First Detection of Paralytic Shellfish Toxins from Alexandrium pacificum above the Regulatory Limit in Blue Mussels (Mytilus galloprovincialis) in New South Wales, Australia
Abstract
1. Introduction
2. Materials and Methods
2.1. Water and Shellfish Sampling
2.2. Alexandrium Isolation and Culture Maintenance
2.3. Species Identification and Enumeration
2.4. Molecular Characterisation
2.4.1. Amplicon Sequencing and qPCR
2.4.2. Bioinformatic Analysis of Amplicon Data
2.4.3. DNA Extraction, PCR Amplification and DNA Sequencing
2.4.4. Sequence Analysis and Phylogenetic Reconstruction
2.5. Biotoxin Analysis
2.5.1. Biotoxin Testing of Shellfish (Mussel) Samples from Twofold Bay
2.5.2. Toxin Profile Determination of Alexandrium Culture
2.6. Oceanographic and Water Mass Characteristics
3. Results
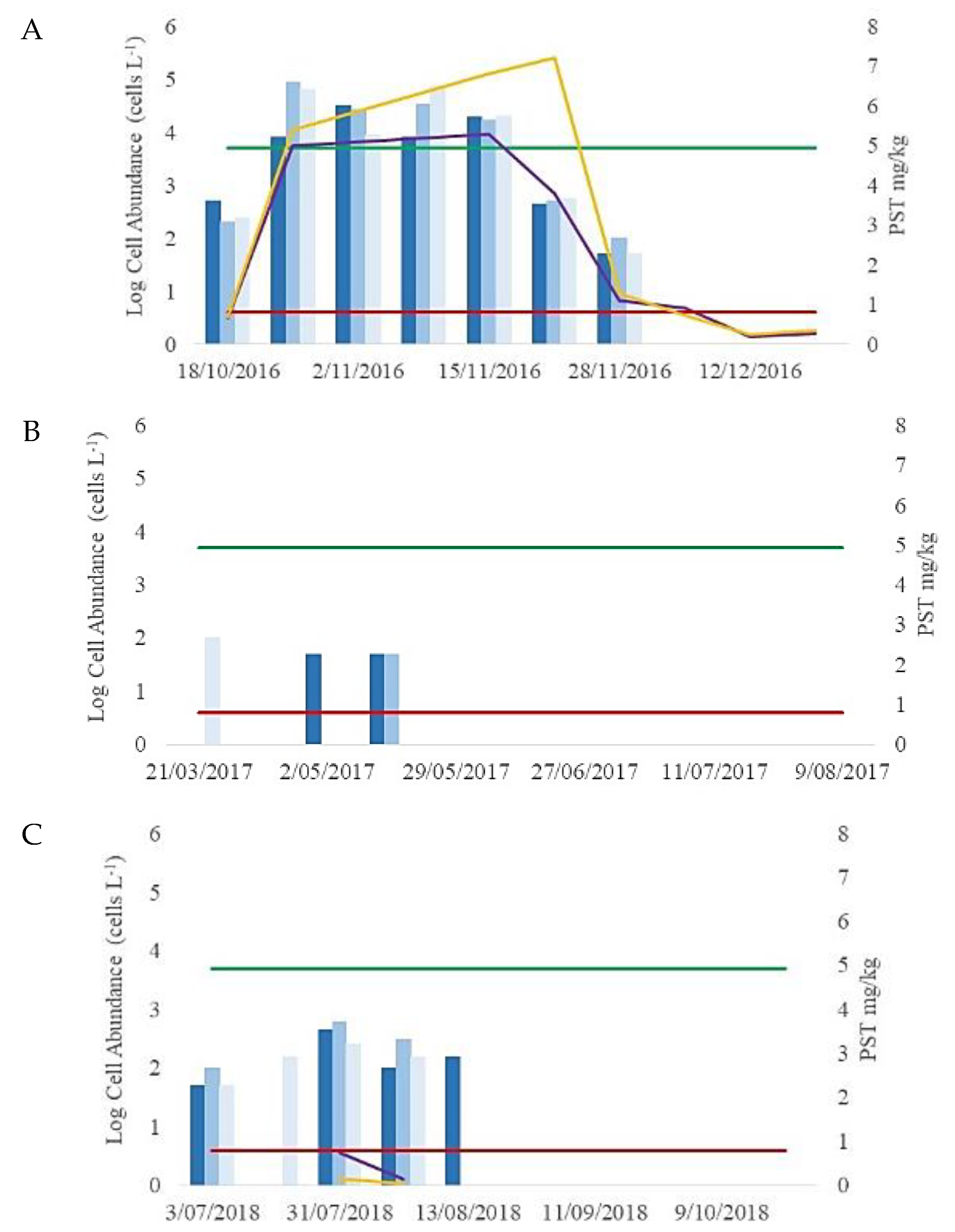
3.1. Initial Light Microscope Identification and Cell Abundances (2016–2018)
3.2. Mussel Toxin Analysis (2016–2018)
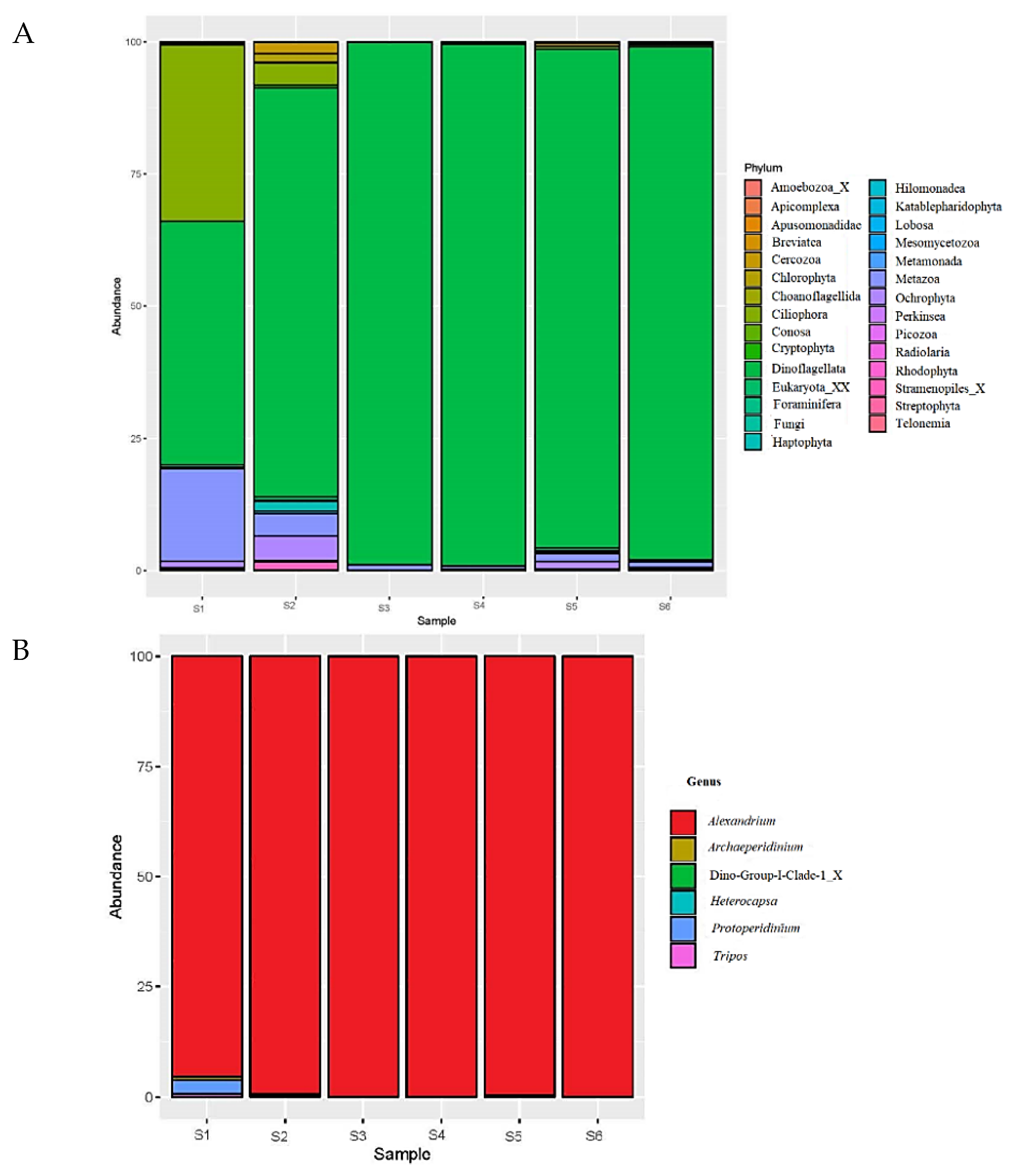
3.3. Amplicon Sequencing and qPCR (2016)
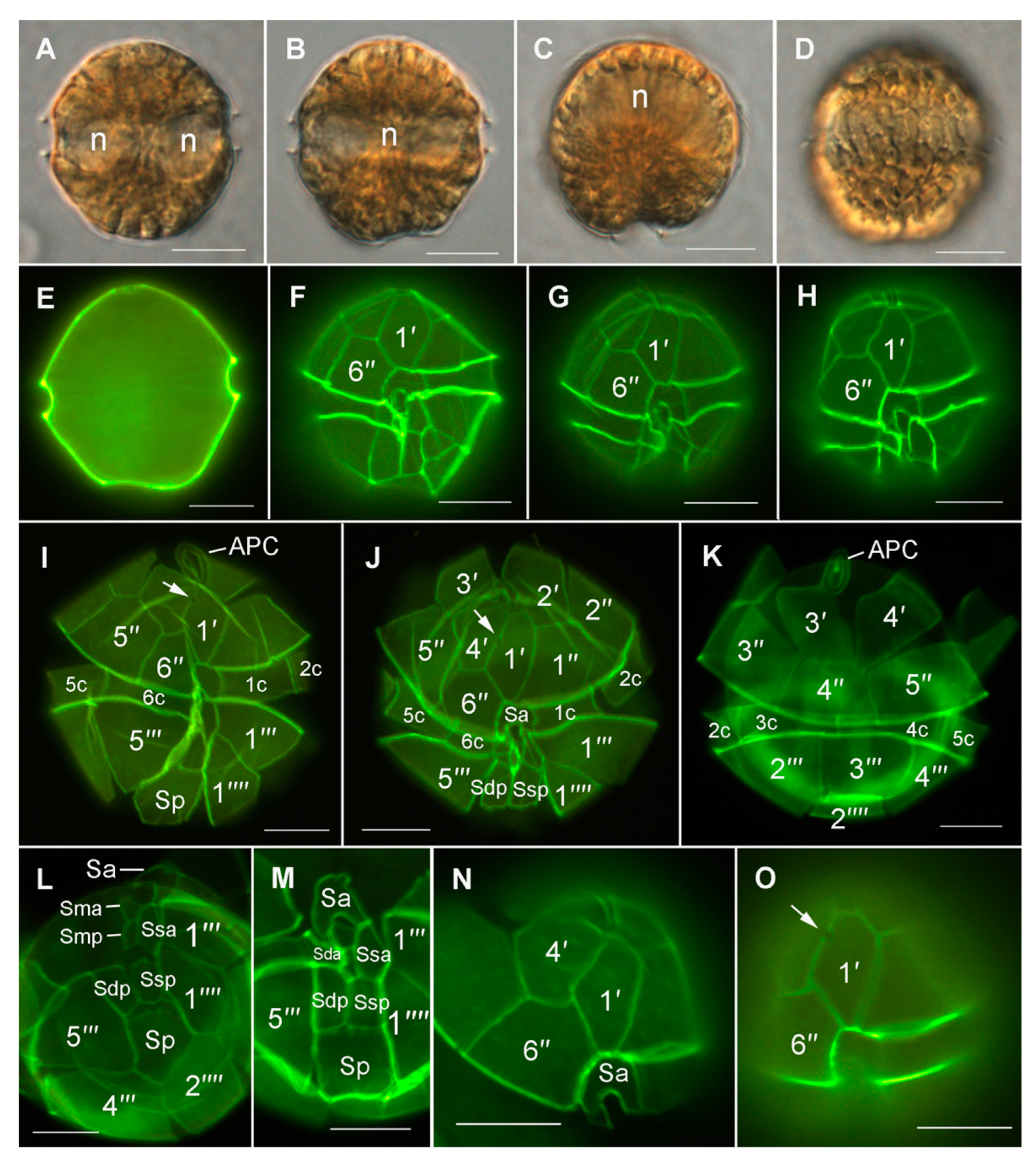
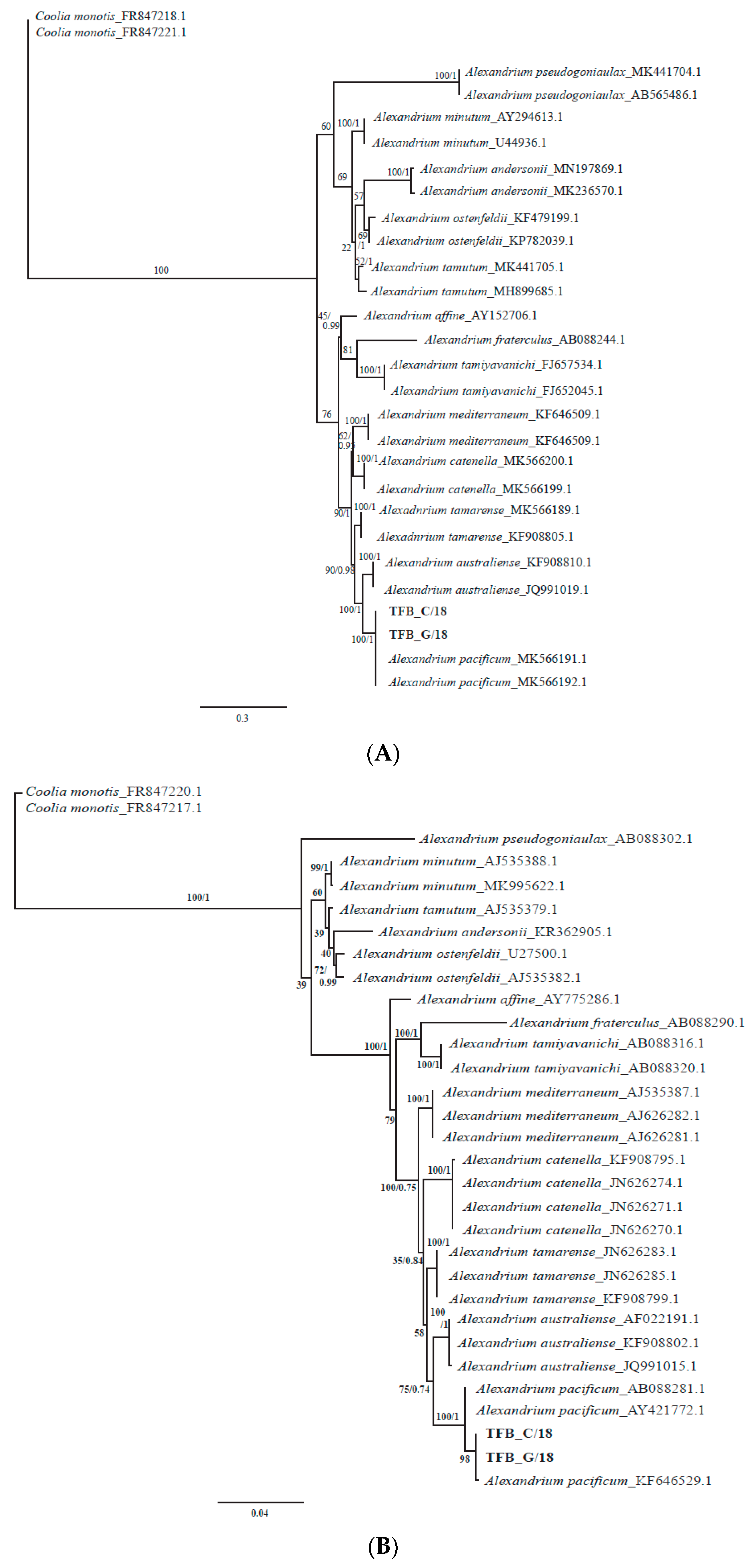
3.4. Morphological and Phylogenetic Analysis of Cultured Strains
3.5. Toxin Profile of Alexandrium pacificum Strains
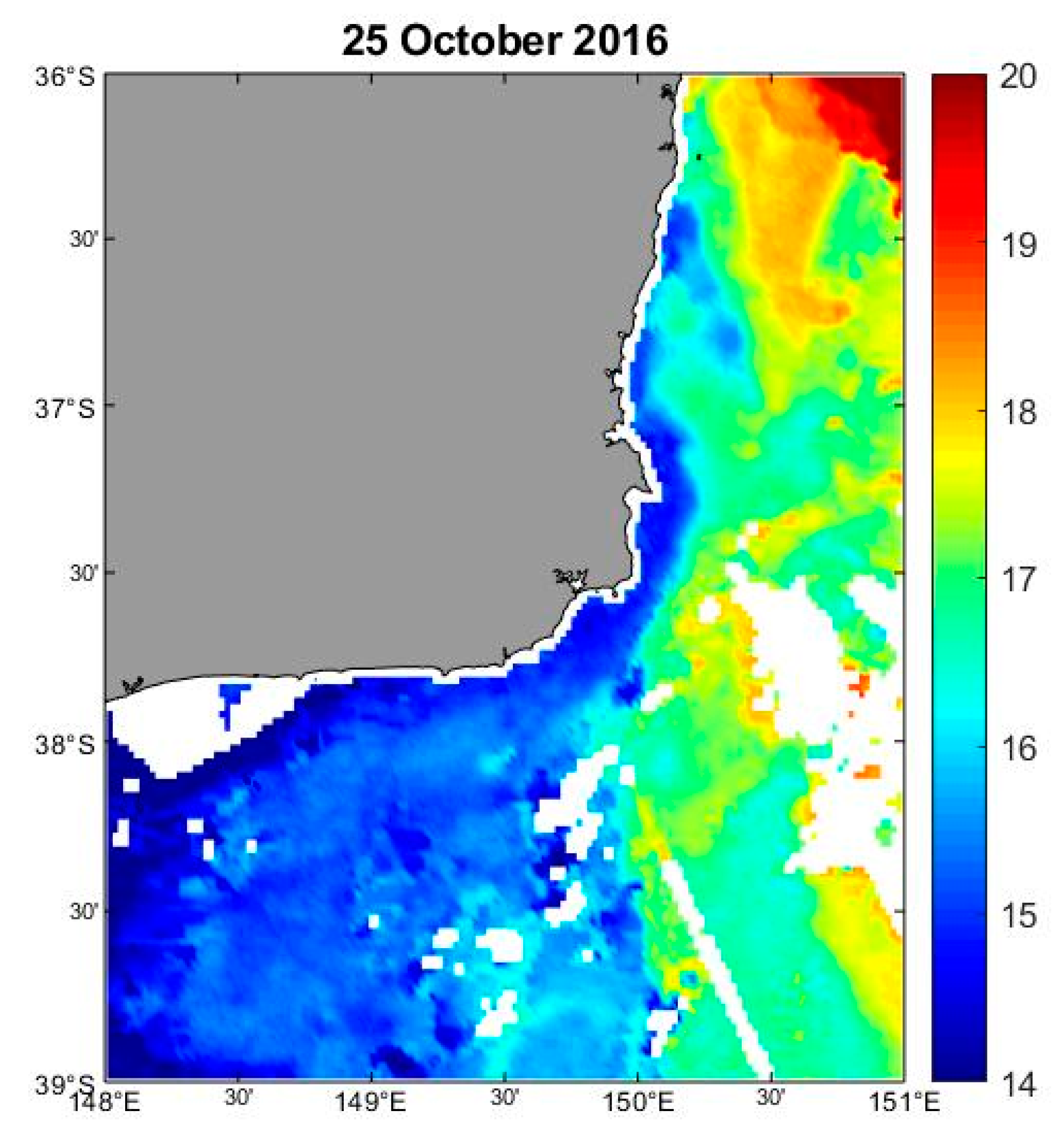
3.6. Oceanographic and Water Mass Characteristics
4. Discussion
4.1. The 2016–2018 Alexandrium Bloom Events
4.2. Alexandrium pacificum Species Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ajani, P.A.; Harwood, T.; Murray, S.A. Recent Trends in Marine Phycotoxins from Australian Coastal Waters. Mar. Drugs 2017, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.Y. Paralytic shellfish poisoning. In Algal Toxins in Seafood and Drinking Water; Falconer, I.R., Ed.; University of Adelaide: Adelaide, Australia, 1993; Volume 75, p. 86. [Google Scholar]
- Deeds, J.R.; Landsberg, J.H.; Etheridge, S.M.; Pitcher, G.C.; Longan, S.W. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 2008, 6, 308–348. [Google Scholar] [CrossRef] [PubMed]
- Oshima, Y.; Blackburn, S.I.; Hallegraeff, G.M. Comparative study on paralytic shellfish toxin profiles of the dinoflagellate Gymnodinium catenatum from three different countries. Mar. Boil. 1993, 116, 471–476. [Google Scholar] [CrossRef]
- Usup, G.; Kulis, D.; Anderson, N.M. Growth and toxin production of the toxic dinoflagellatePyrodinium bahamense var.compressum in laboratory cultures. Nat. Toxins 1994, 2, 254–262. [Google Scholar] [CrossRef]
- Landsberg, J.H.; Hall, S.; Johannessen, J.N.; White, K.D.; Conrad, S.M.; Abbott, J.P.; Flewelling, L.J.; Richardson, R.W.; Dickey, R.W.; Jester, E.L.; et al. Saxitoxin Puffer Fish Poisoning in the United States, with the First Report of Pyrodinium bahamense as the Putative Toxin Source. Environ. Health Perspect. 2006, 114, 1502–1507. [Google Scholar] [CrossRef]
- Anderson, N.M. Bloom dynamics of toxic Alexandrium species in the northeastern U.S. Limnol. Oceanogr. 1997, 42, 1009–1022. [Google Scholar] [CrossRef]
- Townsend, D.W.; Pettigrew, N.R.; Thomas, A.C. Offshore blooms of the red tide dinoflagellate, Alexandrium sp., in the Gulf of Maine. Cont. Shelf Res. 2001, 21, 347–369. [Google Scholar] [CrossRef]
- Taylor, F.J.R.; Fukuyo, Y.; Larsen, J.; Hallegraeff, G.M. Taxonomy of harmful dinoflagellates. In Manual on Harmful Marine Microalgae, 2nd ed.; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; IOC-UNESCO: Paris, France, 2003; Volume 11, pp. 389–432. [Google Scholar]
- Balech, E. The Genus Alexandrium Halim (Dinoflagellata); Sherkin Island Marine Station: County Cork, Ireland, 1995; Volume 452. [Google Scholar]
- Leaw, C.P.; Lim, P.T.; Ng, B.K.; Cheah, M.Y.; Ahmad, A.; Usup, G. Phylogenetic analysis of Alexandriumspecies and Pyrodinium bahamense (Dinophyceae) based on theca morphology and nuclear ribosomal gene sequence. Phycologia 2005, 44, 550–565. [Google Scholar] [CrossRef]
- Anderson, D.M.; Cembella, A.; Hallegraeff, G.M. Progress in understanding harmful algal blooms: Paradigm shifts and new technologies for research, monitoring, and management. Annu. Rev. Mar. Sci. 2012, 4, 143–176. [Google Scholar] [CrossRef]
- Tomas, C.; Van Wagoner, R.; O Tatters, A.; White, K.D.; Hall, S.; Wright, J.L.C. Alexandrium peruvianum (Balech and Mendiola) Balech and Tangen a new toxic species for coastal North Carolina. Harmful Algae 2012, 17, 54–63. [Google Scholar] [CrossRef]
- John, U.; Litaker, R.W.; Montresor, M.; Murray, S.A.; Brosnahan, M.L.; Anderson, N.M. Formal revision of the Alexandrium tamarense species complex (Dinophyceae) taxonomy: The introduction of five species with emphasis on molecular-based (rDNA) classification. Protist 2014, 165, 779–804. [Google Scholar] [CrossRef]
- Le Messurier, D.H. A Survey of Mussels on a Portion of the Australian Coast. Med. J. Aust. 1935, 1, 490–492. [Google Scholar] [CrossRef]
- Hallegraeff, G.M. Harmful algal blooms in the Australian region. Mar. Pollut. Bull. 1992, 25, 186–190. [Google Scholar] [CrossRef]
- Ajani, P.; Hallegraeff, G.; Pritchard, T. Historic Overview of Algal Blooms in Marine and Estuarine Waters of New South Wales, Australia; Linnean Society of New South Wales: New South Wales, Australia, 2001; pp. 1–22. [Google Scholar]
- Hallegraeff, G.M.; Bolch, C. Transport of diatom and dinoflagellate resting spores in ships’ ballast water: Implications for plankton biogeography and aquaculture. J. Plankton Res. 1992, 14, 1067–1084. [Google Scholar] [CrossRef]
- Farrell, H.; Brett, S.; Ajani, P.A.; Murray, S.A. Distribution of the genus Alexandrium (Halim) and paralytic shellfish toxins along the coastline of New South Wales, Australia. Mar. Pollut. Bull. 2013, 72, 133–145. [Google Scholar] [CrossRef]
- Ajani, P.; Brett, S.; Krogh, M.; Scanes, P.; Webster, G.; Armand, L.J.E. The Risk of Harmful Algal Blooms (HABs) in the Oyster-Growing Estuaries of New South Wales. Environ. Monit. Assess. 2013, 185, 5295–5316. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Bolch, C.J.S.; Hill, D.R.A.; Jameson, I.; LeRoi, J.M.; McMinn, A.; Murray, S.; de Salas, M.F.; Saunders, K. Algae of Australia: Phytoplankton of Temperate Coastal Waters; ABRS: Canberra, Australia; CSIRO Publishing: Melbourne, Australia, 2010. [Google Scholar]
- Hallegraeff, G.; Bolch, C.J.; Blackburn, S.I.; Oshima, Y. Species of the Toxigenic Dinoflagellate Genus Alexandrium in Southeastern Australian Waters. Bot. Mar. 1991, 34, 575–588. [Google Scholar] [CrossRef]
- Murray, S.A.; Hoppenrath, M.; Orr, R.J.S.; Bolch, C.; John, U.; Diwan, R.; Yauwenas, R.; Harwood, T.; De Salas, M.; Neilan, B.A.; et al. Alexandrium diversaporum sp. nov., a new non-saxitoxin producing species: Phylogeny, morphology and sxtA genes. Harmful Algae 2014, 31, 54–65. [Google Scholar] [CrossRef]
- Murray, S.A.; Wiese, M.; Neilan, B.A.; Orr, R.J.S.; De Salas, M.; Brett, S.; Hallegraeff, G.M. A reinvestigation of saxitoxin production and sxtA in the ‘non-toxic’ Alexandrium tamarense Group V clade. Harmful Algae 2012, 18, 96–104. [Google Scholar] [CrossRef]
- NSWDPI. Aquaculture Production Report 2018–2019. 2019. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0015/1191021/Provisional-Production-Report-2018-2019.pdf (accessed on 16 April 2020).
- Campbell, A.; McLeod, C.; Pointon, A.; Hudson, D.; Nicholls, C. The 2012–13 Paralytic Shellfish Toxin Event in Tasmania Associated with Dinoflagellate Alga, Alexandrium Tamarense; A SafeFish Review, FRDC Project 2012/060; FRDC: Adelaide, Australia, 2013.
- NSWFA. Marine Biotoxin Management Plan. 2015. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/industry/marine_biotoxin_management_plan.pdf (accessed on 16 April 2020).
- ASQAAC. The Australian Shellfish Quality Assurance Program Operations Manual; Australian Shellfish Quality Assurance Advisory Committee: Canberra, Australia, 2016. [Google Scholar]
- Keller, M.D.; Selvin, R.C.; Clauß, W.; Guillard, R.R.L. Media for the Culture of Oceanic Ultraphytoplankton1,2. J. Phycol. 2007, 23, 633–638. [Google Scholar] [CrossRef]
- Chomérat, N.; Gatti, C.M.I.; Nézan, É.; Chinain, M. Studies on the benthic genus Sinophysis (Dinophysales, Dinophyceae) II. S. canaliculata from Rapa Island (French Polynesia). Phycologia 2017, 56, 193–203. [Google Scholar] [CrossRef]
- Ruvindy, R.; Bolch, C.J.; MacKenzie, L.; Smith, K.F.; Murray, S.A. qPCR Assays for the Detection and Quantification of Multiple Paralytic Shellfish Toxin-Producing Species of Alexandrium. Front. Microbiol. 2018, 9, 3153. [Google Scholar] [CrossRef]
- Blackburn, S.I.; Bolch, C.J.S.; Haskard, K.A.; Hallegraeff, G.M. Reproductive compatibility among four global populations of the toxic dinoflagellate Gymnodinium catenatum (Dinophyceae). Phycologia 2001, 40, 78–87. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. QIIME 2: Reproducible, Interactive, Scalable, and Extensible Microbiome Data Science. PeerJ Preprints 2018. [Google Scholar] [CrossRef]
- McMurdie, P.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Paradis, E.; Claude, J.; Strimmer, K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 2004, 20, 289–290. [Google Scholar] [CrossRef] [PubMed]
- Bisanz, J.E. Qiime2R: Importing QIIME2 Artifacts and Associated Data into R Sessions. 2018. Available online: https://github.com/jbisanz/qiime2R (accessed on 16 April 2020).
- Wickham, H. Tidyverse:Easily Install and Load “Tidyverse” Packages; R Package Version 100; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Scholin, C.A.; Buck, K.R.; Britschgi, T.; Cangelosi, G.; Chávez, F. Identification of Pseudo-nitzschia australis (Bacillariophyceae) using rRNA-targeted probes in whole cell and sandwich hybridization formats. Phycologia 1996, 35, 190–197. [Google Scholar] [CrossRef]
- Nunn, G.B.; Theisen, B.F.; Christensen, B.; Arctander, P. Simplicity-correlated size growth of the nuclear 28S ribosomal RNA D3 expansion segment in the crustacean order isopoda. J. Mol. Evol. 1996, 42, 211–223. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Larkin, M.; Blackshields, G.; Brown, N.; Chenna, R.; Mcgettigan, P.; McWilliam, H.; Valentin, F.; Wallace, I.; Wilm, A.; López, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Hoehna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Boil. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O.; Dufayard, J.-F. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Boil. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.F.; Niedzwiadek, B.; Menard, C.; De Astudillo, L.R.; Biré, R.; A Burdaspal, P.; Ceredi, A.; Davis, B.; Dias, E.; Eaglesham, G.; et al. Quantitative Determination of Paralytic Shellfish Poisoning Toxins in Shellfish Using Prechromatographic Oxidation and Liquid Chromatography with Fluorescence Detection: Collaborative Study. J. AOAC Int. 2005, 88, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Top, H.V.D.; Gerssen, A.; McCarron, P.; Van Egmond, H. Quantitative determination of marine lipophilic toxins in mussels, oysters and cockles using liquid chromatography-mass spectrometry: Inter-laboratory validation study. Food Addit. Contam. Part A 2011, 28, 1–13. [Google Scholar] [CrossRef]
- Villar-González, A.; Rodríguez-Velasco, M.L.; Gagoo-Martínez, A. Determination of Lipophilic Toxins by LC/MS/MS: Single-Laboratory Validation. J. AOAC Int. 2011, 94, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a sensitive and selective liquid chromatography–mass spectrometry method for high throughput analysis of paralytic shellfish toxins using graphitised carbon solid phase extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef]
- NSWFA. Phytoplankton and Biotoxins in NSW Shellfish Aquaculture Areas. 2017. Available online: https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/phytoplankton_and_biotoxin_risk_assessment.pdf (accessed on 16 April 2020).
- Bolch, C.; Harwood, T.; Untari, L.; Murray, S.; Hallegraeff, G.; Turnbull, A. Alexandrium tamarense Group I as the cause of PST on the east coast of Tasmania, Australia. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–30 October 2014; p. 29. [Google Scholar]
- Zhou, J.; Richlen, M.L.; Sehein, T.R.; Kulis, D.M.; Anderson, N.M.; Cai, Z. Microbial Community Structure and Associations During a Marine Dinoflagellate Bloom. Front. Microbiol. 2018, 9, 9. [Google Scholar] [CrossRef]
- Condie, S.A.; Oliver, E.C.; Hallegraeff, G. Environmental drivers of unprecedented Alexandrium catenella dinoflagellate blooms off eastern Tasmania, 2012–2018. Harmful Algae 2019, 87, 101628. [Google Scholar] [CrossRef]
- The Commonwealth Scientific and Industrial Research Organisation. Curr. Boil. 1997, 7, R126. [CrossRef][Green Version]
- Harwood, D.T.; Boundy, M.; Selwood, A.I.; Van Ginkel, R.; MacKenzie, L.; McNabb, P.S. Refinement and implementation of the Lawrence method (AOAC 2005.06) in a commercial laboratory: Assay performance during an Alexandrium catenella bloom event. Harmful Algae 2013, 24, 20–31. [Google Scholar] [CrossRef]
- Murray, S.A.; Wiese, M.; Stüken, A.; Brett, S.; Kellmann, R.; Hallegraeff, G.M.; Neilan, B.A. sxtA-Based Quantitative Molecular Assay To Identify Saxitoxin-Producing Harmful Algal Blooms in Marine Waters. Appl. Environ. Microbiol. 2011, 77, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Negri, A.P.; Llewellyn, L.; Doyle, J.; Webster, N.S.; Frampton, D.; Blackburn, S. Paralytic Shellfish Toxins are Restricted to Few Species among Australia’s Taxonomic Diversity of Cultured Microalgae1. J. Phycol. 2003, 39, 663–667. [Google Scholar] [CrossRef]
- Orlova, T.Y.; Selina, M.S.; Lilly, E.L.; Kulis, D.; Anderson, N.M. Morphogenetic and toxin composition variability of Alexandrium tamarense (Dinophyceae) from the east coast of Russia. Phycologia 2007, 46, 534–548. [Google Scholar] [CrossRef]
- Orr, R.J.S.; Stüken, A.; Murray, S.A.; Jakobsen, K.S. Evolutionary Acquisition and Loss of Saxitoxin Biosynthesis in Dinoflagellates: The Second “Core” Gene, sxtG. Appl. Environ. Microbiol. 2013, 79, 2128–2136. [Google Scholar] [CrossRef]
- Sebastián, C.R.; Etheridge, S.M.; Cook, P.A.; O’Ryan, C.; Pitcher, G.C. Phylogenetic analysis of toxicAlexandrium(Dinophyceae) isolates from South Africa: Implications for the global phylogeography of theAlexandrium tamarensespecies complex. Phycologia 2005, 44, 49–60. [Google Scholar] [CrossRef]
- Krock, B.; Seguel, C.G.; Cembella, A. Toxin profile of Alexandrium catenella from the Chilean coast as determined by liquid chromatography with fluorescence detection and liquid chromatography coupled with tandem mass spectrometry. Harmful Algae 2007, 6, 734–744. [Google Scholar] [CrossRef]
- Suthers, I.; Young, J.W.; Baird, M.; Roughan, M.; Everett, J.D.; Brassington, G.B.; Byrne, M.; Condie, S.A.; Hartog, J.R.; Hassler, C.S.; et al. The strengthening East Australian Current, its eddies and biological effects—An introduction and overview. Deep. Sea Res. Part II 2011, 58, 538–546. [Google Scholar] [CrossRef]



| Data Collected | 2016 | 2018 |
|---|---|---|
| Morphological | ||
| Cell abundance | x | x |
| Strain isolation | x | |
| Light microscopy | x | x |
| Scanning electron microscopy | x | |
| Molecular | ||
| Amplicon Sequencing | x | |
| qPCR using species specific primers | x | |
| LSU rDNA of cultured strain | x | |
| SSU rDNA of cultured strain | x | |
| Toxicological | ||
| Toxin profile determination by LCMS/MS | x | |
| Mussel biotoxin test | x | x |
| Oceanographic conditions | ||
| Water mass characteristics | x | |
| Physicochemical parameters | x | |
| Nutrients | x |
| Species | Primers | |
|---|---|---|
| Name | Sequence | |
| A. catenella | ACT-US-408-F | 5′-ACT TGA TTT GCT TGG TGG GAG-3′ |
| ACT-US-645-R | 5′-AAG TCC AAG GAA GGA AGC ATC C-3′ | |
| A. pacificum | ACTA-416-F | 5′-TCC TCA GTG AGA TTG TAG TG-3′ |
| ACTA-605-R | 5′-GAC AAG GAC ACA AAC AAA TAC-3′ | |
| A. australiense | AusTv2-F | 5′-CGG TGG GTG CAA TGA TTC-3′ |
| AusTv2-R | 5′-GCA GGA AAA TTA CCA TTC AAG T-3′ | |
| AusTv2-P | 5′-CACAGGTAATCAAATGTCCACATAGAAACTG-3′ | |
| Target Compounds | Strain TFB_C/18 | Strain TFB_G/18 | ||||
|---|---|---|---|---|---|---|
| Total Toxin (ng) | Relative % of Total Toxin | Toxin Per Cell (pg/Cell) | Total Toxin (ng) | Relative % of Total Toxin | Toxin Per Cell (pg/Cell) | |
| C1 | 143.1 | 2.8 | 0.103 | 349.8 | 6.4 | 0.274 |
| C2 | 1893.2 | 36.5 | 1.358 | 821.5 | 15.0 | 0.644 |
| GTX2 | ND | 0.0 | ND | ND | 0.0 | ND |
| GTX1 | 672.2 | 13.0 | 0.482 | 820.8 | 15.0 | 0.643 |
| dcGTX2 | ND | 0.0 | ND | ND | 0.0 | ND |
| GTX3 | ND | 0.0 | ND | ND | 0.0 | ND |
| GTX4 | 692.1 | 13.4 | 0.496 | 728.2 | 13.3 | 0.571 |
| dcGTX3 | ND | 0.0 | ND | ND | 0.0 | ND |
| GTX5 | 1110.4 | 21.4 | 0.796 | 2132.6 | 39.1 | 1.671 |
| GTX6 | ND | 0.0 | ND | ND | 0.0 | ND |
| STX | 83.9 | 2.2 | 0.0602 | ND | 0.0 | ND |
| dcNEO | 112.7 | 3.0 | 0.0808 | ND | 4.3 | ND |
| dcSTX | 157.2 | 6.1 | 0.113 | 235.8 | 6.8 | 0.185 |
| NEO | 816.3 | 1.6 | 0.585 | 370.4 | 0.0 | 0.29 |
| LOR | Min | Max | Mean | ||
|---|---|---|---|---|---|
| A. | Field Parameters | ||||
| DO (%) | 0.1 | 85.9 | 112.6 | 100.9 | |
| DO (mg/L) | 0.01 | 7.0 | 9.0 | 7.9 | |
| Turbidity (ntu) | 0.1 | 0.0 | 7.4 | 1.0 | |
| Secchi Depth (m) | 0.1 | 1.4 | 16.0 | 4.8 | |
| pH | 0.01 | 8.0 | 8.2 | 8.1 | |
| Salinity (ppt) | - | 34.9 | 35.9 | 35.5 | |
| Conductivity (µS/cm) | - | 52,973.0 | 54,203.0 | 53,675.9 | |
| Temperature (°C) | 0.01 | 14.6 | 22.7 | 16.7 | |
| B. | Laboratory parameters | ||||
| Suspended Solid (mg/L) | 1 | 1.0 | 40.0 | 6.4 | |
| Turbidity (ntu) | 0.1 | 0.2 | 3.2 | 0.7 | |
| Ammonia (mg/L) | 0.02 | 0.020 | 0.1 | 0.0 | |
| Ammonium (mg/L) | 0.02 | 0.019 | 0.1 | 0.0 | |
| Total Nitrogen (mg/L) | 0.05 | 0.1 | 0.6 | 0.2 | |
| Total Phosphorus (mg/L) | 0.005 | 0.005 | 0.015 | 0.0 | |
| Chl a (µg/L) | 1.0 | 1.0 | 6.0 | 1.9 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barua, A.; Ajani, P.A.; Ruvindy, R.; Farrell, H.; Zammit, A.; Brett, S.; Hill, D.; Sarowar, C.; Hoppenrath, M.; Murray, S.A. First Detection of Paralytic Shellfish Toxins from Alexandrium pacificum above the Regulatory Limit in Blue Mussels (Mytilus galloprovincialis) in New South Wales, Australia. Microorganisms 2020, 8, 905. https://doi.org/10.3390/microorganisms8060905
Barua A, Ajani PA, Ruvindy R, Farrell H, Zammit A, Brett S, Hill D, Sarowar C, Hoppenrath M, Murray SA. First Detection of Paralytic Shellfish Toxins from Alexandrium pacificum above the Regulatory Limit in Blue Mussels (Mytilus galloprovincialis) in New South Wales, Australia. Microorganisms. 2020; 8(6):905. https://doi.org/10.3390/microorganisms8060905
Chicago/Turabian StyleBarua, Abanti, Penelope A. Ajani, Rendy Ruvindy, Hazel Farrell, Anthony Zammit, Steve Brett, David Hill, Chowdhury Sarowar, Mona Hoppenrath, and Shauna A. Murray. 2020. "First Detection of Paralytic Shellfish Toxins from Alexandrium pacificum above the Regulatory Limit in Blue Mussels (Mytilus galloprovincialis) in New South Wales, Australia" Microorganisms 8, no. 6: 905. https://doi.org/10.3390/microorganisms8060905
APA StyleBarua, A., Ajani, P. A., Ruvindy, R., Farrell, H., Zammit, A., Brett, S., Hill, D., Sarowar, C., Hoppenrath, M., & Murray, S. A. (2020). First Detection of Paralytic Shellfish Toxins from Alexandrium pacificum above the Regulatory Limit in Blue Mussels (Mytilus galloprovincialis) in New South Wales, Australia. Microorganisms, 8(6), 905. https://doi.org/10.3390/microorganisms8060905





