Heliocephala variabilis and Pseudopenidiella vietnamensis: Two New Hyphomycetous Species in the Microthyriaceae (Dothideomycetes) from Vietnam
Abstract
1. Introduction
2. Material and Methods
2.1. Samples and Isolates
2.2. DNA Extraction, PCR, Sequencing and Phylogenetic Analyses
2.3. Phenotypic Study
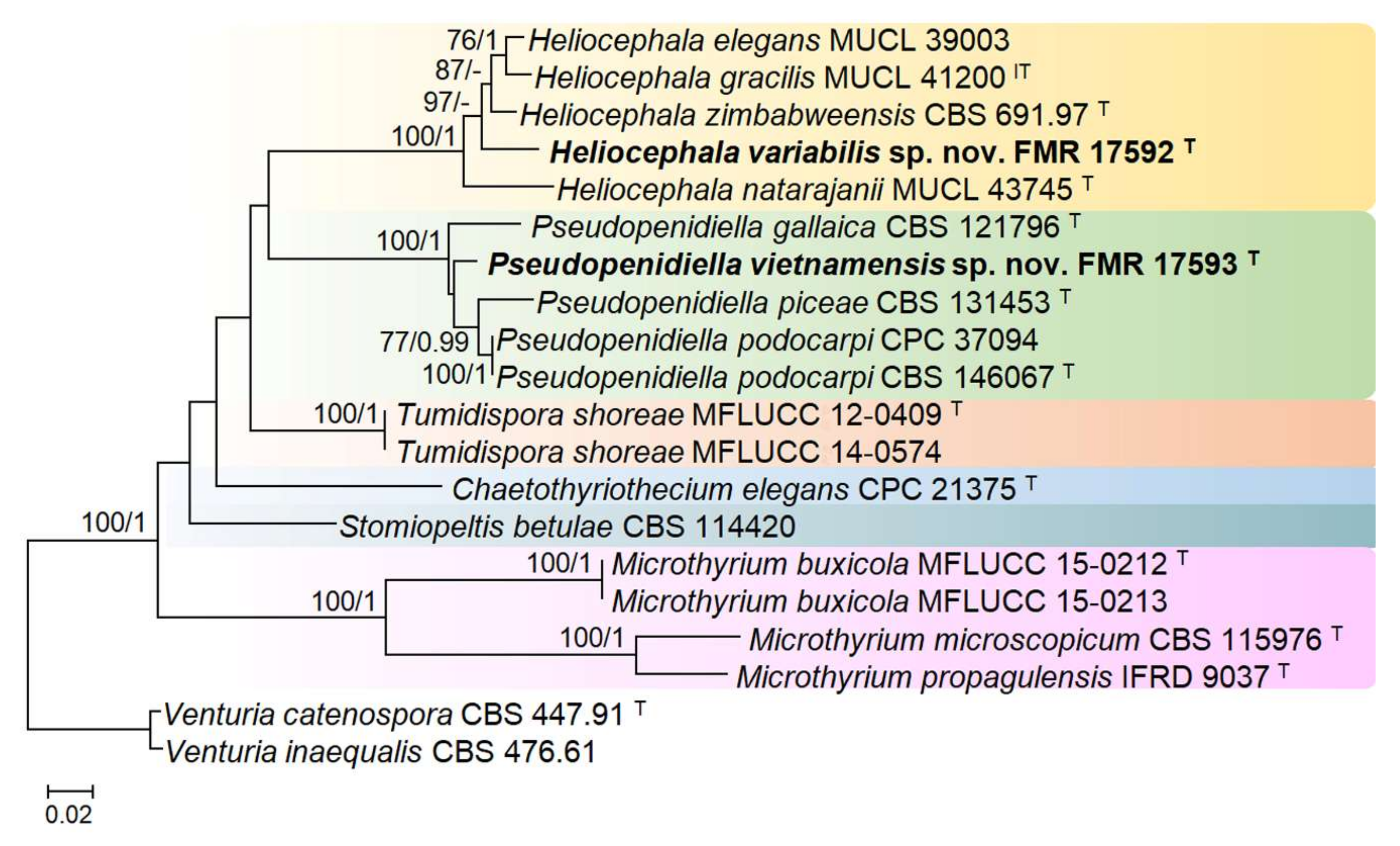
3. Results
Taxonomy
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mittermeier, R.A.; Gill, P.R.; Hoffman, M.; Pilgrim, J.; Brooks, T.; Mittermeier, C.G.; Lamoreux, J.; Da Fonseca, G.A.B. Hotspots Revisited: Earths Biologically Richest and Most Endangered Terrestrial Ecoregions; Conservation International: Washington, DC, USA, 2005; p. 392. [Google Scholar]
- Sterling, E.J.; Hurley, M.M. Conserving Biodiversity in Vietnam: Applying Biogeography to Conservation Research. Proc. Calif. Acad. Sci. 2005, 56, 98–114. [Google Scholar]
- De Queiroz, J.S.; Griswold, D.; Nguyen, D.T.; Hall, P. Vietnam Tropical Forest and Biodiversity Assessment; USAID: Quito, Ecuador, 2013; p. 79. [Google Scholar]
- Mel‘nik, V.A.; Crous, P.W. Braunomyces dictyosporus gen. sp. nov. from Vietnam. IMA Fungus 2014, 5, 1–5. [Google Scholar] [CrossRef]
- Mel’nik, V.A.; Alexandrova, A.V.; Braun, U. Two new species and new records of hyphomycetes from Vietnam. Mycosphere 2014, 5, 591–600. [Google Scholar] [CrossRef]
- Brandt, S.C.; Ellinger, B.; van Nguyen, T.; Thi, Q.D.; van Nguyen, G.; Baschien, C.; Yurkov, A.; Hahnke, R.L.; Schäfer, W.; Gand, M. A unique fungal strain collection from Vietnam characterized for high performance degraders of bioecological important biopolymers and lipids. PLoS ONE 2018, 13, e0202695. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Luangsa-ard, J.J.; Wingfield, M.J.; Carnegie, A.J.; Hernández-Restrepo, M.; Lombard, L.; Roux, J.; Barreto, R.W.; Baseia, I.G.; Cano-Lira, J.F.; et al. Fungal Planet description sheets: 785–867. Persoonia 2018, 41, 238–417. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.G.; Reddy, K.A.; de Hoog, G.S. Heliocephala, a new genus of dematiaceous Hyphomycetes. Persoonia 1984, 12, 239–242. [Google Scholar]
- Heredia-Abarca, G.; Castañeda-Ruiz, R.F.; Arias-Mota, R.M.; Becerra-Hernandez, C.I.; Gómez, S.; Bogale, M.; Untereiner, W.A. A new species of Heliocephala from México with an assessment of the systematic positions of the anamorph genera Heliocephala and Holubovaniella. Mycologia 2011, 103, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, R.F. Deuteromycotina de Cuba, Hyphomycetes, III; Instituto de Investigaciones Fundamentales en Agricultura Tropical Alejandro de Humboldt: Havana, Cuba, 1985; p. 72. [Google Scholar]
- Kumaresan, V.; Srinivasan, M. Heliocephala natarajanii sp. nov. from India. Cryptogam. Mycol. 2002, 23, 329–333. [Google Scholar]
- Crous, P.W.; Summerell, B.A.; Shivas, R.G.; Burgess, T.I.; Decock, C.A.; Dreyer, L.L.; Granke, L.L.; Guest, D.I.; Hardy, G.E.S.J.; Hausbeck, M.K.; et al. Fungal Planet Description Sheets: 107–127. Persoonia 2012, 28, 138–182. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Wingfield, M.J.; Lombard, L.; Roets, F.; Swart, W.J.; Alvarado, P.; Carnegie, A.J.; Moreno, G.; Luangsa-Ard, J.; Thangavel, R.; et al. Fungal Planet description sheets: 951–1041. Persoonia 2019, 43, 223–425. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Silvera-Simón, C.; Mena-Portales, J.; Mercado-Sierra, A.; Guarro, j.; Gené, J. Three new species and a new record of Diplococcium from plant debris in Spain. Mycol. Prog. 2012, 11, 191–199. [Google Scholar] [CrossRef]
- Müller, F.M.; Werner, K.E.; Kasai, M.; Francesconi, A.; Chanock, S.J.; Walsh, T.J. Rapid extraction of genomic DNA from medically important yeasts and filamentous fungi by high-speed cell disruption. J. Clin. Microbiol. 1998, 36, 1625–1629. [Google Scholar] [CrossRef] [PubMed]
- Cano, J.; Guarro, J.; Gené, J. Molecular and morphological identification of Colletotrichum species of clinical interest. J. Clin. Microbiol. 2004, 42, 2450–2454. [Google Scholar] [CrossRef] [PubMed]
- Crous, P.W.; Schoch, C.L.; Hyde, K.D.; Wood, A.R.; Gueidan, C.; de Hoog, G.S.; Groenewald, J.Z. Phylogenetic lineages in the Capnodiales. Stud. Mycol. 2009, 64, 17–47. [Google Scholar] [CrossRef] [PubMed]
- Ariyawansa, H.A.; Hyde, K.D.; Jayasiri, S.C.; Buyck, B.; Chethana, K.W.T.; Dai, D.Q.; Dai, Y.C.; Daranagama, D.A.; Jayawardena, R.S.; Lücking, R.; et al. Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 2015, 75, 27–274. [Google Scholar] [CrossRef]
- Hernández-Restrepo, M.; Gené, J.; Castañeda-Ruiz, R.F.; Mena-Portales, J.; Crous, P.W.; Guarro, J. Phylogeny of saprobic microfungi from Southern Europe. Stud. Mycol. 2017, 86, 53–97. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Groenewald, J.Z.; Cai, L.; Chen, Q.; Marincowitz, S.; Barnes, I.; Bensch, K.; Braun, U.; Camporesi, E.; Damm, U.; et al. Genera of phytopathogenic fungi: GOPHY 1. Stud. Mycol. 2017, 86, 99–216. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic Model Averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Kornerup, A.; Wanscher, J.H. Methuen Handbook of Colour, 3rd ed.; Methuen Publishing Ltd.: London, UK, 1978; p. 256. [Google Scholar]
- Crous, P.W.; Gams, W.; Stalpers, J.A.; Robert, V.; Stegehuis, G. MycoBank: An online initiative to launch mycology into the 21st century. Stud. Mycol. 2004, 50, 19–22. [Google Scholar]
- Mel´nik, V.A.; Castañeda-Ruiz, R.F.; Granados, M. A new species of Heliocephala from Vietnam. Mycotaxon 2013, 123, 281–284. [Google Scholar] [CrossRef]
- Decock, C.; Robert, V.; Masuka, J.A. Heliocephala zimbabweensis sp. nov. from southern Africa. Mycologia 1998, 90, 330–333. [Google Scholar] [CrossRef]
- Crous, P.W.; Braun, U.; Groenewald, J.Z. Mycosphaerella is polyphyletic. Stud. Mycol. 2007, 58, 1–32. [Google Scholar] [CrossRef]
- Quaedvlieg, W.; Binder, M.; Groenewald, J.Z.; Summerell, B.A.; Carnegie, A.J.; Burgess, T.I.; Crous, P.W. Introducing the Consolidated Species Concept to resolve species in the Teratosphaeriaceae. Persoonia 2014, 33, 1–40. [Google Scholar] [CrossRef]
- Kirk, P.M. Nomenclatural novelties. Index Fungorum 2014, 120, 1. [Google Scholar]
- Kirk, P.M. New or interesting microfungi IX. Dematiaceous Hyphomycetes from Esher Common. T. Brit. Mycol. Soc. 1983, 80, 449–467. [Google Scholar] [CrossRef]


| Species | Strain Number 1 | Substrate | Country | Genbank Accession No. 2 | |
|---|---|---|---|---|---|
| ITS | LSU | ||||
| Chaetothyriothecium elegans | CPC 21375 T | Leaves of Castanopsis sp. | Thailand | - | KF268420 |
| Heliocephala elegans | MUCL 39003 | Fallen leaf of Andira inermis | Cuba | HQ333478 | HQ333478 |
| H. gracilis | CBS 369.86 IT | Fallen leaf of Matayba oppositifolia | Cuba | HQ333479 | HQ333479 |
| H. natarajanii | MUCL 43745 T | Basideocarp of Pisolithus tinctorius | India | HQ333480 | HQ333480 |
| H. zimbabweensis | CBS 691.97 T | Unidentified leaf litter | Zimbabwe | HQ333481 | HQ333481 |
| H. variabilis sp. nov. | FMR 17592 T | Unidentified dead leaves | Vietnam | LR536989 | LR588212 |
| Microthyrium buxicola | MFLUCC 15-0212 T | Leaves of Buxus sp. | Italy | - | KT306551 |
| MFLUCC 15-0213 | Leaves of Buxus sp. | Italy | - | KT306552 | |
| M. microscopicum | CBS 115976 T | - | The Netherlands | - | GU301846 |
| M. propagulensis | IFRD 9037 T | Fallen leaves of Castanopsis histrix | China | - | KU948989 |
| Pseudopenidiella piceae | CBS 131453 T | Needle litter of Picea abies | Czech Republic | JX069868 | JX069852 |
| P. gallaica | CBS 121796 T | Unidentified dead leaves | Spain | LT984842 | LT984843 |
| P. podocarpi | CBS 146067 T | Leaves of Podocarpus latifolius | South Africa | MN562140 | MN567647 |
| CPC 37094 | Leaves of P. latifolius | South Africa | MN562141 | MN567648 | |
| P. vietnamensis sp. nov. | FMR 17593 T | Unidentified dead leaves | Vietnam | LR536990 | LR536991 |
| Stomiopeltis betulae | CBS 114420 | Betula sp. | Sweden | GU214701 | GU214701 |
| Tumidispora shoreae | MFLUCC 12-0409 T | Dead leaves of Shorea sp. | Thailand | - | KT314073 |
| MFLUCC 14-0574 | Dead leaves of Shorea sp. | Thailand | - | KT314074 | |
| Venturia catenospora | CBS 447.91 T | Leaf spot of Salix triandra | Germany | EU035427 | MH873940 |
| V. inaequalis | CBS 476.61 | Fruit of Malus sylvestris | Belgium | EU282478 | GU456336 |
| Species | Conidiophore Size * | Conidia | References | |||
|---|---|---|---|---|---|---|
| Size * | Septum No. | Ornamentation | Rostrum | |||
| H. elegans | 250–700 × 7–11 | 8–25 × 3–4 | 1–3 | Smooth | Present, straight | [10] |
| H. gracilis | 80–350 × 7–10 | 4–12.5 × 2–5 | 0–1 | Smooth | Absent | [10] |
| H. natarajanii | up to 109 × 1.5–3.5 | (8.5–)17–34(–103) × (1.5–) 2.5–4.5(–6.5) | 2(–3) | Basal cell verruculose | Present, straight, curved or uncinate | [11] |
| H. proliferans | up to 210 × 3.5–4 | (10–)15–50(–200) × 3–4 | 2 | Basal cell verruculose | Present, straight or curved | [8] |
| H. triseptata | 21–40 × 7–19 | 15–27 × 3.5–4.5 | 3 | Smooth | Present, straight | [9] |
| H. variabilis | up to 153 × 4–6 | 4–26 × 3–6 | (0–)1–3(–4) | Smooth to verruculose | Absent | Present study |
| H. vietnamensis | 210–340 × 6–8 | 14–17 × 2.8–3.8 | 3 | Smooth | Absent | [28] |
| H. zimbabweensis | 180–240 × 3–4 | 23–125 × 3.5–5.3 | 2 | Smooth | Present, straight | [29] |
| Species | Macroconidiophore Size * | Microconidiophore | Ramoconidia Size * | Conidia | References | |
|---|---|---|---|---|---|---|
| Size * | Ornamentation | |||||
| P. gallaica | up to 120 × 2–3 | Present | 7.5–11 × 2–3 | 6–12 × 1–3 | Smooth to verruculose | [7] |
| P. piceae | up to 150 x 3–4 | Present | 8–12 × 2–3 | (6–)7–9(–10) × (2.5–)3 | Finely verruculose | [12] |
| P. podocarpi | 10–110 × 3–4 | Absent | (9–)12–13 × (2.5–)3–3.5 | (9–)11–12(–15) × 2.5(–3) | Verruculose | [13] |
| P. vietnamensis | up to 236 × 3–5 | Absent | 7–13 × 3–4 | 5–10 × 2–3 | Smooth | Present study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iturrieta-González, I.; García, D.; Guarro, J.; Gené, J. Heliocephala variabilis and Pseudopenidiella vietnamensis: Two New Hyphomycetous Species in the Microthyriaceae (Dothideomycetes) from Vietnam. Microorganisms 2020, 8, 478. https://doi.org/10.3390/microorganisms8040478
Iturrieta-González I, García D, Guarro J, Gené J. Heliocephala variabilis and Pseudopenidiella vietnamensis: Two New Hyphomycetous Species in the Microthyriaceae (Dothideomycetes) from Vietnam. Microorganisms. 2020; 8(4):478. https://doi.org/10.3390/microorganisms8040478
Chicago/Turabian StyleIturrieta-González, Isabel, Dania García, Josep Guarro, and Josepa Gené. 2020. "Heliocephala variabilis and Pseudopenidiella vietnamensis: Two New Hyphomycetous Species in the Microthyriaceae (Dothideomycetes) from Vietnam" Microorganisms 8, no. 4: 478. https://doi.org/10.3390/microorganisms8040478
APA StyleIturrieta-González, I., García, D., Guarro, J., & Gené, J. (2020). Heliocephala variabilis and Pseudopenidiella vietnamensis: Two New Hyphomycetous Species in the Microthyriaceae (Dothideomycetes) from Vietnam. Microorganisms, 8(4), 478. https://doi.org/10.3390/microorganisms8040478






