The Not so Good, the Bad and the Ugly: Differential Bacterial Adhesion and Invasion Mediated by Salmonella PagN Allelic Variants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Plasmid Constructions
2.2. Protein Expression and Antibody Preparation
2.3. Cell Cultures
2.4. Bacterial Binding and Invasion Assays
2.5. Microscopy
2.6. Binding to Extracellular Matrix Proteins
2.7. Bacterial Genomes and PagN Sequences
2.8. PagN Structure Analysis
2.9. Statistical Analysis
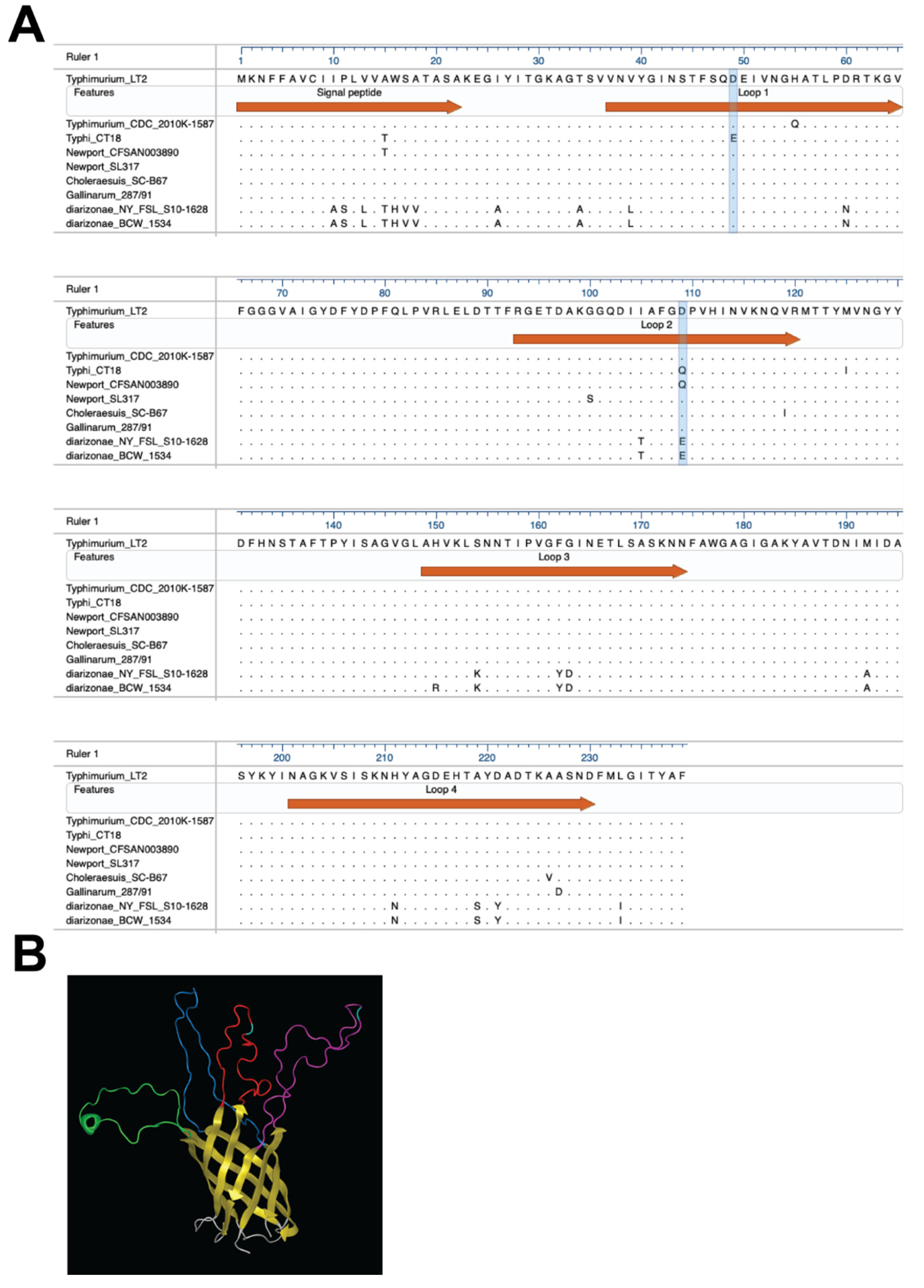
3. Results and Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tsolis, R.M.; Xavier, M.N.; Santos, R.L.; Baumler, A.J. How to become a top model: Impact of animal experimentation on human Salmonella disease research. Infect. Immun. 2011, 79, 1806–1814. [Google Scholar] [CrossRef] [Green Version]
- Alikhan, N.F.; Zhou, Z.; Sergeant, M.J.; Achtman, M. A genomic overview of the population structure of Salmonella. PLoS Genet. 2018, 14, e1007261. [Google Scholar] [CrossRef] [Green Version]
- Grimont, P.A.D.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars; WHO Collaborating Center for Reference and Research on Salmonella; Institut Pasteur, 28 rue du Dr. Roux: Paris, France, 2007; pp. 1–166. [Google Scholar]
- Yue, M.; Schifferli, D.M. Allelic variation in Salmonella: An underappreciated driver of adaptation and virulence. Front. Microbiol. 2014, 4, 419. [Google Scholar] [CrossRef]
- Giner-Lamia, J.; Vinuesa, P.; Betancor, L.; Silva, C.; Bisio, J.; Soleto, L.; Chabalgoity, J.A.; Puente, J.L.; Salmonella, C.N.; Garcia-Del Portillo, F. Genome analysis of Salmonella enterica subsp. diarizonae isolates from invasive human infections reveals enrichment of virulence-related functions in lineage ST1256. BMC Genomics 2019, 20, 99. [Google Scholar] [CrossRef] [Green Version]
- Uelze, L.; Borowiak, M.; Deneke, C.; Jacobs, C.; Szabo, I.; Tausch, S.H.; Malorny, B. First complete genome sequence and comparative analysis of Salmonella enterica subsp. diarizonae serovar 61:k:1,5,(7) indicates host adaptation traits to sheep. Gut Pathog. 2019, 11, 48. [Google Scholar] [CrossRef] [Green Version]
- Bäumler, A.J.; Tsolis, R.M.; Ficht, T.A.; Adams, L.G. Evolution of host adaptation in Salmonella enterica. Infect. Immun. 1998, 66, 4579–4587. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, K.L.; Threlfall, E.J. Frequency and polymorphism of sopE in isolates of Salmonella enterica belonging to the ten most prevalent serotypes in England and Wales. J. Med. Microbiol. 2004, 53, 539–543. [Google Scholar] [CrossRef]
- Heffron, F.; Niemann, G.; Yoon, H.; Kidwai, A.; Brown, R.N.E.; McDermott, J.D.; Smith, R.; Adlkins, J.N. Salmonella-Secreted Virulence Factors. In Salmonella: From Genome to Function; Porwollik, S., Ed.; Caister Academic Press: Norfolk, UK, 2011; pp. 187–223. [Google Scholar]
- Rakov, A.V.; Mastriani, E.; Liu, S.L.; Schifferli, D.M. Association of Salmonella virulence factor alleles with intestinal and invasive serovars. BMC Genom. 2019, 20, 429. [Google Scholar] [CrossRef]
- Elhadad, D.; Desai, P.; Grassl, G.A.; McClelland, M.; Rahav, G.; Gal-Mor, O. Differences in Host Cell Invasion and Salmonella Pathogenicity Island 1 Expression between Salmonella enterica Serovar Paratyphi A and Nontyphoidal S. Typhimurium. Infect. Immun. 2016, 84, 1150–1165. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.A.; Smith, S.G. The PagN protein of Salmonella enterica serovar Typhimurium is an adhesin and invasin. BMC Microbiol. 2008, 8, 142. [Google Scholar] [CrossRef] [Green Version]
- Cirillo, D.M.; Heffernan, E.J.; Wu, L.; Harwood, J.; Fierer, J.; Guiney, D.G. Identification of a domain in Rck, a product of the Salmonella typhimurium virulence plasmid, required for both serum resistance and cell invasion. Infect. Immun. 1996, 64, 2019–2023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velge, P.; Wiedemann, A.; Rosselin, M.; Abed, N.; Boumart, Z.; Chausse, A.M.; Grepinet, O.; Namdari, F.; Roche, S.M.; Rossignol, A.; et al. Multiplicity of Salmonella entry mechanisms, a new paradigm for Salmonella pathogenesis. Microbiologyopen 2012, 1, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Wiedemann, A.; Mijouin, L.; Ayoub, M.A.; Barilleau, E.; Canepa, S.; Teixeira-Gomes, A.P.; Le Vern, Y.; Rosselin, M.; Reiter, E.; Velge, P. Identification of the epidermal growth factor receptor as the receptor for Salmonella Rck-dependent invasion. FASEB J. 2016, 30, 4180–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heffernan, E.J.; Wu, L.; Louie, J.; Okamoto, S.; Fierer, J.; Guiney, D.G. Specificity of the complement resistance and cell association phenotypes encoded by the outer membrane protein genes rck from Salmonella typhimurium and ail from Yersinia enterocolitica. Infect. Immun. 1994, 62, 5183–5186. [Google Scholar] [CrossRef] [Green Version]
- Ho, D.K.; Jarva, H.; Meri, S. Human complement factor H binds to outer membrane protein Rck of Salmonella. J. Immunol. 2010, 185, 1763–1769. [Google Scholar] [CrossRef] [Green Version]
- Glaubman, J.; Hofmann, J.; Bonney, M.E.; Park, S.; Thomas, J.M.; Kokona, B.; Ramos Falcon, L.I.; Chung, Y.K.; Fairman, R.; Okeke, I.N. Self-association motifs in the enteroaggregative Escherichia coli heat-resistant agglutinin 1. Microbiology 2016, 162, 1091–1102. [Google Scholar] [CrossRef]
- Bhargava, S.; Johnson, B.B.; Hwang, J.; Harris, T.A.; George, A.S.; Muir, A.; Dorff, J.; Okeke, I.N. Heat-resistant agglutinin 1 is an accessory enteroaggregative Escherichia coli colonization factor. J. Bacteriol. 2009, 191, 4934–4942. [Google Scholar] [CrossRef] [Green Version]
- Fleckenstein, J.M.; Kopecko, D.J.; Warren, R.L.; Elsinghorst, E.A. Molecular characterization of the tia invasion locus from enterotoxigenic Escherichia coli. Infect. Immun. 1996, 64, 2256–2265. [Google Scholar] [CrossRef] [Green Version]
- Fagan, R.P.; Lambert, M.A.; Smith, S.G. The hek outer membrane protein of Escherichia coli strain RS218 binds to proteoglycan and utilizes a single extracellular loop for adherence, invasion, and autoaggregation. Infect. Immun. 2008, 76, 1135–1142. [Google Scholar] [CrossRef] [Green Version]
- Mancini, J.; Weckselblatt, B.; Chung, Y.K.; Durante, J.C.; Andelman, S.; Glaubman, J.; Dorff, J.D.; Bhargava, S.; Lijek, R.S.; Unger, K.P.; et al. The heat-resistant agglutinin family includes a novel adhesin from enteroaggregative Escherichia coli strain 60A. J. Bacteriol. 2011, 193, 4813–4820. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.; Chakraborty, K.; Nagaraja, T.; Basak, S.; Koley, H.; Dutta, S.; Mitra, U.; Das, S. An adhesion protein of Salmonella enterica serovar Typhi is required for pathogenesis and potential target for vaccine development. Proc. Natl. Acad. Sci. USA 2011, 108, 3348–3353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.L.; Tsui, I.S.; Yip, C.M.; Fung, A.W.; Wong, D.K.; Dai, X.; Yang, Y.; Hackett, J.; Morris, C. Salmonella enterica serovar typhi uses type IVB pili to enter human intestinal epithelial cells. Infect. Immun. 2000, 68, 3067–3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, M.; Han, X.; Masi, L.D.; Zhu, C.; Ma, X.; Zhang, J.; Wu, R.; Schmieder, R.; Kaushik, R.S.; Fraser, G.P.; et al. Allelic variation contributes to bacterial host specificity. Nat. Commun. 2015, 6, 8754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, A.; Cao, S.; Tu, L.; Chen, P.; Zhang, C.; Jia, A.; Yang, W.; Liu, Z.; Chen, H.; Schifferli, D.M. FimH alleles direct preferential binding of Salmonella to distinct mammalian cells or to avian cells. Microbiology 2009, 155, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Hersh, D.; Monack, D.M.; Smith, M.R.; Ghori, N.; Falkow, S.; Zychlinsky, A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc. Natl. Acad. Sci. USA 1999, 96, 2396–2401. [Google Scholar] [CrossRef] [Green Version]
- Hermant, D.; Menard, R.; Arricau, N.; Parsot, C.; Popoff, M.Y. Functional Conservation of the Salmonella and Shigella Effectors of Entry into Epithelial-Cells. Mol. Microbiol. 1995, 17, 781–789. [Google Scholar] [CrossRef]
- Cunrath, O.; Meinel, D.M.; Maturana, P.; Fanous, J.; Buyck, J.M.; Saint Auguste, P.; Seth-Smith, H.M.B.; Korner, J.; Dehio, C.; Trebosc, V.; et al. Quantitative contribution of efflux to multi-drug resistance of clinical Escherichia coli and Pseudomonas aeruginosa strains. EBio Med. 2019, 41, 479–487. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.G.; Young, L.; Chuang, R.Y.; Venter, J.C.; Hutchison, C.A., 3rd; Smith, H.O. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Edwards, R.A.; Keller, L.H.; Schifferli, D.M. Improved allelic exchange vectors and their use to analyze 987P fimbria gene expression. Gene 1998, 207, 149–157. [Google Scholar] [CrossRef]
- Blomfield, I.C.; McClain, M.S.; Eisenstein, B.I. Type 1 fimbriae mutants of Escherichia coli K12: Characterization of recognized afimbriate strains and construction of new fim deletion mutants. Mol. Microbiol. 1991, 5, 1439–1445. [Google Scholar] [CrossRef] [Green Version]
- Harms, A.; Liesch, M.; Korner, J.; Quebatte, M.; Engel, P.; Dehio, C. A bacterial toxin-antitoxin module is the origin of inter-bacterial and inter-kingdom effectors of Bartonella. PLoS Genet. 2017, 13, e1007077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoiseth, S.K.; Stocker, B.D. Aromatic-dependent Salmonella typhimurium are nonvirulent and effective as live vaccines. Nature 1981, 291, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Khan, A.S.; Bayer, M.E.; Schifferli, D.M. Ordered translocation of 987P fimbrial subunits through the outer membrane of Escherichia coli. J. Bacteriol. 1995, 177, 3704–3713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nair, M.K.; De Masi, L.; Yue, M.; Galvan, E.M.; Chen, H.; Wang, F.; Schifferli, D.M. Adhesive properties of YapV and paralogous autotransporter proteins of Yersinia pestis. Infect. Immun. 2015, 83, 1809–1819. [Google Scholar] [CrossRef] [Green Version]
- Schifferli, D.M.; Alrutz, M.A. Permissive linker insertion sites in the outer membrane protein of 987P fimbriae of Escherichia coli. J. Bacteriol. 1994, 176, 1099–1110. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Chen, H.; Galván, E.M.; Lasaro, M.A.; Schifferli, D.M. Effects of Psa and F1 on the adhesive and invasive interactions of Yersinia pestis with human respiratory tract epithelial cells. Infect. Immun. 2006, 74, 5636–5644. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, C.E.; Kruczkiewicz, P.; Laing, C.R.; Lingohr, E.J.; Gannon, V.P.; Nash, J.H.; Taboada, E.N. The Salmonella In Silico Typing Resource (SISTR): An Open Web-Accessible Tool for Rapidly Typing and Subtyping Draft Salmonella Genome Assemblies. PLoS ONE 2016, 11, e0147101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Freddolino, P.L.; Zhang, Y. COFACTOR: Improved protein function prediction by combining structure, sequence and protein-protein interaction information. Nucleic Acids Res. 2017, 45, W291–W299. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef] [Green Version]
- Colovos, C.; Yeates, T.O. Verification of protein structures: Patterns of nonbonded atomic interactions. Protein Sci. 1993, 2, 1511–1519. [Google Scholar] [CrossRef] [Green Version]
- Goswami, N.; Hussain, M.I.; Borah, P. Molecular dynamics approach to probe the antigenicity of PagN—An outer membrane protein of Salmonella Typhi. J. Biomol. Struct. Dyn. 2018, 36, 2131–2146. [Google Scholar] [CrossRef] [PubMed]
- Yue, M.; Rankin, S.C.; Blanchet, R.T.; Nulton, J.D.; Edwards, R.A.; Schifferli, D.M. Diversification of the Salmonella fimbriae: A model of macro- and microevolution. PLoS ONE 2012, 7, e38596. [Google Scholar] [CrossRef] [PubMed]
- Hansmeier, N.; Miskiewicz, K.; Elpers, L.; Liss, V.; Hensel, M.; Sterzenbach, T. Functional expression of the entire adhesiome of Salmonella enterica serotype Typhimurium. Sci. Rep. 2017, 7, 10326. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.C.; Halang, P.; Fleury, C.; Jalalvand, F.; Morgelin, M.; Riesbeck, K. Haemophilus Protein F Orthologs of Pathogens Infecting the Airways: Exploiting Host Laminin at Heparin-Binding Sites for Maximal Adherence to Epithelial Cells. J. Infect. Dis. 2017, 216, 1303–1307. [Google Scholar] [CrossRef]
- Izquierdo, M.; Navarro-Garcia, F.; Nava-Acosta, R.; Nataro, J.P.; Ruiz-Perez, F.; Farfan, M.J. Identification of cell surface-exposed proteins involved in the fimbria-mediated adherence of enteroaggregative Escherichia coli to intestinal cells. Infect. Immun. 2014, 82, 1719–1724. [Google Scholar] [CrossRef] [Green Version]
- Samadder, P.; Xicohtencatl-Cortes, J.; Saldana, Z.; Jordan, D.; Tarr, P.I.; Kaper, J.B.; Giron, J.A. The Escherichia coli ycbQRST operon encodes fimbriae with laminin-binding and epithelial cell adherence properties in Shiga-toxigenic E. coli O157:H7. Environ. Microbiol. 2009, 11, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Gunn, J.S.; Belden, W.J.; Miller, S.I. Identification of PhoP-PhoQ activated genes within a duplicated region of the Salmonella typhimurium chromosome. Microb. Pathog. 1998, 25, 77–90. [Google Scholar] [CrossRef]
- Park, S.Y.; Groisman, E.A. Signal-specific temporal response by the Salmonella PhoP/PhoQ regulatory system. Mol. Microbiol. 2014, 91, 135–144. [Google Scholar] [CrossRef] [Green Version]
- Golubeva, Y.A.; Ellermeier, J.R.; Cott Chubiz, J.E.; Slauch, J.M. Intestinal Long-Chain Fatty Acids Act as a Direct Signal To Modulate Expression of the Salmonella Pathogenicity Island 1 Type III Secretion System. mBio 2016, 7, e02170-15. [Google Scholar] [CrossRef] [Green Version]
- Kouzi-Koliakos, K.; Koliakos, G.G.; Tsilibary, E.C.; Furcht, L.T.; Charonis, A.S. Mapping of three major heparin-binding sites on laminin and identification of a novel heparin-binding site on the B1 chain. J. Biol. Chem. 1989, 264, 17971–17978. [Google Scholar]
- Lambert, M.A.; Smith, S.G. The PagN protein mediates invasion via interaction with proteoglycan. FEMS Microbiol. Lett. 2009, 297, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Masi, L.; Yue, M.; Hu, C.; Rakov, A.V.; Rankin, S.C.; Schifferli, D.M. Cooperation of Adhesin Alleles in Salmonella-Host Tropism. mSphere 2017, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayden, H.S.; Matamouros, S.; Hager, K.R.; Brittnacher, M.J.; Rohmer, L.; Radey, M.C.; Weiss, E.J.; Kim, K.B.; Jacobs, M.A.; Sims-Day, E.H.; et al. Genomic Analysis of Salmonella enterica Serovar Typhimurium Characterizes Strain Diversity for Recent U.S. Salmonellosis Cases and Identifies Mutations Linked to Loss of Fitness under Nitrosative and Oxidative Stress. MBio 2016, 7, e00154. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Strain | Genus, Species, Serovar and/or Relevant Genotype | Source |
|---|---|---|
| AAEC189 | E. coli MM294 ∆lac recA endA ∆fim (rK− mK+) | [32] |
| BL21(DE3) | E. coli str. B F− ompT gal dcm lon hsdSB(rB−mB−) λ(DE3 [lacI lacUV5-T7p07 ind1 sam7 nin5]) [malB+]K-12(λS) | Novagen |
| JKE201 | E. coli MG1655 RP4-2-Tc::[ΔMu1::Δaac(3)IV::lacIq-ΔaphA-Δnic35-ΔMu2::zeo] ΔdapA::(erm-pir) ΔrecA ΔmcrA Δ(mrr-hsdRMS-mcrBC)) | [29,33] |
| SL1344 | S. enterica subsp. enterica serovar Typhimurium | [34] |
| S. Typhi | S. enterica subsp. enterica serovar Typhi 9,12,[Vi]:d:[Z66] | Salmonella Reference collection C (SARC) no2 |
| S. diarizonae | S. enterica subsp. diarizonae 50:k:z | SARC no7 |
| DMS1507 | S. enterica subsp. enterica serovar Typhimurium LB5010 sipB::aphA-3 | [27,28] |
| DMS1949 | SL1344 sipB ΔpagN | This study |
| Plasmid | Description | Source |
| pMG81 | Expression plasmid with tetR and tetA promoter upstream envZ, ApR | Mark Goulian |
| pRS1 | pMG81∆envZ | This study |
| pDMS1973 | S. Typhi pagN in pRS1 | This study |
| pDMS1974 | SL1344 pagN in pRS1 | This study |
| pDMS2062 | S. diarizonae pagN in pRS1 | This study |
| pDMS2081 | SL1344 pagND49E in pRS1 | This study |
| pDMS2082 | SL1344 pagND109Q in pRS1 | This study |
| pDMS2083 | SL1344 pagND109E in pRS1 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Hu, Q.; Dehinwal, R.; Rakov, A.V.; Grams, N.; Clemens, E.C.; Hofmann, J.; Okeke, I.N.; Schifferli, D.M. The Not so Good, the Bad and the Ugly: Differential Bacterial Adhesion and Invasion Mediated by Salmonella PagN Allelic Variants. Microorganisms 2020, 8, 489. https://doi.org/10.3390/microorganisms8040489
Wu Y, Hu Q, Dehinwal R, Rakov AV, Grams N, Clemens EC, Hofmann J, Okeke IN, Schifferli DM. The Not so Good, the Bad and the Ugly: Differential Bacterial Adhesion and Invasion Mediated by Salmonella PagN Allelic Variants. Microorganisms. 2020; 8(4):489. https://doi.org/10.3390/microorganisms8040489
Chicago/Turabian StyleWu, Yanping, Qiaoyun Hu, Ruchika Dehinwal, Alexey V. Rakov, Nicholas Grams, Erin C. Clemens, Jennifer Hofmann, Iruka N. Okeke, and Dieter M. Schifferli. 2020. "The Not so Good, the Bad and the Ugly: Differential Bacterial Adhesion and Invasion Mediated by Salmonella PagN Allelic Variants" Microorganisms 8, no. 4: 489. https://doi.org/10.3390/microorganisms8040489






