In Depth Natural Product Discovery from the Basidiomycetes Stereum Species
Abstract
:1. Introduction
2. Secondary Metabolites from Stereum and Their Activities
2.1. Sesquiterpenoids
2.1.1. Hirsutane
2.1.2. Illudalane and Norilludalane
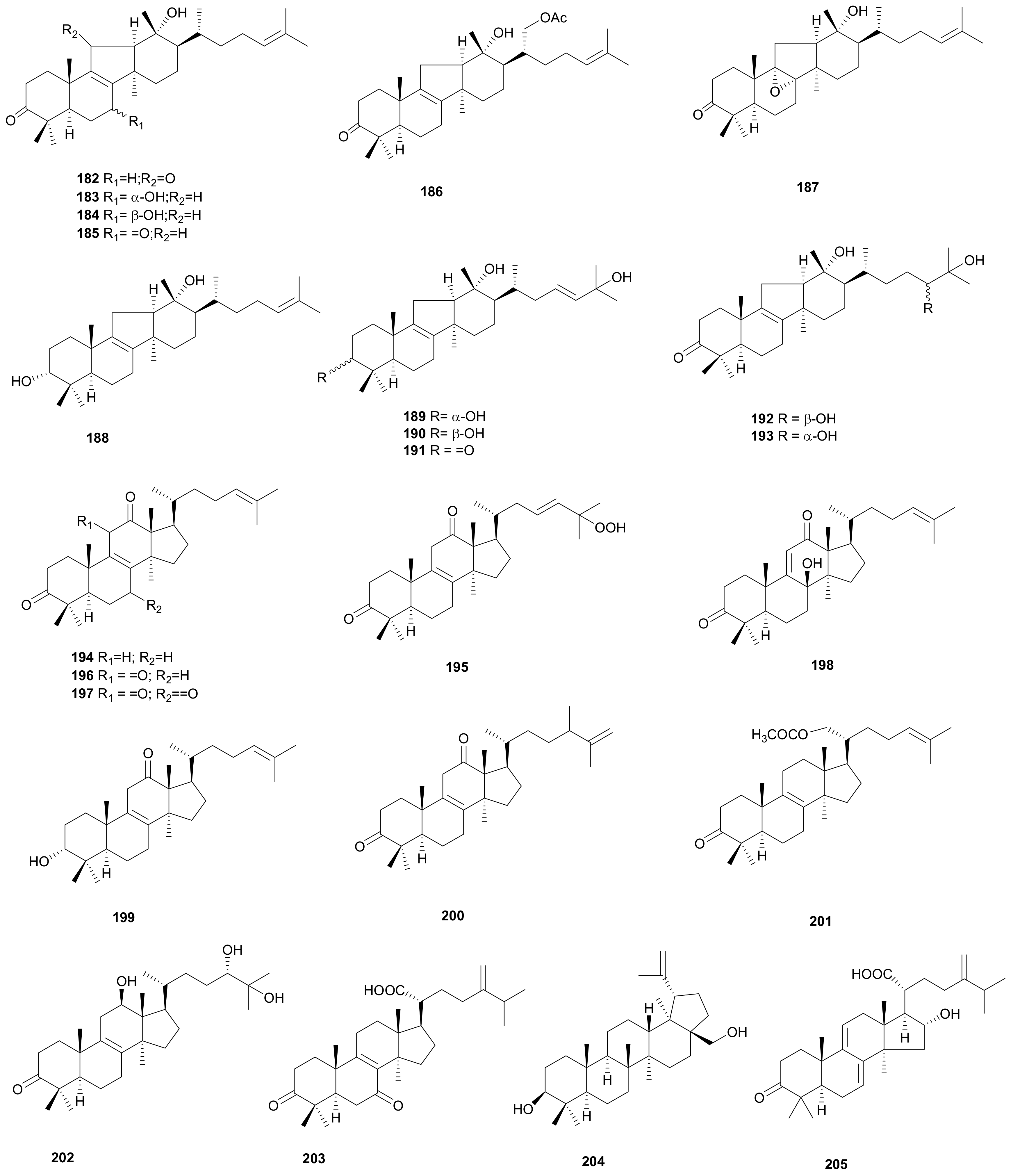
2.1.3. Stereumane and Cadinane
2.1.4. Sterpurane and Isolactarane
2.1.5. Drimane
2.1.6. Acetylenic Sesquiterpenoids
2.1.7. Unclassified Sesquiterpenoids
2.2. Polyketides and Their Derivatives
2.3. Aromatics and Their Derivatives
2.3.1. Benzofuran
2.3.2. Phenol Derivatives and Other Aromatic Compounds
2.4. Vibralactones and Derivatives
2.5. Lanostane Triterpenoids
2.6. Sterols
2.7. Carboxylic Acids and Saccharides
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, H.; Zhu, G.; Fan, Y.; Du, Y.; Lan, M.; Xu, Y.; Zhu, W. Natural Products Research in China from 2015 to 2016. Front. Chem. 2018, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saket, S.; Ravishankar, V. Evaluation of Antimicrobial, Enzyme Inhibitory, Antioxidant and Cytotoxic Activities of Partially Purified Volatile Metabolites of Marine Streptomyces sp. S2A. Microorganisms 2018, 6, 72. [Google Scholar]
- Wang, H.; Sun, T.; Song, W.; Guo, X.; Zhao, J. Taxonomic Characterization and Secondary Metabolite Analysis of NEAU-wh3-1: An Embleya Strain with Antitumor and Antibacterial Activity. Microorganisms 2020, 8, 441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sandargo, B.; Chepkirui, C.; Cheng, T.; Chaverra-Muñoz, L.; Thongbai, B.; Stadler, M.; Hüttel, S. Biological and chemical diversity go hand in hand: Basidomycota as source of new pharmaceuticals and agrochemicals. Biotechnol. Adv. 2019, 37, 107344. [Google Scholar] [CrossRef]
- Reid, D. A Monographic Study of the Stipitate Species of the Genus Stereum. Ph.D. Thesis, University of London (External), London, UK, 1965. [Google Scholar]
- Liu, D.Z.; Wang, F.; Liao, T.G.; Tang, J.G.; Steglich, W.; Zhu, H.J.; Liu, J.K. Vibralactone: A lipase inhibitor with an unusual fused β-lactone produced by cultures of the basidiomycete Boreostereum vibrans. Org. Lett. 2006, 8, 5749–5752. [Google Scholar] [CrossRef]
- Bai, N.; Li, G.H.; Luo, S.L.; Du, L.C.; Zhao, P.J. Functional characterization of Vib-PT, an aromatic prenyltransferase involved in the biosynthesis of vibralactone from Stereum vibrans. Appl. Environ. Microb. 2020, 86, 1–18. [Google Scholar] [CrossRef]
- Larignon, P.; Dubos, B. Fungi associated with esca disease in grapevine. Eur. J. Plant. Pathol. 1997, 103, 147–157. [Google Scholar] [CrossRef]
- Yun, B.S.; Lee, I.K.; Cho, Y.; Cho, S.M.; Yoo, I.D. New Tricyclic Sesquiterpenes from the Fermentation Broth of Stereum hirsutum. J. Nat. Prod. 2002, 65, 786–788. [Google Scholar] [CrossRef]
- Yoo, N.H.; Kim, J.P.; Yun, B.S.; Ryoo, I.J.; Lee, I.K.; Yoon, E.S.; Koshino, H.; Yoo, I.D. Hirsutenols, D, E and F, new sesquiterpenes from the culture broth of Stereum hirsutum. J. Antibiot. 2006, 59, 110–113. [Google Scholar] [CrossRef]
- Heatley, N.; Jennings, M.; Florey, H. Antibiotics from Stereum hirsutum. Br. J. Exp. Pathol. 1947, 28, 35–46. [Google Scholar]
- Qi, Q.Y.; Bao, L.; Ren, J.W.; Han, J.J.; Zhang, Z.Y.; Li, Y.; Yao, Y.J.; Cao, R.; Liu, H.W. Sterhirsutins A and B, two new heterodimeric sesquiterpenes with a new skeleton from the culture of Stereum hirsutum collected in Tibet Plateau. Org. Lett. 2014, 16, 5092–5095. [Google Scholar] [CrossRef]
- Qi, Q.Y.; Ren, J.W.; Sun, L.W.; He, L.W.; Bao, L.; Yue, W.; Sun, Q.M.; Yao, Y.J.; Yin, W.B.; Liu, H.W. Stucturally Diverse Sesquiterpenes Produced by a Chinese Tibet Fungus Stereum hirsutum and Their Cytotoxic and Immunosuppressant Activities. Org. Lett. 2015, 17, 3098–3101. [Google Scholar] [CrossRef]
- Ma, K.; Bao, L.; Han, J.; Jin, T.; Yang, X.; Zhao, F.; Li, S.; Song, F.; Liu, M.; Liu, H. New benzoate derivatives and hirsutane type sesquiterpenoids with antimicrobial activity and cytotoxicity from the solid-state fermented rice by the medicinal mushroom Stereum hirsutum. Food Chem. 2014, 143, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Comer, F.W.; Mccapra, F.; Qureshi, I.H.; Scott, A.I. Structure and chemistry of hirsutic acid. Tetrahedron 1968, 23, 4761–4768. [Google Scholar] [CrossRef]
- Coyle, J.T.; Puttfarcken, P. Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993, 262, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M. The importance of free radicals and catalytic metal ions in human diseases. Mol. Asp. Med. 1985, 8, 89–193. [Google Scholar] [CrossRef]
- Hammond, B.; Kontos, H.A.; Hess, M.L. Oxygen radicals in the adult respiratory distress syndrome, in myocardial ischemia and reperfusion injury, and in cerebral vascular damage. Can. J. Physiol. Pharmacol. 1985, 63, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Kutateladze, A.G.; Kuznetsov, D.M. Triquinanes and related sesquiterpenes revisited computationally: Structure corrections of hirsutanols B and D, hirsutenol E, cucumin B, antrodins C–E, chondroterpenes A and H, chondrosterins C and E, dichrocephone A, and pethybrene. J. Org. Chem. 2017, 82, 10795–10802. [Google Scholar] [CrossRef] [Green Version]
- Liermann, J.C.; Schüffler, A.; Wollinsky, B.; Birnbacher, J.; Kolshorn, H.; Anke, T.; Opatz, T. Hirsutane-Type Sesquiterpenes with Uncommon Modifications from Three Basidiomycetes. J. Org. Chem. 2010, 75, 2955–2961. [Google Scholar] [CrossRef]
- Mantle, P.; Mellows, G. Production of hirsutanes by Stereum complicatum. Trans. Br. Mycol. Soc. 1973, 61, 513–519. [Google Scholar] [CrossRef]
- Mehta, G.; Srikrishna, A. Synthesis of polyquinane natural products: An update. Chem. Rev. 1997, 97, 671–720. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Tsuzuki, K.; Sakan, F.; Shirahama, H.; Matsumoto, T. Total synthesis of d1-hirsutic acid. Tetrahedron Lett. 1974, 15, 3745–3748. [Google Scholar] [CrossRef]
- Feline, T.C.; Mellows, G.; Jones, R.B.; Phillips, L. Biosynthesis of hirsutic acid C using 13 C nuclear magnetic resonance spectroscopy. J. Chem. Soc. Chem. Commun. 1974, 63–64. [Google Scholar] [CrossRef]
- Greene, A.E.; Luche, M.J.; Serra, A.A. An efficient, enantioconvergent total synthesis of natural Hirsutic acid C. J. Org. Chem. 1985, 50, 3957–3962. [Google Scholar] [CrossRef]
- Austin, K.A.; Banwell, M.G.; Harfoot, G.J.; Willis, A.C. Chemoenzymatic syntheses of the linear triquinane-type sesquiterpenes (+)-hirsutic acid and (−)-complicatic acid. Tetrahedron Lett. 2006, 47, 7381–7384. [Google Scholar] [CrossRef]
- Banwell, M.G.; Austin, K.A.; Willis, A.C. Chemoenzymatic total syntheses of the linear triquinane-type natural products (+)-hirsutic acid and (−)-complicatic acid from toluene. Tetrahedron 2007, 63, 6388–6403. [Google Scholar] [CrossRef]
- Flynn, C.M.; Schmidt-Dannert, C. Sesquiterpene synthase–3-hydroxy-3-methylglutaryl coenzyme A synthase fusion protein responsible for hirsutene biosynthesis in Stereum hirsutum. Appl. Environ. Microbiol. 2018, 84, e00036-00018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isaka, M.; Srisanoh, U.; Choowong, W.; Boonpratuang, T. Sterostreins A–E, new terpenoids from cultures of the basidiomycete Stereum ostrea BCC 22955. Org. Lett. 2011, 13, 4886–4889. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Srisanoh, U.; Sappan, M.; Supothina, S.; Boonpratuang, T. Sterostreins F-O, illudalanes and norilludalanes from cultures of the Basidiomycete Stereum ostrea BCC 22955. Phytochemistry 2012, 79, 116–120. [Google Scholar] [CrossRef]
- Tian, M.Q.; Liu, R.; Li, J.F.; Zhang, K.Q.; Li, G.H. Three new sesquiterpenes from the fungus Stereum sp. YMF1.1686. Phytochem. Lett. 2016, 15, 186–189. [Google Scholar] [CrossRef]
- Li, J.F.; Qin, Y.K.; Tian, M.Q.; Zhang, K.Q.; Li, G.H. Two new sesquiterpenes from the fungus Stereum sp. NN048997. Phytochem. Lett. 2014, 10, 32–34. [Google Scholar] [CrossRef]
- Duan, Y.C.; Feng, J.; Bai, N.; Li, G.H.; Zhang, K.Q.; Zhao, P.J. Four novel antibacterial sesquiterpene-alpha-amino acid quaternary ammonium hybrids from the mycelium of mushroom Stereum hirsutum. Fitoterapia 2018, 128, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.Q.; Wang, X.; Yan, J.M.; Zhang, K.Q.; Li, G.H. A New Sesquiterpenoid from the Fungus Stereum sp. YMF1.1686. Chem. Nat. Compd. 2018, 54, 286–288. [Google Scholar] [CrossRef]
- Yan, J.M.; Wang, Y.L.; Zhang, K.Q.; Li, G.H. A New Sesquiterpene from Stereum sp. YMF1.04734. Chem. Nat. Compd. 2019, 55, 669–670. [Google Scholar] [CrossRef]
- Yan, J.M.; Wu, Q.L.; Zhao, P.J.; Zhang, K.Q.; Li, G.H. Chemical constituents of the fungus Stereum rugosum ATCC64657. Phytochem. Lett. 2018, 27, 105–107. [Google Scholar] [CrossRef]
- Li, G.H.; Liu, F.F.; Shen, L.; Zhu, H.J.; Zhang, K.Q. Stereumins H–J, Stereumane-Type Sesquiterpenes from the Fungus Stereum sp. J. Nat. Prod. 2011, 74, 296–299. [Google Scholar] [CrossRef]
- Li, G.H.; Duan, M.; Yu, Z.F.; Li, L.; Dong, J.Y.; Wang, X.B.; Guo, J.W.; Huang, R.; Wang, M.; Zhang, K.Q. Stereumin A-E, sesquiterpenoids from the fungus Stereum sp. CCTCC AF 207024. Phytochemistry 2008, 69, 1439–1445. [Google Scholar] [CrossRef]
- Liu, F.F.; Li, G.H.; Yang, Z.S.; Zheng, X.; Yang, Y.; Zhang, K.Q. Two new sesquiterpenes from the fungus Stereum sp. Helv. Chim. Acta 2010, 93, 1737–1741. [Google Scholar] [CrossRef]
- Zheng, X.; Li, G.H.; Xie, M.J.; Wang, X.; Sun, R.; Lu, H.; Zhang, K.Q. Stereumins K-P, sesquiterpenes from the fungus Stereum sp. CCTCC AF 2012007. Phytochemistry 2013, 86, 144–150. [Google Scholar] [CrossRef]
- Bunyapaiboonsri, T.; Yoiprommarat, S.; Nopgason, R.; Komwijit, S.; Veeranondha, S.; Puyngain, P.; Boonpratuang, T. Cadinane sesquiterpenoids from the basidiomycete Stereum cf. sanguinolentum BCC 22926. Phytochemistry 2014, 105, 123–128. [Google Scholar] [CrossRef]
- Huang, J.R.; Yang, B.J.; Mo, M.H.; Zhang, K.Q.; Li, G.H. Secondary metabolites from the fungus Stereum gausapatum ATCC60954. Phytochem. Lett. 2020, 35, 171–174. [Google Scholar] [CrossRef]
- Li, G.H.; Zhang, K.Q. A novel sesquiterpene isolated from Stereum sp. 8954. Chinese Chem. Lett. 2005, 16, 1615–1617. [Google Scholar]
- Ayer, W.A.; Saeedi-Ghomi, M.H. The sterepolides: New isolactaranes from Stereum purpureum. Tetrahedron Lett. 1981, 22, 2071–2074. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Dai, Z. Isolation and identification of two new metabolites from silver leaf fungus Stereum purpureum. J. Org. Chem. 1992, 57, 2313–2316. [Google Scholar] [CrossRef]
- Ayer, W.A.; Nakashima, T.T.; Saeedi-Ghomi, M.H. Studies on the biosynthesis of the sterpurenes. Can. J. Chem. 1984, 62, 531–533. [Google Scholar] [CrossRef]
- Zhao, S.K.; Helquist, P. Short synthesis of (.+-.)-sterpurene. J. Org. Chem. 1990, 55, 5820–5821. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Okamura, W.H. A short enantioselective synthesis of (+)-sterpurene: Complete intramolecular transfer of central to axial to central chiral elements. J. Am. Chem. Soc. 1988, 110, 4062–4063. [Google Scholar] [CrossRef]
- Harmata, M.; Bohnert, G.J. A 4+ 3 cycloaddition approach to the synthesis of (±)-sterpurene. Org. Lett. 2003, 5, 59–61. [Google Scholar] [CrossRef]
- Lan, P.; White, L.E.; Taher, E.S.; Guest, P.E.; Banwell, M.G.; Willis, A.C. Chemoenzymatic synthesis of (+)-asperpentyn and the enantiomer of the structure assigned to aspergillusol A. J. Nat. Prod. 2015, 78, 1963–1968. [Google Scholar] [CrossRef] [PubMed]
- Opatz, T.; Kolshorn, H.; Anke, H. Sterelactones: New isolactarane type sesquiterpenoids with antifungal activity from Stereum sp. IBWF 01060. J. Antibiot. 2008, 61, 563–567. [Google Scholar] [CrossRef]
- Kim, Y.H.; Yun, B.S.; Ryoo, I.J.; Kim, J.P.; Koshino, H.; Yoo, I.D. Methoxylaricinolic acid, a new sesquiterpene from the fruiting bodies of Stereum ostrea. J. Antibiot. 2006, 59, 432–434. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Yangchum, A.; Choeyklin, R.; Anaphon, S. Acetylenic sesquiterpenoids from cultures of the basidiomycete Stereum cf. hirsutum BCC 26597. Nat. Prod. Res. 2019, 1–7. [Google Scholar] [CrossRef]
- Yan, J.M.; Wang, X.; Tian, M.Q.; Liu, C.M.; Zhang, K.Q.; Li, G.H. Chemical constituents from the fungus Stereum sp. YMF1.04183. Phytochem. Lett. 2017, 22, 6–8. [Google Scholar] [CrossRef]
- Rashid, S.; Bhat, B.A.; Mehta, G. A vicarious, one-pot synthesis of benzo- and naphthofurans: Applications to the syntheses of stereumene B and paeoveitols. Tetrahedron Lett. 2019, 60, 1122–1125. [Google Scholar] [CrossRef]
- Risdian, C.; Mozef, T.; Wink, J. Biosynthesis of Polyketides in Streptomyces. Microorganisms 2019, 7, 124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lackner, G.; Bohnert, M.; Wick, J.; Hoffmeister, D. Assembly of melleolide antibiotics involves a polyketide synthase with cross-coupling activity. Chem. Biol. 2013, 20, 1101–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurasawa, S.; Naganawa, H.; Takeuchi, T.; Umezawa, H. The structure of MS-3: A glyoxalase I inhibitor produced by a mushroom. Agric. Biol. Chem. 1975, 39, 2009–2014. [Google Scholar]
- Kurasawa, S.; Takeuchi, T.; Umezawa, H. Studies on glyoxalase inhibitor isolation of a new active agent, MS-3, from a mushroom culture. Agric. Biol. Chem. 1975, 39, 2003–2008. [Google Scholar] [CrossRef]
- Aqueveque, P.; Cespedes, C.L.; Becerra, J.; Davila, M.; Sterner, O. Bioactive compounds isolated from submerged fermentations of the Chilean fungus Stereum rameale. Z. Naturforsch. C. Biosci. 2015, 70, 97–102. [Google Scholar] [CrossRef]
- Li, G.H.; Li, L.; Duan, M.; Zhang, K.Q. The chemical constituents of the fungus Stereum sp. Chem. Biodivers. 2006, 3, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Ito-Kobayashi, M.; Aoyagi, A.; Tanaka, I.; Muramatsu, Y.; Umetani, M.; Takatsu, T. Sterenin A, B, C and D, Novel 11 β-Hydroxysteroid Dehydrogenase Type 1 Inhibitors from Stereum sp. SANK 21205. J. Antibiot. 2008, 61, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.T.; Qi, Q.Y.; Ma, K.; Pei, Y.F.; Han, J.J.; Xu, W.; Li, E.W.; Liu, H.W. Depside α-glucosidase inhibitors from a culture of the mushroom Stereum hirsutum. Planta Med. 2014, 80, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Aqueveque, P.; Cespedes, C.L.; Becerra, J.; Aranda, M.; Sterner, O. Antifungal activities of secondary metabolites isolated from liquid fermentations of Stereum hirsutum (Sh134-11) against Botrytis cinerea (grey mould agent). Food Chem. Toxicol. 2017, 109, 1048–1054. [Google Scholar] [CrossRef] [PubMed]
- Shinozuka, T.; Yamamoto, Y.; Hasegawa, T.; Saito, K.; Naito, S. First total synthesis of sterenins A, C and D. Tetrahedron Lett. 2008, 49, 1619–1622. [Google Scholar] [CrossRef]
- Keay, B.; Hopkins, J. Furans and their Benzo Derivatives: Applications. Comprehensive Heterocyclic Chemistry III; Elsevier: Amsterdam, The Netherlands, 2008; Volume 3. [Google Scholar]
- Bu’Lock, J.; Kaye, B.; Hudson, A. New benzofurans from Stereum subpileatum, their biosynthesis, and related processes of aromatic aminoacid metabolism in a basidiomycete. Phytochemistry 1971, 10, 1037–1046. [Google Scholar] [CrossRef]
- Sun, R.; Zheng, X.; Wang, X.; Dang, L.Z.; Yang, Z.S.; Luo, S.L.; Zhang, K.Q.; Li, G.H. Two new benzofuran derivatives from the fungus Stereum sp. YMF1. 1684. Phytochem. Lett. 2011, 4, 320–322. [Google Scholar] [CrossRef]
- Tian, M.Q.; Wu, Q.L.; Wang, X.; Zhang, K.Q.; Li, G.H. A new compound from Stereum insigne CGMCC5.57. Nat. Prod. Res. 2017, 31, 932–937. [Google Scholar] [CrossRef]
- Yu, Y.C.; Liu, B.; Wu, Y. Synthesis of Phenostereum A and Related Diol and Attempted Assignment of Relative Configurations of Remote Stereocenters by 13C NMR. ChemistrySelect 2016, 1, 3120–3123. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Tian, M.Q.; Li, J.F.; Li, G.H. Study on the chemical constituents of Stereum sp. YMF1. 1660. Chem. Bioeng. 2015, 32, 29–32. [Google Scholar]
- Omolo, J.O.; Anke, H.; Sterner, O. Hericenols A–D and a chromanone from submerged cultures of a Stereum species. Phytochemistry 2002, 60, 431–435. [Google Scholar] [CrossRef]
- Duan, Y.C.; Meng, X.X.; Yang, Y.L.; Yang, Y.H.; Zhao, P.J. Two new phenol derivatives from Stereum hirsutum FP-91666. J. Asian Nat. Prod. Res. 2015, 17, 324–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.P.; Zhao, Z.Z.; Yin, R.H.; Yin, X.; Feng, T.; Li, Z.H.; Wei, K.; Liu, J.K. Six New Vibralactone Derivatives from Cultures of the Fungus Boreostereum vibrans. Nat. Prod. Bioprospect. 2014, 4, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.P.; Jiang, M.Y.; Zhao, Z.Z.; Feng, T.; Li, Z.H.; Liu, J.K. Vibralactone Biogenesis-Associated Analogues from Submerged Cultures of the Fungus Boreostereum vibrans. Nat. Prod. Bioprospect. 2018, 8, 37–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordes, J.; Calo, F.; Anderson, K.; Pfaffeneder, T.; Laclef, S.; White, A.J.; Barrett, A.G. Total syntheses of angelicoin A, hericenone J, and hericenol A via migratory prenyl-and geranylation–aromatization sequences. J. Org. Chem. 2012, 77, 652–657. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tamanoi, H.; Hasegawa, Y.; Segawa, Y.; Masuyama, A. Divergent synthesis of bioactive resorcinols isolated from the fruiting bodies of Hericium erinaceum: Total syntheses of hericenones A, B, and I, hericenols B–D, and erinacerins A and B. J. Org. Chem. 2014, 79, 5227–5238. [Google Scholar] [CrossRef]
- Dubin, G.M.; Fkyerat, A.; Tabacchi, R. Acetylenic aromatic compounds from Stereum hirsutum. Phytochemistry 2000, 53, 571–574. [Google Scholar] [CrossRef]
- Nair, M.; Anchel, M. An antibacterial quinone hydroquinone pair from the ascomycete, Nectria coryli. Tetrahedron Lett. 1972, 9, 795–796. [Google Scholar] [CrossRef]
- Nair, M.; Anchel, M. Frustulosinol, an antibiotic metabolite of Stereum frustulosum: Revised structure of frustulosin. Phytochemistry 1977, 16, 390–392. [Google Scholar] [CrossRef]
- Goddard, M.L.; Tabacchi, R. Total synthesis of bioactive frustulosin and frustulosinol. Tetrahedron Lett. 2006, 47, 909–911. [Google Scholar] [CrossRef]
- Orr, A.F. Synthesis of the antibiotic isopentenynyl hydroquinones frustulosin and frustulosinol. J. Chem. Soc. Chem. Commun. 1979, 40–41. [Google Scholar] [CrossRef]
- Ronald, R.C.; Lansinger, J.M.; Lillie, T.S.; Wheeler, C.J. Total synthesis of frustulosin and aurocitrin. J. Org. Chem. 1982, 47, 2541–2549. [Google Scholar] [CrossRef]
- Hadvary, P.; Lengsfeld, H.; Wolfer, H. Inhibition of pancreatic lipase in vitro by the covalent inhibitor tetrahydrolipstatin. Biochem. J. 1988, 256, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiler, E.; Braun, N.; Böttcher, T.; Kastenmüller, A.; Weinkauf, S.; Sieber, S.A. Vibralactone as a tool to study the activity and structure of the ClpP1P2 complex from Listeria monocytogenes. Angew. Chem. Int. Ed. 2011, 50, 11001–11004. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Snider, B.B. Synthesis of (±)-and (−)-Vibralactone and Vibralactone C. J. Org. Chem. 2008, 73, 8049–8056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leeder, A.J.; Heap, R.J.; Brown, L.J.; Franck, X.; Brown, R.C. A short diastereoselective total synthesis of (±)-vibralactone. Org. Lett. 2016, 18, 5971–5973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, M.Y.; Wang, F.; Yang, X.L.; Fang, L.Z.; Dong, Z.J.; Zhu, H.J.; Liu, J.K. Derivatives of vibralactone from cultures of the basidiomycete Boreostereum vibrans. Chem. Pharm. Bull. 2008, 56, 1286–1288. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.Y.; Zhang, L.; Dong, Z.J.; Yang, Z.L.; Leng, Y.; Liu, J.K. Vibralactones D–F from Cultures of the Basidiomycete Boreostereum vibrans. Chem. Pharm. Bull. 2010, 58, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.Q.; Wei, K.; Feng, T.; Li, Z.H.; Zhang, L.; Wang, Q.A.; Liu, J.K. Vibralactones G–J from cultures of the basidiomycete Boreostereum vibrans. J. Asian Nat. Prod. Res. 2012, 14, 115–120. [Google Scholar] [CrossRef]
- Wang, G.Q.; Wei, K.; Zhang, L.; Li, Z.H.; Wang, Q.A.; Liu, J.K. Three new vibralactone-related compounds from cultures of Basidiomycete Boreostereum vibrans. J. Asian Nat. Prod. Res. 2014, 16, 447–452. [Google Scholar] [CrossRef]
- Zhao, P.J.; Yang, Y.L.; Du, L.; Liu, J.K.; Zeng, Y. Elucidating the Biosynthetic Pathway for Vibralactone: A Pancreatic Lipase Inhibitor with a Fused Bicyclic β-Lactone. Angew. Chem. Int. Ed. 2013, 52, 2298–2302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Zhou, H.; Du, G.; Feng, K.N.; Feng, T.; Fu, X.L.; Liu, J.K.; Zeng, Y. A monooxygenase from Boreostereum vibrans catalyzes oxidative decarboxylation in a divergent vibralactone biosynthesis pathway. Angew. Chem. Int. Ed. 2016, 55, 5463–5466. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, D.; Brandt, P.; Blanchette, R.A.; Nett, M.; Hoffmeister, D. Unexpected metabolic versatility in a combined fungal fomannoxin/vibralactone biosynthesis. J. Nat. Prod. 2016, 79, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Matsuda, M.; Seki, M.; Hirose, M.; Kikuchi, T. Sterepinic Acids A–C, New Carboxylic Acids Produced by a Marine Alga-Derived Fungus. Molecules 2018, 23, 1336. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.S.; Kim, J.P. Ostalactones A-C, beta- and epsilon-Lactones with Lipase Inhibitory Activity from the Cultured Basidiomycete Stereum ostrea. J. Nat. Prod. 2016, 79, 3148–3151. [Google Scholar] [CrossRef]
- Yao, J.N.; Chen, L.; Tang, Y.; Chen, H.P.; Zhao, Z.Z.; Li, Z.H.; Feng, T.; Liu, J.K. Lanostane triterpenoids from fruiting bodies of basidiomycete Stereum sp., structures and biological activities. J. Antibiot. 2017, 70, 1104–1111. [Google Scholar] [CrossRef]
- Hu, Z.X.; Hu, K.; Shi, Y.M.; Wang, W.G.; Du, X.; Li, Y.; Zhang, Y.H.; Pu, J.X.; Sun, H.D. Rearranged 6/6/5/6-fused triterpenoid acids from the stems of Kadsura coccinea. J. Nat. Prod. 2016, 79, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Lian-niang, L.; Hong, X.; Kangouri, K.; Ikeda, A.; Omura, S. Triterpenoid Acids fromKadsura longipedunculata. Neokadsuranic Acids B and C: Two Novel Triterpenoids with 14 (13 → 12)abeo-Lanostane Skeletons. Planta Med. 1989, 55, 294–296. [Google Scholar] [CrossRef]
- Yao, J.N.; Chen, L.; Chen, H.P.; Zhao, Z.Z.; Zhang, S.B.; Huang, Y.; Tang, Y.; Isaka, M.; Li, Z.H.; Feng, T.; et al. Miscellaneous lanostane triterpenoids with cytotoxicities from fruiting bodies of the basidiomycete Stereum sp. Fitoterapia 2018, 125, 227–234. [Google Scholar] [CrossRef]
- Hybelbauerová, S.; Sejbal, J.; Dračínský, M.; Hahnová, A.; Koutek, B. Chemical Constituents of Stereum subtomentosum and Two Other Birch-Associated Basidiomycetes: An Interspecies Comparative Study. Chem. Biodivers. 2008, 5, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.; Carvalho, J.F.; Neves, M.A.; Silvestre, S.M.; Leitao, A.J.; Silva, M.M.C.; Melo, M.L.S. Anticancer steroids: Linking natural and semi-synthetic compounds. Nat. Prod. Rep. 2013, 30, 324–374. [Google Scholar] [CrossRef] [PubMed]
- Duecker, F.L.; Reuß, F.; Heretsch, P. Rearranged ergostane-type natural products: Chemistry, biology, and medicinal aspects. Org. Biom. Chem. 2019, 17, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Chen, H.P.; Wu, B.; Zhang, L.; Li, Z.H.; Feng, T.; Liu, J.K. Matsutakone and matsutoic acid, two (nor) steroids with unusual skeletons from the edible mushroom Tricholoma matsutake. J. Org. Chem. 2017, 82, 7974–7979. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.Z.; Han, K.Y.; Li, Z.H.; Feng, T.; Chen, H.P.; Liu, J.K. Cytotoxic ergosteroids from the fungus Stereum hirsutum. Phytochem. Lett. 2019, 30, 143–149. [Google Scholar] [CrossRef]
- Cateni, F.; Doljak, B.; Zacchigna, M.; Anderluh, M.; Piltaver, A.; Scialino, G.; Banfi, E. New biologically active epidioxysterols from Stereum hirsutum. Bioorg. Med. Chem. Lett. 2007, 17, 6330–6334. [Google Scholar] [CrossRef] [PubMed]
- Woldemichael, G.M.; Franzblau, S.G.; Zhang, F.; Wang, Y.; Timmermann, B.N. Inhibitory effect of sterols from Ruprechtia triflora and diterpenes from Calceolaria pinnifolia on the growth of Mycobacterium tuberculosis. Planta Med. 2003, 69, 628–631. [Google Scholar]
- Kuria, K.A.; Chepkwony, H.; Govaerts, C.; Roets, E.; Busson, R.; de Witte, P.; Zupko, I.; Hoornaert, G.; Quirynen, L.; Maes, L. The Antiplasmodial Activity of Isolates from Ajuga remota. J. Nat. Prod. 2002, 65, 789–793. [Google Scholar] [CrossRef]
- Kuo, Y.; Weng, S.; Chou, C.; Chang, T.; Tsai, W. Activation and proliferation signals in primary human T lymphocytes inhibited by ergosterol peroxide isolated from Cordyceps cicadae. Br. J. Pharmacol. 2003, 140, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.S.; Jo, Y.S.; Kim, Y.H.; Hyun, J.W.; Kim, H.W. Cytotoxic activities of acetoxyscirpenediol and ergosterol peroxide from Paecilomyces tenuipes. Life Sci. 2001, 69, 229–237. [Google Scholar] [CrossRef]
- Mizushina, Y.; Watanabe, I.; Togashi, H.; Hanashima, L.; Takemura, M.; Ohta, K.; Sugawara, F.; Koshino, H.; Esumi, Y.; Uzawa, J. An ergosterol peroxide, a natural product that selectively enhances the inhibitory effect of linoleic acid on DNA polymerase β. Biol. Pharm. Bull. 1998, 21, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Kobori, M.; Yoshida, M.; Ohnishi-Kameyama, M.; Takei, T.; Shinmoto, H. 5α, 8α-Epidioxy-22E-ergosta-6, 9 (11), 22-trien-3β-ol from an edible mushroom suppresses growth of HL60 leukemia and HT29 colon adenocarcinoma cells. Biol. Pharm. Bull. 2006, 29, 755–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gloer, J.B. The chemistry of fungal antagonism and defense. Can. J. Bot. 1995, 73, 1265–1274. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, Y.X.; Li, Y.; Zhang, R.; Hu, T.Y.; Su, P.; Zhou, M.; Tang, T.; Zeng, Y.; Yang, Y.L.; et al. Rapid discovery and functional characterization of diterpene synthases from basidiomycete fungi by genome mining. Fungal Genet. Biol. 2019, 128, 36–42. [Google Scholar] [CrossRef] [PubMed]











© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, M.; Zhao, P.; Li, G.; Zhang, K. In Depth Natural Product Discovery from the Basidiomycetes Stereum Species. Microorganisms 2020, 8, 1049. https://doi.org/10.3390/microorganisms8071049
Tian M, Zhao P, Li G, Zhang K. In Depth Natural Product Discovery from the Basidiomycetes Stereum Species. Microorganisms. 2020; 8(7):1049. https://doi.org/10.3390/microorganisms8071049
Chicago/Turabian StyleTian, Mengqing, Peiji Zhao, Guohong Li, and Keqin Zhang. 2020. "In Depth Natural Product Discovery from the Basidiomycetes Stereum Species" Microorganisms 8, no. 7: 1049. https://doi.org/10.3390/microorganisms8071049




