exogenous enzymes to improve nonruminant animal production. J. Anim. Sci. 2011, 89, 3189–3218. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Corless, A.; Sell, S.L. Digestive system development in post-hatch poultry. Worlds Poult. Sci. J. 1998, 54, 335–345. [Google Scholar] [CrossRef]
- Mahmood, T.; Mirza, M.A.; Nawaz, H.; Shahid, M. Effect of difference exogenous proteases on growth performance, nutrient digestibility, and carcass response in broiler chickens fed poultry by-product meal-based diets. Livest Sci. 2017, 200, 71–75. [Google Scholar] [CrossRef]
- Isaksen, M.F.; Cowieson, A.J.; Kragh, K.M. Starch- and protein-degrading enzymes: Biochemistry, enzymology and characteristics relevant to animal feed use. In Enzymes in Farm Animal Nutrition, 2nd ed.; Bedford, M.R., Partridge, G.G., Eds.; CAB International: Oxfordshire, UK, 2010; pp. 85–95. [Google Scholar]
- Cowieson, A.J.; Acamovic, T.; Bedford, M.R. Using the precision-feeding bioassay to determine the efficacy of exogenous enzymes—A new perspective. Anim. Feed Sci. Technol. 2006, 129, 149–158. [Google Scholar] [CrossRef]
- Cowieson, A.J. Strategic selection of exogenous enzymes for corn/soy-based poultry diets. J. Poult. Sci. 2010, 47, 1–7. [Google Scholar] [CrossRef]
- Jozefiak, D.; Rutkowski, A.; Martin, S.A. Carbohydrate fermentation in the avian ceca: A review. Anim. Feed Sci. Technol. 2004, 113, 1–15. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef]
- Oakley, B.B.; Kogut, M.H. Spatial and temporal changes in broiler chicken cecal and fecal microbiomes and correlations of bacterial taxa with cytokine gene expression. Front. Vet. Sci. 2016, 3. [Google Scholar] [CrossRef]
- Kiarie, E.; Romero, L.F.; Nyachoti, C.M. The role of added feed enzymes in promoting gut health in swine and poultry. Nutr. Res. Rev. 2013, 26, 71–88. [Google Scholar] [CrossRef]
- Freitas, D.M.; Viera, S.L.; Angel, C.R.; Fabero, A.; Maiorka, A. Performance and nutrient utilization of broilers fed diets supplemented with a novel mono-component protease. J. Appl. Poult. Res. 2011, 20, 322–334. [Google Scholar] [CrossRef]
- Angel, C.R.; Saylor, W.; Viera, S.L.; Ward, N. Effects of a monocomponent protease on performance and protein utilization in 7- to 22-day-old broiler chickens. Poult. Sci. 2011, 90, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Walk, C.L.; Juntunen, K.; Paloheimo, M.; Ledoux, D.R. Evaluation of novel protease enzymes on growth performance and nutrient digestibility of poultry: Enzyme dose response. Poult. Sci. 2019, 98, 5525–5532. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Munyaka, P.M.; Nandha, N.K.; Kiarie, E.; Nyachoti, C.M.; Khafipour, E. Impact of combined β-glucanase and xylanase enzymes on growth performance, nutrients utilization and gut microbiota in broiler chickens fed corn or wheat-based diets. Poult. Sci. 2016, 95, 528–540. [Google Scholar] [CrossRef]
- Yin, D.; Yin, X.; Wang, X.; Lei, Z.; Wang, M.; Guo, Y.; Aggrey, S.E.; Nie, W. Supplementation of amylase combined with glucoamylase or protease changes intestinal microbiota diversity and benefits for broilers fed a diet of newly harvested corn. J. Anim. Sci. Biotechnol. 2018, 9, 24. [Google Scholar] [CrossRef]
- Giannenas, I.; Bonos, E.; Anestis, V.; Filioussis, G.; Papanastasiou, D.K.; Bartzanas, T.; Papaioannou, N.; Tzora, A.; Skoufos, I. Effects of protease addition and replacement of soybean meal by corn gluten meal on the growth of broilers and on the environmental performances of a broiler production system in greece. PLoS ONE 2017, 12, e0169511. [Google Scholar] [CrossRef]
- Cobb-Vantress. Broiler Management Guide Cobb 500; Cobb-Vantress: Siloam Springs, AR, USA, 2018; Available online: https://www.cobb-vantress.com/assets/Cobb-Files/product-guides/bdc20a5443/70dec630-0abf-11e9-9c88-c51e407c53ab.pdf (accessed on 11 February 2020).
- NRC. Nutrient Requirements of Poultry, 9th ed.; The National Academies Press: Washington, DC, USA, 1994.
- Applegate, T.J.; Angel, R. Nutrient requirements of poultry publication: History and need for an update. J. Appl. Poult. Res. 2014, 23, 567–575. [Google Scholar] [CrossRef]
- Weatherburn, M.W. The phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Rothrock, M.J., Jr.; Hiett, K.L.; Gamble, J.; Caudill, A.C.; Cicconi-Hogan, K.M.; Caporaso, J.G. A hybrid DNA extraction method for the qualitative and quantitative assessment of bacterial communities from poultry production samples. J. Vis. Exp. 2014, 94. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Bittinger, K.; Bushman, F.D.; DeSantis, T.Z.; Andersen, G.L.; Knight, R. PyNAST: A flexible tool for aligning sequences to a template alignment. Bioinformatics 2010, 26, 266–267. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Baeza, Y.; Pirrung, M.; Gonzalez, A.; Knight, R. EMPeror: A tool for visualizing high-throughput microbial community data. Gigascience 2013, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Namroud, N.F.; Shivazad, M.; Zaghari, M. Impact of dietary crude protein and amino acids status on performance and some excreta characteristics of broiler chicks during 10–28 days of age. J. Anim. Physiol. Anim. Nutr. 2010, 94, 280–286. [Google Scholar] [CrossRef]
- Rawlings, N.D. Protease Families, Evolution, and Mechanism of Action. In Proteases: Structure and Function; Brix, K., Stocker, W., Eds.; Springer: Heidelberg, Germany, 2013; pp. 1–36. [Google Scholar]
- Law, F.L.; Zulkifli, I.; Soleimani, A.F.; Liang, J.B.; Awad, E.A. The effects of low-protein diets and protease supplementation on broiler chickens in a hot and humid tropical environment. Asian Australas. J. Anim. Sci. 2018, 31, 1291–1300. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Kamran, J.; Abd El-Hack, M.E.; Alagawany, M.; Bhatti, S.A.; Ahmad, G.; Saleem, A.; Ullah, Z.; Yameen, R.M.K.; Ding, C. Influence of low-protein and low-amino acid diets with different sources of protease on performance, carcasses, and nitrogen retention of broiler chickens. Anim. Prod. Sci. 2017, 58, 1625–1631. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Lu, H.; Ajuwon, K.M.; Knap, I.; Adeola, O. Interactive effects of dietary protein source and exogenous protease on growth performance, immune competence and jejunal health of broiler chickens. Anim. Prod. Sci. 2016, 57, 252–261. [Google Scholar] [CrossRef]
- Noy, Y.; Sklan, D. Digestion and Absorption in the Young Chick. Poult. Sci. 1995, 74, 366–373. [Google Scholar] [CrossRef]
- Kaczmarek, S.A.; Rogiewicz, A.; Mogielnicka, M.; Rutkowski, A.; Jones, R.O.; Slominski, B.A. The effect of protease, amylase, and nonstarch polysaccharide-degrading enzyme supplementation on nutrient utilization and growth performance of broiler chickens fed corn soybean meal-based diets. Poult. Sci. 2014, 93, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Mahagna, M.; Nir, I.; Larbier, M.; Nitsan, Z. Effect of age and exogenous amalyse and protease on development of the digestive tract, pancreatic enzyme activities and digestibility of nutrients in young meat-type chicks. Reprod. Nutr. Dev. 1995, 35, 201–212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yuan, L.; Wang, M.; Zhang, X.; Wang, Z. Effects of protease and non-starch polysaccharide enzyme on performance, digestive function, activity and gene expression of endogenous enzyme of broilers. PLoS ONE 2017, 12, e0173941. [Google Scholar] [CrossRef] [PubMed]
- Qaisrani, S.N.; Van Krimpen, M.M.M.; Kwakkel, R.P.; Verstegen, M.W.A.; Hendriks, W.H. Dietary factors affecting hindgut protein fermentation in broilers: A review. Worlds Poult. Sci. J. 2015, 71, 139–160. [Google Scholar] [CrossRef]
- Lee, S.A.; Bedford, M.R.; Walk, C.L. Meta-analysis: Explicit value of mono-component proteases in monogastric diets. Poult. Sci. 2018, 97, 2078–2085. [Google Scholar] [CrossRef]
- Paster, B.J.; Russell, J.B.; Yang, C.M.J.; Chow, J.M.; Woese, C.R.; Tanner, R. Phylogeny of the Ammonia-Producing Ruminal Bacteria Peptostreptococcus anaerobius, Clostridium sticklandii, and Clostridium aminophilum sp. nov. Int. J. Syst. Evol. Microbiol. 1993, 43, 107–110. [Google Scholar] [CrossRef]
- Apajalahti, J.; Vienola, K. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 2016, 221, 323–330. [Google Scholar] [CrossRef]
- Fan, P.; Li, L.; Rezaei, A.; Eslamfam, S.; Che, D.; Ma, X. Metabolites of Dietary Protein and Peptides by Intestinal Microbes and their Impacts on Gut. Curr. Protein Pept. Sci. 2015, 16, 646–654. [Google Scholar] [CrossRef]
- Tong, P.; Ji, X.; Chen, L.; Liu, J.; Xu, L.; Zhu, L.; Zhou, W.; Liu, G.; Wang, S.; Guo, X.; et al. Metagenome analysis of antibiotic resistance genes in fecal microbiota of chickens. Agri Gene 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.M.; Reddy, B.; Deshpande, S.; Rank, D.N.; Joshi, C.G. Taxonomic and gene-centric metagenomics of the fecal microbiome of low and high feed conversation ratio (FCR) broilers. J. Appl. Genet. 2014, 55, 145–154. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Rothrock, M.J., Jr.; Fluharty, F.L.; Callaway, T.R. The Successional Changes in the Gut Microbiome of Pasture-Raised Chickens Fed Soy-Containing and Soy-Free Diets. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Rothrock, M.J., Jr.; Sanad, Y.M.; Callaway, T.R. The Effects of Feeding a Soybean-Based or a Soy-Free Diet on the Gut Microbiome of Pasture-Raised Chickens Throughout Their Lifecycle. Front. Sustain. Food Syst. 2019, 3. [Google Scholar] [CrossRef]
- Shang, Y.; Kumar, S.; Oakley, B.; Kim, W.K. Chicken Gut Microbiota: Importance and Detection Technology. Front. Vet. Sci. 2018, 5, 00254. [Google Scholar] [CrossRef] [PubMed]
- Jumpertz, R.; Le, S.D.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–64. [Google Scholar] [CrossRef]
- Brenner, D.J.; Farmer, J.J. Enterobacteriaceae. In Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef]
- Hammes, W.P.; Hertel, C. Lactobacillus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Penner, J.L. Proteus. In Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Juni, E. Acinetobacter. In Bergey’s Manual of Systematics of Archaea and Bacteria; Springer: New York, NY, USA, 2015. [Google Scholar] [CrossRef]
- Singh, K.M.; Shah, T.; Deshpande, S.; Jakhesara, S.J.; Koringa, P.G.; Rank, D.N.; Joshi, C.G. High through put 16S rRNA gene-based pyrosequencing analysis of the fecal microbiota of high FCR and low FCR broiler growers. Mol. Biol. Rep. 2012, 39, 10595–10602. [Google Scholar] [CrossRef]
- Kumar, P.; Sharma, B.K.; Uppadhyay, B.; Surnar, S.R.; Yadav, R. Growth Promoting and Immunostimulatory Effects of Feed Probiotic (Proteus) in Labeorohita (Hamilton) Fingerlings. Int. J. Sci. Environ. Technol. 2016, 5, 3038–3047. [Google Scholar]
| Item | Diet 1 | |
|---|---|---|
| Ingredient, % of Inclusion | Adequate Protein | Low Protein |
| Corn | 53.85 | 63.25 |
| Soybean meal | 29.70 | 21.75 |
| Dried distillers’ grains with solubles | 10.00 | 10.00 |
| Fat | 2.52 | 1.08 |
| Limestone | 1.29 | 1.31 |
| Dicalcium phosphate | 1.47 | 1.52 |
| Salt | 0.30 | 0.30 |
| Vitamin premix 2 | 0.25 | 0.25 |
| Mineral premix 3 | 0.075 | 0.075 |
| L-lysine | 0.27 | 0.28 |
| DL-methionine | 0.28 | 0.18 |
| Calculated Chemical Composition | ||
| Metabolizable Energy, kcal/kg | 3010 | 3010 |
| Crude Protein, % | 22.30 | 19.23 |
| Ether Extract, % | 5.56 | 4.40 |
| Crude Fiber, % | 3.23 | 3.13 |
| Ca, % | 0.90 | 0.90 |
| Available P, % | 0.40 | 0.45 |
| Lysine, % | 1.30 | 1.10 |
| Total sulfur amino acids, % | 0.96 | 0.81 |
| Threonine, % | 0.86 | 0.73 |
| Tryptophan, % | 0.28 | 0.23 |
| Analyzed Composition4 | ||
| Crude Protein, % | 20.83 | 18.95 |
| Lysine, % | 1.31 | 1.15 |
| Methionine, % | 0.55 | 0.46 |
| Cysteine, % | 0.34 | 0.30 |
| Threonine, % | 0.75 | 0.67 |
| Tryptophan, % | 0.23 | 0.21 |
| Item | Adequate Protein | Low Protein | SEM 3 | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|---|
| No Protease | 200 g/t Protease | No Protease | 200 g/t Protease | Diet | Protease | Diet × Protease | ||
| Body weight day 0, g | 48.5 | 48.2 | 48.9 | 49.0 | 0.33 | 0.07 | 0.91 | 0.50 |
| Body weight day 7, g | 181.7 | 178.4 | 171.8 | 174.5 | 3.24 | 0.05 | 0.94 | 0.37 |
| Body weight day 14, g | 416.1 | 423.1 | 392.8 | 383.4 | 12.92 | 0.03 | 0.93 | 0.53 |
| ADG 4 day 0 to 7, g | 19.0 | 18.6 | 17.6 | 17.9 | 0.45 | 0.03 | 0.95 | 0.39 |
| ADG 4 day 7 to 14, g | 33.5 | 35.0 | 31.6 | 29.8 | 1.60 | 0.04 | 0.93 | 0.33 |
| ADG 4 day 0 to 14, g | 26.3 | 26.8 | 24.6 | 23.9 | 0.92 | 0.02 | 0.93 | 0.52 |
| Daily feed intake day 0 to 7, g | 23.0 | 22.7 | 23.0 | 24.4 | 0.59 | 0.16 | 0.39 | 0.19 |
| Daily feed intake day 7 to 14, g | 46.0 | 49.7 | 46.7 | 44.4 | 2.35 | 0.34 | 0.78 | 0.21 |
| Daily feed intake day 0 to 14, g | 34.5 | 36.2 | 34.9 | 34.4 | 1.37 | 0.61 | 0.67 | 0.43 |
| FCR 5 day 0 to 7 | 1.21 | 1.22 | 1.31 | 1.36 | 0.01 | <0.001 | 0.03 | 0.19 |
| FCR 5 day 7 to 14 | 1.37 | 1.42 | 1.48 | 1.51 | 0.04 | 0.04 | 0.41 | 0.80 |
| FCR 5 day 0 to 14 | 1.31 | 1.35 | 1.42 | 1.45 | 0.03 | <0.01 | 0.27 | 0.82 |
| Item | Adequate Protein | Low Protein | SEM 3 | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|---|
| No Protease | 200 g/t Protease | No Protease | 200 g/t Protease | Diet | Protease | Diet × Protease | ||
| Ammonia-N (mg/g) | 1.17 | 1.06 | 0.88 | 1.05 | 0.10 | 0.13 | 0.76 | 0.17 |
| Total SCFA, mM | 58.27 | 58.70 | 46.52 | 52.05 | 5.11 | 0.09 | 0.57 | 0.63 |
| Acetate | 89.29 | 89.49 | 89.89 | 87.80 | 1.31 | 0.68 | 0.48 | 0.39 |
| Propionate | 1.31 | 0.97 | 1.16 | 1.99 | 0.23 | 0.08 | 0.31 | 0.02 |
| Butyrate | 6.88 | 8.04 | 6.22 | 8.14 | 1.14 | 0.81 | 0.20 | 0.74 |
| Isobutyrate | 0.43 | 0.27 | 0.22 | 0.29 | 0.06 | 0.14 | 0.50 | 0.09 |
| Valerate | 0.32 | 0.10 | 0.61 | 0.50 | 0.31 | 0.24 | 0.54 | 0.79 |
| Isovalerate | 0.95 | 0.52 | 0.53 | 0.47 | 0.13 | 0.09 | 0.09 | 0.18 |
| Caproate | 0.81 | 0.65 | 1.38 | 0.81 | 0.82 | 0.67 | 0.66 | 0.80 |
| Item | Adequate Protein | Low Protein | SEM 3 | p-Value 2 | ||||
|---|---|---|---|---|---|---|---|---|
| No Protease | 200 g/t Protease | No Protease | 200 g/t Protease | Diet | Protease | Diet × Protease | ||
| Observed OTUs | 1132 | 1184 | 919 | 1206 | 76.76 | 0.23 | 0.04 | 0.15 |
| Chao1 | 1992 | 2052 | 1600 | 2030 | 135.79 | 0.15 | 0.09 | 0.19 |
| Phylogenetic diversity 4 | 47.2 | 48.9 | 39.5 | 49.7 | 2.83 | 0.24 | 0.05 | 0.15 |
| Shannon index | 5.0 | 5.1 | 4.3 | 4.9 | 0.23 | 0.07 | 0.15 | 0.23 |
| Evenness | 0.49 | 0.50 | 0.44 | 0.48 | 0.02 | 0.06 | 0.22 | 0.29 |
| Phyla | Adequate Protein | Low Protein | SEM 4 | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|---|
| No Protease | 200 g/t Protease | No Protease | 200 g/t Protease | Diet | Protease | Diet × Protease | ||
| Firmicutes | 46.5 | 46.9 | 40.9 | 53.6 | 5.93 | 0.93 | 0.29 | 0.31 |
| Proteobacteria | 48.4 | 45.0 | 53.9 | 34.0 | 6.42 | 0.67 | 0.09 | 0.22 |
| Bacteroidetes | 2.8 | 6.5 | 4.0 | 10.8 | 2.93 | 0.36 | 0.09 | 0.61 |
| Actinobacteria | 1.1 | 0.5 | 0.1 | 0.3 | 0.32 | 0.09 | 0.55 | 0.20 |
| Firmicutes:Bacteroidetes | 58.7 | 140.5 | 25.4 | 18.9 | 69.56 | 0.28 | 0.60 | 0.54 |
| Genera | Adequate Protein | Low Protein | SEM 4 | p-Value 3 | ||||
|---|---|---|---|---|---|---|---|---|
| No Protease | 200 g/t Protease | No Protease | 200 g/t Protease | Diet | Protease | Diet × Protease | ||
| Unclassified, Family Enterobacteriaceae | 30.1 | 31.7 | 45.3 | 27.1 | 6.29 | 0.41 | 0.21 | 0.14 |
| Lactobacillus | 22.3 | 11.6 | 13.0 | 25.8 | 4.39 | 0.58 | 0.82 | 0.02 |
| Enterococcus | 8.1 | 12.6 | 7.1 | 6.8 | 2.03 | 0.12 | 0.32 | 0.26 |
| Bacteroides | 1.2 | 5.4 | 3.9 | 10.2 | 3.00 | 0.23 | 0.10 | 0.74 |
| Unclassified, Family Planococcaceae | 5.1 | 7.3 | 6.6 | 1.6 | 3.14 | 0.52 | 0.67 | 0.27 |
| Klebsiella | 5.3 | 3.9 | 5.6 | 2.5 | 1.29 | 0.66 | 0.10 | 0.52 |
| Ruminococcus | 2.6 | 2.3 | 3.6 | 4.8 | 1.43 | 0.23 | 0.75 | 0.62 |
| Proteus | 4.8 | 4.2 | 1.2 | 0.6 | 1.16 | 0.01 | 0.61 | 0.97 |
| Acinetobacter | 2.4 | 3.7 | 1.0 | 1.1 | 0.59 | 0.01 | 0.26 | 0.32 |
| Unclassified, Family Ruminococcaceae | 1.8 | 1.0 | 1.8 | 2.9 | 0.82 | 0.26 | 0.82 | 0.27 |
| Unclassified, Family Alcaligenaceae | 3.7 | 0.1 | 0.1 | 1.5 | 2.00 | 0.58 | 0.58 | 0.22 |
| Unclassified, Order Clostridiales | 0.5 | 1.0 | 1.7 | 2.1 | 0.80 | 0.17 | 0.53 | 0.93 |
| Blautia | 0.9 | 0.9 | 0.8 | 1.3 | 0.39 | 0.69 | 0.53 | 0.45 |
| Oscillospira | 0.2 | 0.4 | 0.8 | 1.6 | 0.58 | 0.15 | 0.40 | 0.62 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lourenco, J.M.; Nunn, S.C.; Lee, E.J.; Dove, C.R.; Callaway, T.R.; Azain, M.J. Effect of Supplemental Protease on Growth Performance and Excreta Microbiome of Broiler Chicks. Microorganisms 2020, 8, 475. https://doi.org/10.3390/microorganisms8040475
Lourenco JM, Nunn SC, Lee EJ, Dove CR, Callaway TR, Azain MJ. Effect of Supplemental Protease on Growth Performance and Excreta Microbiome of Broiler Chicks. Microorganisms. 2020; 8(4):475. https://doi.org/10.3390/microorganisms8040475
Chicago/Turabian StyleLourenco, Jeferson M., S. Claire Nunn, Eliza. J. Lee, C. Robert Dove, Todd R. Callaway, and Michael J. Azain. 2020. "Effect of Supplemental Protease on Growth Performance and Excreta Microbiome of Broiler Chicks" Microorganisms 8, no. 4: 475. https://doi.org/10.3390/microorganisms8040475
APA StyleLourenco, J. M., Nunn, S. C., Lee, E. J., Dove, C. R., Callaway, T. R., & Azain, M. J. (2020). Effect of Supplemental Protease on Growth Performance and Excreta Microbiome of Broiler Chicks. Microorganisms, 8(4), 475. https://doi.org/10.3390/microorganisms8040475






