Comparison of the Vitreous Fluid Bacterial Microbiomes between Individuals with Post Fever Retinitis and Healthy Controls
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. DNA Extraction and Sequencing of the Samples
2.3. Whole Metagenome Analysis
2.4. Statistical Analysis
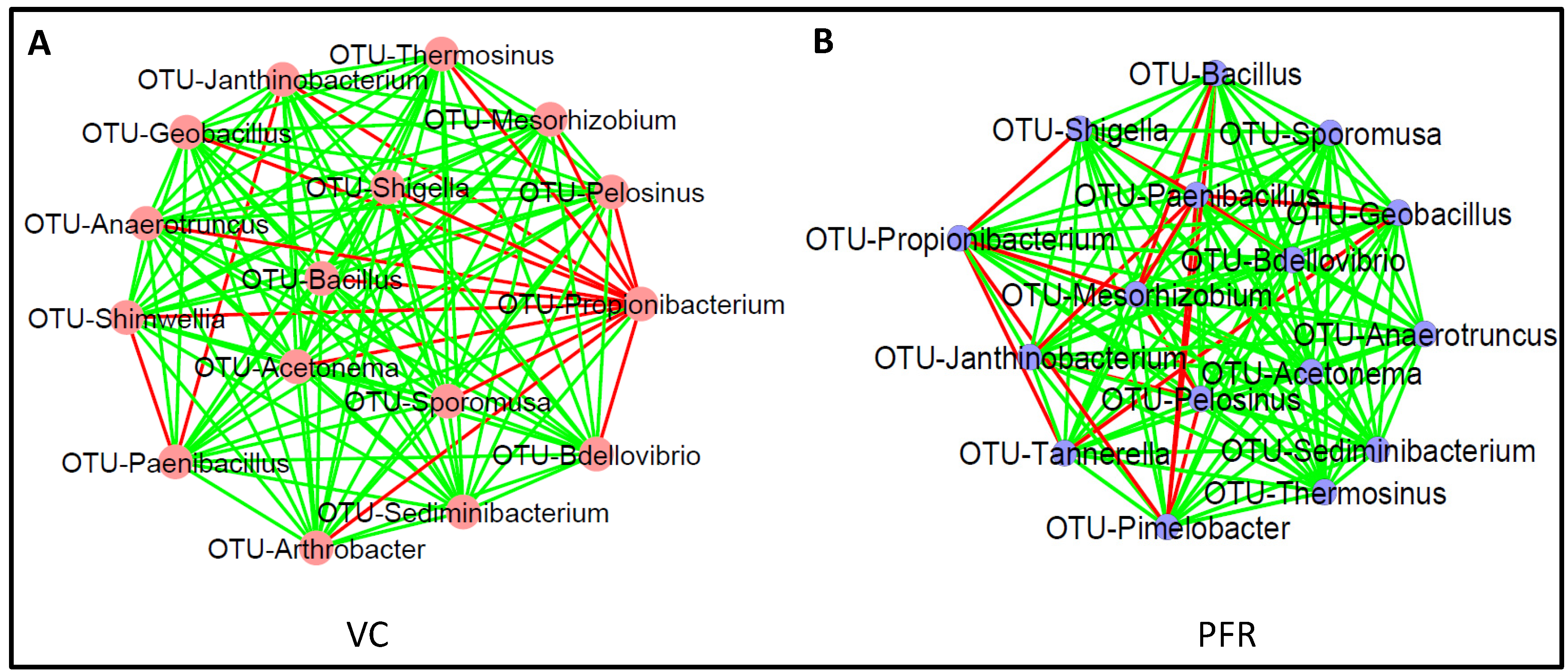
2.5. Correlation Network Analysis of Bacterial Genera
3. Results
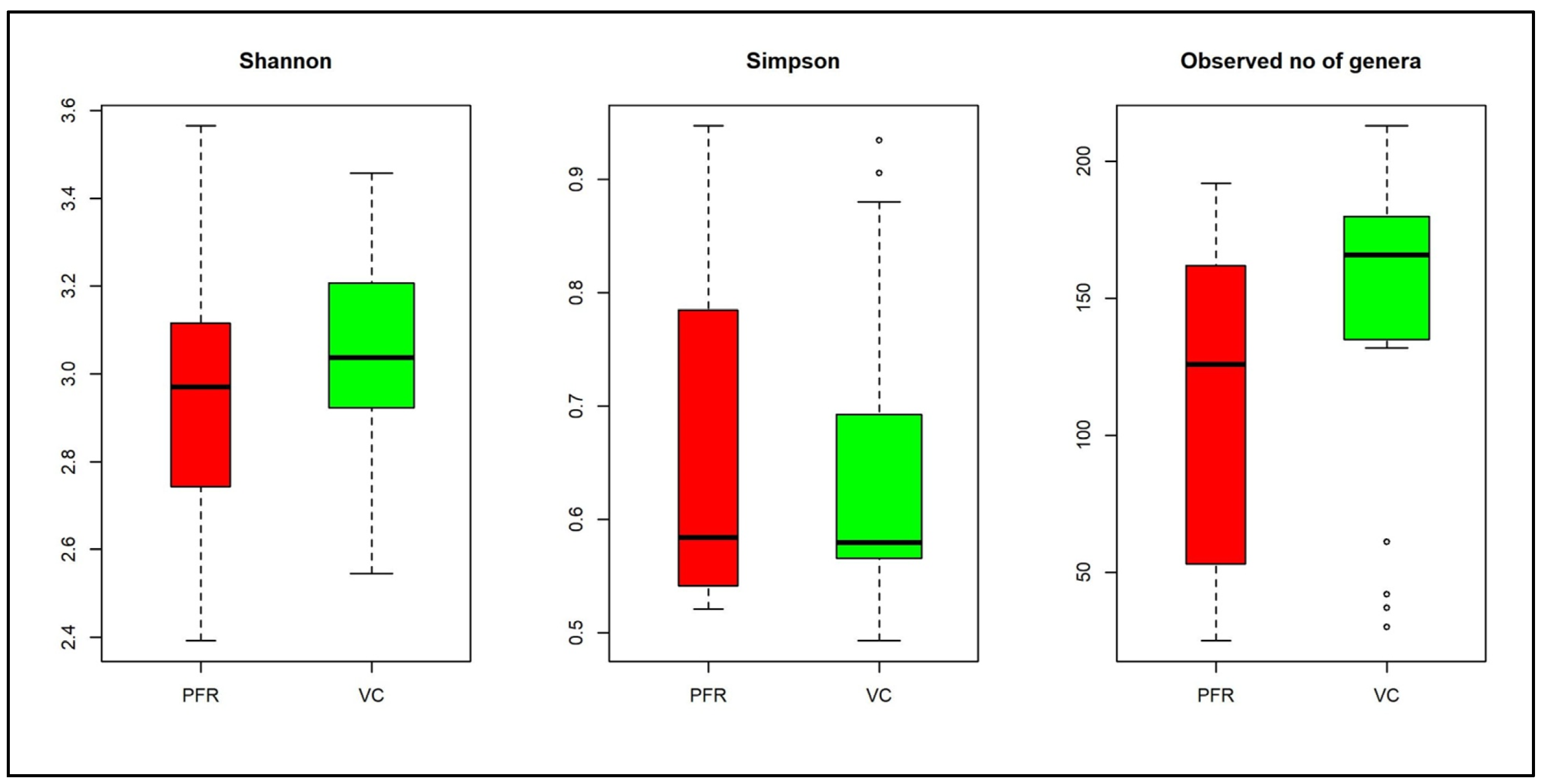
3.1. Bacterial Microbiomes in the Vitreous of Control and Postfever Retinitis Groups
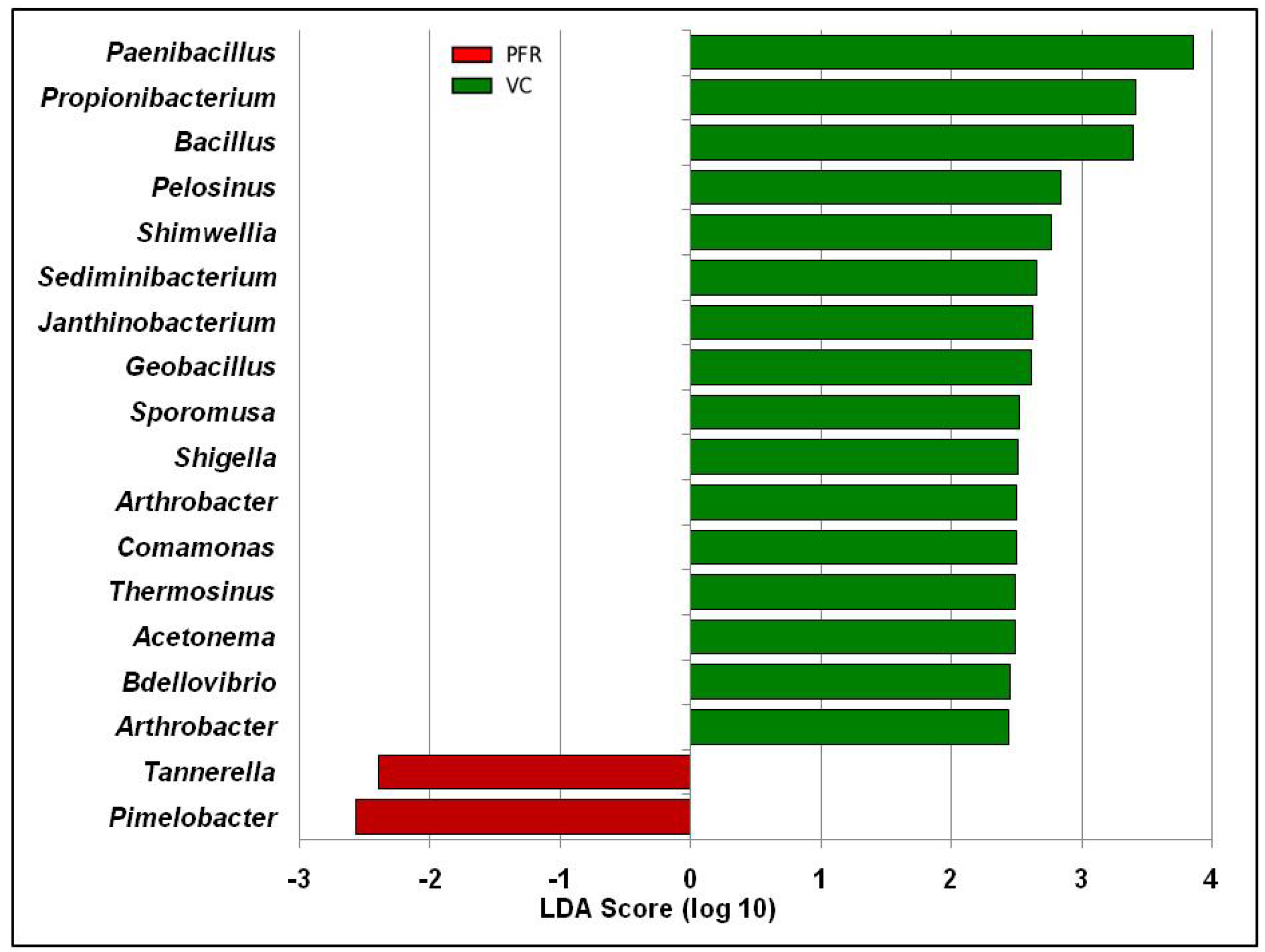
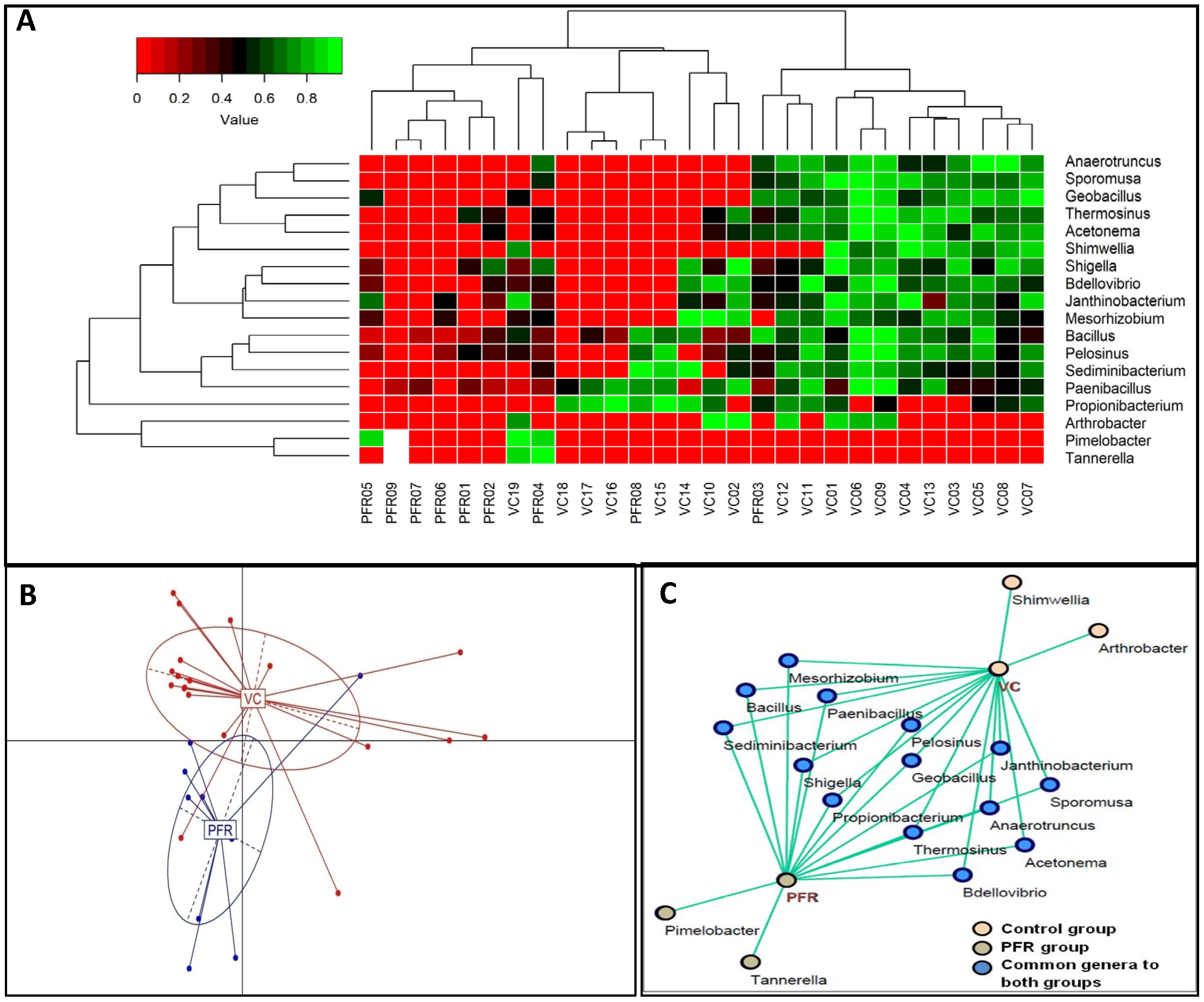
3.2. Differentially Abundant Bacterial Genera in the Vitreous Fluid of Controls and Postfever Retinitis Individuals
3.3. Interactive Network of Bacterial Genera in Control and PFR Group
3.4. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathways Analysis
4. Discussion
5. Conclusions
- (i)
- Demonstrates that the bacterial microbiomes of vitreous fluid from VC and PFR individuals could be discriminated at the genera level.
- (ii)
- The vitreous fluid of VC individuals showed increase in abundance of anti-inflammatory and antimicrobial genera and decrease in proinflammatory genera.
- (iii)
- The vitreous fluid of PFR individuals showed decrease in abundance of anti-inflammatory and increase in proinflammatory genera.
- (iv)
- KEGG pathway analysis identified significant increase in signalling pathways in PFR group that are associated with inflammatory cytokines.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Panchal, B. Commentary: Post fever retinitis and vasculitis: A morphological conundrum. Indian J. Ophthalmol. 2018, 66, 1844. [Google Scholar] [CrossRef]
- Vishwanath, S.; Badami, K.; Sriprakash, K.S.; Sujatha, B.L.; Shashidhar, S.D.; Shilpa, Y.D. Post-fever retinitis: A single center experience from south India. Int. Ophthalmol. 2014, 34, 851–857. [Google Scholar] [CrossRef]
- Chan, D.P.; Teoh, S.C.; Tan, C.S.; Nah, G.K.; Rajagopalan, R.; Prabhakaragupta, M.K.; Chee, C.K.; Lim, T.H.; Goh, K.Y.; Eye Institute Dengue-Related Ophthalmic Complications Workgroup. Ophthalmic complications of dengue. Emerg. Infect. Dis. 2006, 12, 285–289. [Google Scholar] [CrossRef]
- Chawla, R.; Pundlik, G.A.; Chaudhry, R.; Thakur, C. Rickettsial retinitis: Direct bacterial infection or an immune-mediated response? Indian J. Ophthalmol. 2017, 65, 1038–1041. [Google Scholar] [CrossRef]
- Duke-Elder, S.; Perkins, E.S. ’System of Ophthalmology’, Diseases of the Uveal Tract; Henry Kimpton: London, UK, 1968; Volume 9. [Google Scholar]
- Shah, S.M.; Howard, R.S.; Sarkies, N.J.; Graham, E.M. Tuberculosis presenting as retinal vasculitis. J. R. Soc. Med. 1988, 81, 232–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velaitham, P.; Vijayasingham, N. Central retinal vein occlusion concomitant with dengue fever. Int. J. Retin. Vitr. 2016, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, Z.; Zhu, M.; Zhang, Y. Effects of the probiotic Arthrobacter sp. CW9 on the survival and immune status of white shrimp (Penaeus vannamei). Lett. Appl. Microbiol. 2014, 58, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Taravati, P.; Deborah, L.; Russell, N. Van Gelder Role of molecular diagnostics in ocular microbiology. Curr. Ophthalmol. Rep. 2013, 1, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doan, T.; Akileswaran, L.; Andersen, D.; Johnson, B.; Ko, N.; Shrestha, A.; Shestopalov, V.; Lee, C.S.; Lee, A.Y.; Van Gelder, R.N. Paucibacterial Microbiome and Resident DNA Virome of the Healthy Conjunctiva. Invest. Ophthalmol. Vis. Sci. 2016, 57, 5116–5126. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Hu, H.; Kang, Y.; Chen, W.; Fang, W.; Wang, K.; Zhang, Q.; Fu, A.; Zhou, S.; Cheng, C.; et al. Identification of pathogens in culture-negative infective endocarditis cases by metagenomic analysis. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 43. [Google Scholar] [CrossRef] [Green Version]
- Jayasudha, R.; Chakravarthy, S.K.; Prashanthi, G.S.; Sharma, S.; Garg, P.; Murthy, S.I.; Shivaji, S. Alterations in gut bacterial and fungal microbiomes are associated with bacterial Keratitis, an inflammatory disease of the human eye. J. Biosci. 2018, 43, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using Cytoscape. F1000Research 2016, 5, 1519. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Prashanthi, G.S.; Jayasudha, R.; Chakravarthy, S.K.; Padakandla, S.R.; SaiAbhilash, C.R.; Sharma, S.; Bagga, B.; Murthy, S.I.; Garg, P.; Shivaji, S. Alterations in the Ocular Surface Fungal Microbiome in Fungal Keratitis Patients. Microorganisms 2019, 7, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shivaji, S.; Jayasudha, R.; Prashanthi, G.S.; Chakravarthy, K.S.; Sharma, S. The Human Ocular Surface Fungal Microbiome. Invest. Ophthalmol. Vis. Sci. 2019, 60, 451–459. [Google Scholar] [CrossRef] [Green Version]
- Cavuoto, K.M.; Banerjee, S.; Miller, D.; Galor, A. Composition and Comparison of the Ocular Surface Microbiome in Infants and Older Children. Transl. Vis. Sci. Technol. 2018, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Ozkan, J.; Willcox, M.; Wemheuer, B.; Wilcsek, G.; Coroneo, M.; Thomas, T. Biogeography of the human ocular microbiota. Ocul. Surf. 2019, 17, 111–118. [Google Scholar] [CrossRef]
- Kirstahler, P.; Bjerrum, S.S.; Friis-Moller, A.; la Cour, M.; Aarestrup, F.M.; Westh, H.; Pamp, S.J. Genomics-Based Identification of Microorganisms in Human Ocular Body Fluid. Sci. Rep. 2018, 8, 4126. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, D.; Joseph, J.; Chakrabarti, M.; Sharma, S.; Jayasudha, R.; Sama, K.C.; Sontam, B.; Tyagi, M.; Narayanan, R.; Shivaji, S. New insights into culture negative endophthalmitis by unbiased next generation sequencing. Sci. Rep. 2019, 9, 844. [Google Scholar] [CrossRef] [Green Version]
- Wen, X.; Hu, X.; Miao, L.; Ge, X.; Deng, Y.; Bible, P.W.; Wei, L. Epigenetics, microbiota, and intraocular inflammation: New paradigms of immune regulation in the eye. Prog. Retin. Eye Res. 2018, 64, 84–95. [Google Scholar] [CrossRef]
- Walujkar, S.A.; Dhotre, D.P.; Marathe, N.P.; Lawate, P.S.; Bharadwaj, R.S.; Shouche, Y.S. Characterization of bacterial community shift in human Ulcerative Colitis patients revealed by Illumina based 16S rRNA gene amplicon sequencing. Gut Pathog. 2014, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Patrone, V.; Puglisi, E.; Cardinali, M.; Schnitzler, T.S.; Svegliati, S.; Festa, A.; Gabrielli, A.; Morelli, L. Gut microbiota profile in systemic sclerosis patients with and without clinical evidence of gastrointestinal involvement. Sci. Rep. 2017, 7, 14874. [Google Scholar] [CrossRef] [Green Version]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Ranjith, K.; Dutta, A.; Pinna, N.K.; Mande, S.S.; Sharma, S.; Garg, P.; Murthy, S.I.; Shivaji, S. Alterations in the gut bacterial microbiome in fungal Keratitis patients. PLoS ONE 2018, 13, e0199640. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ye, Z.; Cao, Q.; Su, G.; Wang, Q.; Deng, J.; Zhou, C.; Kijlstra, A.; Yang, P. Gut Microbiota Composition and Fecal Metabolic Phenotype in Patients With Acute Anterior Uveitis. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1523–1531. [Google Scholar] [CrossRef] [Green Version]
- Jayasudha, R.; Kalyana Chakravarthy, S.; Sai Prashanthi, G.; Sharma, S.; Tyagi, M.; Shivaji, S. Implicating Dysbiosis of the Gut Fungal Microbiome in Uveitis, an Inflammatory Disease of the Eye. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Kalyana Chakravarthy, S.; Jayasudha, R.; Sai Prashanthi, G.; Ali, M.H.; Sharma, S.; Tyagi, M.; Shivaji, S. Dysbiosis in the Gut Bacterial Microbiome of Patients with Uveitis, an Inflammatory Disease of the Eye. Indian J. Microbiol. 2018, 58, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Caspi, R.R. Microbiome and Autoimmune Uveitis. Front. Immunol. 2019, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Rowan, S.; Taylor, A. Gut microbiota modify risk for dietary glycemia-induced age-related macular degeneration. Gut Microbes 2018, 9, 452–457. [Google Scholar] [CrossRef]
- Zinkernagel, M.S.; Zysset-Burri, D.C.; Keller, I.; Berger, L.E.; Leichtle, A.B.; Largiader, C.R.; Fiedler, G.M.; Wolf, S. Association of the Intestinal Microbiome with the Development of Neovascular Age-Related Macular Degeneration. Sci. Rep. 2017, 7, 40826. [Google Scholar] [CrossRef] [Green Version]
- Mendez, R.; Watane, A.; Farhangi, M.; Cavuoto, K.M.; Leith, T.; Budree, S.; Galor, A.; Banerjee, S. Gut microbial dysbiosis in individuals with Sjögren’s disease. bioRxiv 2019. [Google Scholar] [CrossRef]
- Ge, C.; Wei, C.; Yang, B.X.; Cheng, J.; Huang, Y.S. Conjunctival microbiome changes associated with fungal keratitis: Metagenomic analysis. Int. J. Ophthalmol. 2019, 12, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, J.; Nielsen, S.; Diez-Vives, C.; Coroneo, M.; Thomas, T.; Willcox, M. Temporal Stability and Composition of the Ocular Surface Microbiome. Sci. Rep. 2017, 7, 9880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A. Virulence mechanisms of Tannerella forsythia. Periodontol. 2000 2010, 54, 106–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ziganshina, E.E.; Sharifullina, D.M.; Lozhkin, A.P.; Khayrullin, R.N.; Ignatyev, I.M.; Ziganshin, A.M. Bacterial Communities Associated with Atherosclerotic Plaques from Russian Individuals with Atherosclerosis. PLoS ONE 2016, 11, e0164836. [Google Scholar] [CrossRef] [PubMed]
- Horner-Devine, M.C.; Silver, J.M.; Leibold, M.A.; Bohannan, B.J.; Colwell, R.K.; Fuhrman, J.A.; Green, J.L.; Kuske, C.R.; Martiny, J.B.; Muyzer, G.; et al. A comparison of taxon co-occurrence patterns for macro- and microorganisms. Ecology 2007, 88, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achermann, Y.; Goldstein, E.J.; Coenye, T.; Shirtliff, M.E. Propionibacterium acnes: From commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 2014, 27, 419–440. [Google Scholar] [CrossRef] [Green Version]
- Graham, G.M.; Farrar, M.D.; Cruse-Sawyer, J.E.; Holland, K.T.; Ingham, E. Proinflammatory cytokine production by human keratinocytes stimulated with Propionibacterium acnes and P. acnes GroEL. Br. J. Dermatol. 2004, 150, 421–428. [Google Scholar] [CrossRef]
- Saffra, N.; Moriarty, E.; Milman, T. Bilateral sequential Propionibacterium acnes exogenous endophthalmitis. J. Ophthalmic. Inflamm. Infect. 2016, 6, 17. [Google Scholar] [CrossRef] [Green Version]
- Kong, C.; Gao, R.; Yan, X.; Huang, L.; Qin, H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition 2019, 60, 175–184. [Google Scholar] [CrossRef]
- Sumi, C.D.; Yang, B.W.; Yeo, I.C.; Hahm, Y.T. Antimicrobial peptides of the genus Bacillus: A new era for antibiotics. Can. J. Microbiol. 2015, 61, 93–103. [Google Scholar] [CrossRef]
- Harini, K.; Ajila, V.; Hegde, S. Bdellovibrio bacteriovorus: A future antimicrobial agent? J. Indian Soc. Periodontol. 2013, 17, 823–825. [Google Scholar] [CrossRef] [PubMed]
- Rebollar, E.A.; Simonetti, S.J.; Shoemaker, W.R.; Harris, R.N. Direct and Indirect Horizontal Transmission of the Antifungal Probiotic Bacterium Janthinobacterium lividum on Green Frog (Lithobates clamitans) Tadpoles. Appl. Environ. Microbiol. 2016, 82, 2457–2466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cochrane, S.A.; Vederas, J.C. Lipopeptides from Bacillus and Paenibacillus spp.: A Gold Mine of Antibiotic Candidates. Med. Res. Rev. 2016, 36, 4–31. [Google Scholar] [CrossRef]
- Killackey, S.A.; Sorbara, M.T.; Girardin, S.E. Cellular Aspects of Shigella Pathogenesis: Focus on the Manipulation of Host Cell Processes. Front. Cell. Infect. Microbiol. 2016, 6, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brzuszkiewicz, E.; Waschkowitz, T.; Wiezer, A.; Daniel, R. Complete genome sequence of the B12-producing Shimwellia blattae strain DSM 4481, isolated from a cockroach. J. Bacteriol. 2012, 194, 4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eş, I.; Khaneghah, A.; Hashemi, M.; Koubaa, S. Current advances in biological production of propionic acid. Biotechnol. Lett. 2017, 39, 635–645. [Google Scholar]
- Ren, W.; Rajendran, R.; Zhao, Y.; Tan, B.; Wu, G.; Bazer, F.W.; Zhu, G.; Peng, Y.; Huang, X.; Deng, J.; et al. Amino Acids As Mediators of Metabolic Cross Talk between Host and Pathogen. Front. Immunol. 2018, 9, 319. [Google Scholar] [CrossRef]
- Samartzidou, H.; Mehrazin, M.; Xu, Z.; Benedik, M.J.; Delcour, A.H. Cadaverine inhibition of porin plays a role in cell survival at acidic pH. J. Bacteriol. 2003, 185, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Marcinkiewicz, J.; Kontny, E. Taurine and inflammatory diseases. Amino Acids 2014, 46, 7–20. [Google Scholar] [CrossRef] [Green Version]
- Kerstholt, M.; Vrijmoeth, H.; Lachmandas, E.; Oosting, M.; Lupse, M.; Flonta, M.; Dinarello, C.A.; Netea, M.G.; Joosten, L.A.B. Role of glutathione metabolism in host defense against Borrelia burgdorferi infection. Proc. Natl. Acad. Sci. USA 2018, 115, E2320–E2328. [Google Scholar] [CrossRef] [Green Version]
- Van Laarhoven, A.; Dian, S.; Aguirre-Gamboa, R.; Avila-Pacheco, J.; Ricano-Ponce, I.; Ruesen, C.; Annisa, J.; Koeken, V.; Chaidir, L.; Li, Y.; et al. Cerebral tryptophan metabolism and outcome of tuberculous meningitis: An observational cohort study. Lancet Infect. Dis. 2018, 18, 526–535. [Google Scholar] [CrossRef]
- Li, S.; Wang, Z.; Li, F.; Yu, K.; Xiang, J. A Novel Vascular Endothelial Growth Factor Receptor Participates in White Spot Syndrome Virus Infection in Litopenaeus vannamei. Front. Immunol. 2017, 8, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Gui, W.; Niu, F.; Chong, S. The MAPK Signaling Pathways as a Novel Way in Regulation and Treatment of Parasitic Diseases. Diseases 2019, 7, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slanina, H.; Mündlein, S.; Hebling, S.; Schubert-Unkmeir, A. Role of epidermal growth factor receptor signaling in the interaction of Neisseria meningitidis with endothelial cells. Infect. Immun. 2014, 82, 1243–1245. [Google Scholar] [CrossRef] [Green Version]



| Samples | Total/ Average | Reads in Millions (Q > 25) | Reads Assigned to Bacteria |
|---|---|---|---|
| VC | Total | 79.24 | 858,444 |
| Average | 4.17 | 45,181.3 | |
| PFR | Total | 47.4 | 282,642 |
| Average | 5.27 | 31,404.7 | |
| VC + PFR | Total | 126.64 | 1,141,086 |
| Average | 4.52 | 40,753 |
| Genera | VC * | PFR * | p Value (Wilcoxon) |
|---|---|---|---|
| Paenibacillus | 3.466785 | 2.272994 | 0.002 |
| Bacillus | 1.423273 | 0.941839 | 0.032 |
| Propionibacterium | 0.567522 | 0.116189 | 0.038 |
| Pelosinus | 0.278704 | 0.159072 | 0.048 |
| Shigella | 0.096847 | 0.042937 | 0.041 |
| Sediminibacterium | 0.094356 | 0.028078 | 0.033 |
| Janthinobacterium | 0.050997 | 0.018052 | 0.019 |
| Thermosinus | 0.040074 | 0.012004 | 0.022 |
| Bdellovibrio | 0.03733 | 0.0074 | 0.005 |
| Acetonema | 0.034731 | 0.010722 | 0.024 |
| Sporomusa | 0.035308 | 0.006305 | 0.028 |
| Mesorhizobium | 0.030874 | 0.004932 | 0.003 |
| Anaerotruncus | 0.02709 | 0.009003 | 0.048 |
| Geobacillus | 0.018645 | 0.004591 | 0.038 |
| Shimwellia | 0.011266 | 0 | 0.011 |
| Arthrobacter | 0.008512 | 0 | 0.045 |
| Tannerella | 0 | 0.00896 | 0.042 |
| Pimelobacter | 0 | 0.002584 | 0.01 |
| Genera | Function | Abundance Increased or Decreased in Vitreous of VC or PFR | References |
|---|---|---|---|
| Acetonema | Unknown | Decreased in PFR | - |
| Anaerotruncus | Probiotic | Decreased in PFR | [40] |
| Arthrobacter | Probiotic | Decreased in PFR | [8] |
| Bacillus | Antimicrobial peptides | Decreased in PFR | [41] |
| Bdellovibrio | Antimicrobial | Decreased in PFR | [42] |
| Geobacillus | Unknown | Decreased in PFR | - |
| Janthinobacterium | Antibacterial, fungal and viral (violacin)/Probiotic | Decreased in PFR | [43] |
| Mesorhizobium | Unknown | Decreased in PFR | - |
| Paenibacillus | Antimicrobial lipopeptides | Decreased in PFR | [44] |
| Pelosinus | Unknown | Decreased in PFR | - |
| Pimelobacter | Unknown | Increased in PFR | - |
| Propionibacterium | Proinflammatory | Decreased in PFR | [38] |
| Sediminibacterium | Unknown | Decreased in PFR | - |
| Shigella | Proinflammatory | Decreased in PFR | [45] |
| Shimwellia | Probiotic | Decreased in PFR | [46] |
| Sporomusa | Antimicrobial and anti-inflammatory | Decreased in PFR | [47] |
| Tannerella | Proinflammatory | Increased in PFR | [34] |
| Thermosinus | Unknown | Decreased in PFR | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arunasri, K.; Mahesh, M.; Sai Prashanthi, G.; Jayasudha, R.; Kalyana Chakravarthy, S.; Tyagi, M.; Pappuru, R.R.; Shivaji, S. Comparison of the Vitreous Fluid Bacterial Microbiomes between Individuals with Post Fever Retinitis and Healthy Controls. Microorganisms 2020, 8, 751. https://doi.org/10.3390/microorganisms8050751
Arunasri K, Mahesh M, Sai Prashanthi G, Jayasudha R, Kalyana Chakravarthy S, Tyagi M, Pappuru RR, Shivaji S. Comparison of the Vitreous Fluid Bacterial Microbiomes between Individuals with Post Fever Retinitis and Healthy Controls. Microorganisms. 2020; 8(5):751. https://doi.org/10.3390/microorganisms8050751
Chicago/Turabian StyleArunasri, Kotakonda, Malleswarapu Mahesh, Gumpili Sai Prashanthi, Rajagopalaboopathi Jayasudha, Sama Kalyana Chakravarthy, Mudit Tyagi, Rajeev R. Pappuru, and Sisinthy Shivaji. 2020. "Comparison of the Vitreous Fluid Bacterial Microbiomes between Individuals with Post Fever Retinitis and Healthy Controls" Microorganisms 8, no. 5: 751. https://doi.org/10.3390/microorganisms8050751
APA StyleArunasri, K., Mahesh, M., Sai Prashanthi, G., Jayasudha, R., Kalyana Chakravarthy, S., Tyagi, M., Pappuru, R. R., & Shivaji, S. (2020). Comparison of the Vitreous Fluid Bacterial Microbiomes between Individuals with Post Fever Retinitis and Healthy Controls. Microorganisms, 8(5), 751. https://doi.org/10.3390/microorganisms8050751






