Inhibition of Salmonella Binding to Porcine Intestinal Cells by a Wood-Derived Prebiotic
Abstract
1. Introduction
2. Material and Methods
2.1. Bacteria
2.2. Prebiotic
2.3. Effects on Binding Activity via Slide Agglutination Tests
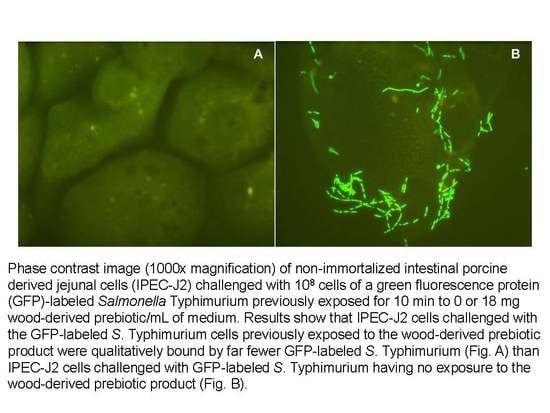
2.4. Effects on Adherence of a GFP-Labeled S. Typhimurium to Intestinal Porcine Epithelial Cells
2.5. Effect on Invasiveness of S. Typhimurium DT104 for Intestinal Porcine Epithelial Cells
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects on Binding Activity via Slide Agglutination Tests
3.2. Effects on Adherence of a GFP-Labeled S. Typhimurium to Intestinal Porcine Epithelial Cells
3.3. Effect on Invasiveness of S. Typhimurium DT104 to Intestinal Porcine Epithelial Cells
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Da Silva, C.; Callegari, M.; Dalto, D. A review of prevention and control methods of salmonella species in swine production and the role of dietary non-nutritional additives. Asian J. Anim. Vet. Adv. 2015, 10, 803–829. [Google Scholar] [CrossRef][Green Version]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Zijlstra, R.T. Dietary starch and fiber as prebiotics in swine diets. In Cutting Edge Nutritional Strategies for Improving Performance, Profitability and Sustainability, Proceedings of the 2018 Animal Nutrition Conference of Canada, Edmonton, AB, Canada, 2–3 May 2018; Animal Nutrition Association of Canada (ANAC): Ottawa, ON, Canada, 2018; pp. 136–142. [Google Scholar]
- Sharon, N. Carbohydrates as future anti-adhesion drugs for infectious diseases. Biochim. Biophys. Acta 2006, 1760, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Oyofo, B.A.; Deloach, J.R.; Corrier, D.E.; Norman, J.O.; Ziprin, R.L.; Mollenhauer, H.H. Effect of Carbohydrates on Salmonella typhimurium Colonization in Broiler Chickens. Avian Dis. 1989, 33, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Anuta, J.; Buentello, A.; Patnaik, S.; Hume, M.; Mustafa, A.; Gatlin, D.; Lawrence, A. Effects of dietary supplementation of a commercial prebiotic Previda®on survival, growth, immune responses and gut microbiota of Pacific white shrimp, Litopenaeus vannamei. Aquac. Nutr. 2014, 22, 410–418. [Google Scholar] [CrossRef]
- Faber, T.A.; Hopkins, A.C.; Middelbos, I.S.; Price, N.P.; Fahey, G.C. Galactoglucomannan oligosaccharide supplementation affects nutrient digestibility, fermentation end-product production, and large bowel microbiota of the dog1. J. Anim. Sci. 2011, 89, 103–112. [Google Scholar] [CrossRef]
- Faber, T.A.; Dilger, R.N.; Hopkins, A.C.; Price, N.P.; Fahey, G.C., Jr. Effects of oligosaccharides in a soybean meal-based diet on fermentative and immune responses in broiler chicks challenged with Eimeria acervuline. Poult. Sci. 2012, 9, 3132–3140. [Google Scholar] [CrossRef]
- Faber, T.A.; Dilger, R.; Iakiviak, M.; Hopkins, A.C.; Price, N.P.; Fahey, G.C. Ingestion of a novel galactoglucomannan oligosaccharide-arabinoxylan (GGMO-AX) complex affected growth performance and fermentative and immunological characteristics of broiler chicks challenged with Salmonella typhimurium1. Poult. Sci. 2012, 91, 2241–2254. [Google Scholar] [CrossRef]
- Anderson, R.C.; Buckley, S.A.; Callaway, T.R.; Genovese, K.J.; Kubena, L.F.; Harvey, R.; Nisbet, D.J. Effect of sodium chlorate on salmonella typhimurium concentrations in the weaned pig gut. J. Food Prot. 2001, 64, 255–258. [Google Scholar] [CrossRef]
- Anderson, R.C.; Buckley, S.A.; Kubena, L.F.; Stanker, L.H.; Harvey, R.; Nisbet, D.J. Bactericidal effect of sodium chlorate on escherichia coli O157:H7 and salmonella typhimurium DT104 in rumen contents in vitro. J. Food Prot. 2000, 63, 1038–1042. [Google Scholar] [CrossRef]
- Byrd, J.; Anderson, R.C.; Callaway, T.R.; Moore, R.W.; Knape, K.D.; Kubena, L.F.; Ziprin, R.L.; Nisbet, D.J. Effect of experimental chlorate product administration in the drinking water on Salmonella typhimurium contamination of broilers. Poult. Sci. 2003, 82, 1403–1406. [Google Scholar] [CrossRef]
- Hautefort, I.; Proença, M.J.; Hinton, J.C.D. Single-copy green fluorescent protein gene fusions allow accurate measurement of salmonella gene expression in vitro and during infection of mammalian cells. Appl. Environ. Microbiol. 2003, 69, 7480–7491. [Google Scholar] [CrossRef]
- Genovese, K.J.; Anderson, R.C.; Harvey, R.B.; Nisbet, D.J. Competitive exclusion treatment reduces the mortality and fecal shedding associated with enterotoxigenic Escherichia coli infection in nursery-raised neonatal pigs. Can. J. Vet. Res. 2001, 64, 204–207. [Google Scholar]
- Mirelman, D.; Altmann, G.; Eshdat, Y. Screening of bacterial isolates for mannose-specific lectin activity by agglutination of yeasts. J. Clin. Microbiol. 1980, 11, 328–331. [Google Scholar] [CrossRef]
- Gedek, B.R. Adherence of Escherichia coli serogroup O 157 and the Salmonella typhimurium mutant DT 104 to the surface of Saccharomyces boulardii. Mycoses 1999, 42, 261–264. [Google Scholar] [CrossRef]
- Koh, S.Y.; George, S.; Brözel, V.; Moxley, R.A.; Francis, D.; Kaushik, R.S. Porcine intestinal epithelial cell lines as a new in vitro model for studying adherence and pathogenesis of enterotoxigenic Escherichia coli. Vet. Microbiol. 2008, 130, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Brosnahan, A.J.; Brown, D.R. Porcine IPEC-J2 intestinal epithelial cells in microbiological investigations. Vet. Microbiol. 2012, 156, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.R.; Price, L.D. Characterization of Salmonella enterica serovar Typhimurium DT104 invasion in an epithelial cell line (IPEC J2) from porcine small intestine. Vet. Microbiol. 2007, 120, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.D.; Kohrt, L.J.; Brown, D. Comparison of growth phase on Salmonella enterica serovar Typhimurium invasion in an epithelial cell line (IPEC J2) and mucosal explants from porcine small intestine. Comp. Immunol. Microbiol. Infect. Dis. 2008, 31, 63–69. [Google Scholar] [CrossRef]
- He, H.; Genovese, K.J.; Swaggerty, C.; Nisbet, D.J.; Kogut, M.H. A comparative study on invasion, survival, modulation of oxidative burst, and nitric oxide responses of macrophages (HD11), and systemic infection in chickens by prevalent poultry salmonella serovars. Foodborne Pathog. Dis. 2012, 9, 1104–1110. [Google Scholar] [CrossRef]
- Wray, C.; Woodward, M.J. Escherichia coli infections in farm animals. In Escherichia Coli, Mechanisms of Virulence; Sussman, M., Ed.; Cambridge University Press: Cambridge, UK, 1997; pp. 49–84. [Google Scholar]
- Tiralongo, J.; Moran, A.P. Bacterial lectin-like interactions in cell recognition and adhesion. In Microbial Glycobiology: Structures, Relevance and Applications; Moran, A.P., Holst, O., Brennan, J., von Itzstein, M., Eds.; Academic Press: London, UK, 2009; pp. 551–565. [Google Scholar]
- Turner, M.W. The role of mannose-binding lectin in health and disease. Mol. Immunol. 2003, 40, 423–429. [Google Scholar] [CrossRef]
- Meng, Q.; Kerley, M.S.; Russel, T.J.; Allee, G.L. Lectin-like activity of Escherichia coli K88, Salmonella choleraesuis, and Bifidobacteria pseudolongum of porcine gastrointestinal origin. J. Anim. Sci. 1998, 76, 551–556. [Google Scholar] [CrossRef][Green Version]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R. Prebiotic galactooligosaccharides reduce adherence of Enteropathogenic Escherichia coli to tissue culture Cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef]
- Firon, N.; Ofek, I.; Sharon, N. Carbohydrate specificity of the surface lectins of Escherichia coli, Klebsiella pneumoniae, and Salmonella typhimurium. Carbohydr. Res. 1983, 120, 235–249. [Google Scholar] [CrossRef]
- Kisiela, D.; Laskowska, A.; Sapeta-Rączka, A.; Kuczkowski, M.; Wieliczko, A.; Ugorski, M. Functional characterization of the FimH adhesin from Salmonella enterica serovar Enteritidis. Microbiology 2006, 152, 1337–1346. [Google Scholar] [CrossRef]
- Rajani, J.; Dastar, B.; Samadi, F.; Torshizi, M.A.K.; Abdulkhani, A.; Esfandyarpour, S. Effect of extracted galactoglucomannan oligosaccharides from pine wood (Pinus brutia) on Salmonella typhimuriumcolonisation, growth performance and intestinal morphology in broiler chicks. Br. Poult. Sci. 2016, 57, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Faber, T.A.; Bauer, L.L.; Price, N.P.; Hopkins, A.C.; Fahey, G.C. In vitro digestion and fermentation characteristics of temulose molasses, a coproduct of fiberboard production, and select temulose fractions using canine fecal inoculum. J. Agric. Food Chem. 2011, 59, 1847–1853. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Mikkelsen, L.L.; Jensen, B.B. Effects of nondigestible oligosaccharides on Salmonella enterica serovar typhimurium and nonpathogenic escherichia coli in the pig small intestine in vitro. Appl. Environ. Microbiol. 2001, 67, 3391–3395. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.T.; Boudry, C.; Everaert, N.; Bindelle, J. Prebiotic potential of novel carbohydrates in an in vitro co-inoculation fermentation model of the bacteria isolated from pig intestine and Salmonella. J. Anim. Sci. 2016, 94, 58–61. [Google Scholar] [CrossRef]
- De Melo, E.B.; Gomes, A.D.S.; Carvalho, I. α- and β-Glucosidase inhibitors: Chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Panwar, H.; Calderwood, D.; Grant, I.R.; Grover, S.; Green, B.D. Lactobacillus strains isolated from infant faeces possess potent inhibitory activity against intestinal alpha- and beta-glucosidases suggesting anti-diabetic potential. Eur. J. Nutr. 2014, 53, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lian, G.; Yu, B. Naturally occurring polyphenolic glucosidase inhibitors. Isr. J. Chem. 2015, 55, 268–284. [Google Scholar] [CrossRef]
- Vizcarra, I.A.; Hosseini, V.; Kollmannsberger, P.; Meier, S.; Weber, S.S.; Arnoldini, M.; Ackermann, M.; Vogel, V. How type 1 fimbriae help Escherichia coli to evade extracellular antibiotics. Sci. Rep. 2016, 6, 18109. [Google Scholar] [CrossRef] [PubMed]



| - | Effect of Wood-Derived Prebiotic (mg/mL) on Agglutination Reaction a | |||
|---|---|---|---|---|
| Bacteria | 10 | 20 | 40 | 80 |
| Salmonella Typhimurium (poultry isolate) | +++ | +++ | ++ | - |
| Salmonella Typhimurium NVSL 95-1776 (swine isolate) | +++ | ++ | - | - |
| Salmonella Typhimurium DT104 | +++ | ++ | - | - |
| Salmonella Typhimurium (GFP-labeled) | +++ | - | - | - |
| Salmonella Typhimurium (unlabeled parent) | +++ | - | - | - |
| Escherichia coli CVM 1569 (F6) | +++ | - | - | - |
| Escherichia coli CVM 1585 (F4) | +++ | - | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Božić, A.; Anderson, R.C.; Crippen, T.L.; Swaggerty, C.L.; Hume, M.E.; Beier, R.C.; He, H.; Genovese, K.J.; Poole, T.L.; Harvey, R.B.; et al. Inhibition of Salmonella Binding to Porcine Intestinal Cells by a Wood-Derived Prebiotic. Microorganisms 2020, 8, 1051. https://doi.org/10.3390/microorganisms8071051
Božić A, Anderson RC, Crippen TL, Swaggerty CL, Hume ME, Beier RC, He H, Genovese KJ, Poole TL, Harvey RB, et al. Inhibition of Salmonella Binding to Porcine Intestinal Cells by a Wood-Derived Prebiotic. Microorganisms. 2020; 8(7):1051. https://doi.org/10.3390/microorganisms8071051
Chicago/Turabian StyleBožić, Aleksandar, Robin C. Anderson, Tawni L. Crippen, Christina L. Swaggerty, Michael E. Hume, Ross C. Beier, Haiqi He, Kenneth J. Genovese, Toni L. Poole, Roger B. Harvey, and et al. 2020. "Inhibition of Salmonella Binding to Porcine Intestinal Cells by a Wood-Derived Prebiotic" Microorganisms 8, no. 7: 1051. https://doi.org/10.3390/microorganisms8071051
APA StyleBožić, A., Anderson, R. C., Crippen, T. L., Swaggerty, C. L., Hume, M. E., Beier, R. C., He, H., Genovese, K. J., Poole, T. L., Harvey, R. B., & Nisbet, D. J. (2020). Inhibition of Salmonella Binding to Porcine Intestinal Cells by a Wood-Derived Prebiotic. Microorganisms, 8(7), 1051. https://doi.org/10.3390/microorganisms8071051