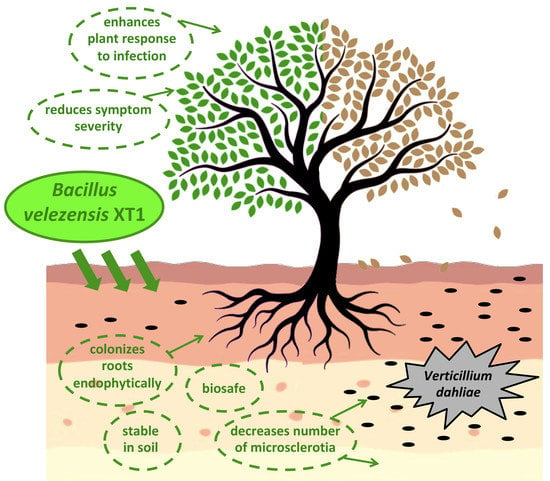
Biological Control of Verticillium Wilt on Olive Trees by the Salt-Tolerant Strain Bacillus velezensis XT1
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strains and Routine Culture Conditions
2.2. Evaluation of the Toxicity of B. velezensis XT1
2.2.1. E. coli MC4100 Sensitivity Assay
2.2.2. A. fischeri NRRL B-11177 Microbial Metabolism Evaluation
2.2.3. C. elegans N2 Pathogenicity Assay
2.3. In Vitro Evaluation of the Antifungal Activity of B. velezensis XT1
2.3.1. Assay in Liquid Media
2.3.2. Assay on Solid Media
2.3.3. Assay on Modified Solid Media
2.3.4. Volatile Compounds Antifungal Test
2.3.5. Crude Lipopeptide Extract Antifungal Test
2.4. Preventive Activity by B. velezensis XT1 of Verticillium Wilt on Olive Trees
2.5. Field Experiments with B. velezensis XT1 on Olive Trees Diseased by V. dahliae
2.6. Permanence of B. velezensis XT1 in Soil
2.7. Ability of B. velezensis XT1 to Colonize Endophytically Olive Roots
2.8. Statistical Analyses
3. Results
3.1. Biosafety of B. velezensis XT1
3.2. In Vitro Antifungal Activity of B. Velezensis XT1
3.3. Prevention of Verticillium Wilt on Olive Trees Treated with B. velezensis XT1
3.4. Reduction in Verticillium Wilt by B. velezensis XT1 on Diseased Olive Trees
3.5. Permanence of B. velezensis XT1 in Soil
3.6. Endophytic Growth of B. velezensis XT1 in Olive Roots
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ruggieri, G. A new disease of olive. L’Italia Agric. 1946, 83, 369–372. [Google Scholar]
- Klosterman, S.J.; Atallah, Z.K.; Vallad, G.E.; Subbarao, K.V. Diversity, pathogenicity and management of Verticillium species. Annu. Rev. Phytopathol. 2009, 47, 39–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keykhasaber, M.; Thomma, B.P.H.J.; Hiemstra, J.A. Verticillium wilt caused by Verticillium dahliae in woody plants with emphasis on olive and shade trees. Eur. J. Plant Pathol. 2018, 150, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Berlanger, I.; Powelson, M.L. Verticillium wilt. Plant Heal. Instr. 2000. [Google Scholar] [CrossRef]
- Landa, B.B.; Pérez, A.G.; Luaces, P.; Montes-Borrego, M.; Navas-Cortés, J.A.; Sanz, C. Insights into the effect of Verticillium dahliae defoliating-pathotype infection on the content of phenolic and volatile compounds related to the sensory properties of virgin olive oil. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- López-Escudero, F.J.; Mercado-Blanco, J. Verticillium wilt of olive: A case study to implement an integrated strategy to control a soil-borne pathogen. Plant Soil 2011, 344, 1–50. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Díaz, R.; Cirulli, M.; Bubici, G.; Jiménez-Gasco, M.; Antoniou, P.; Tjamos, E. Olive, an ancient crop under a major health threat Verticillium wilt on olive: Importance and distribution. Am. Phytopathol. Soc. Plant Dis. 2012, 96, 304–329. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT database, Crops Processed, Data for Olive Oil; FAO: Italy, Rome, 2014. [Google Scholar]
- Ministerio de Agricultura, Alimentación y Medio Ambiente (MAGRAMA). Avances de Superficies y Producciones de Cultivos: Spain. 2015. Available online: https://www.mapa.gob.es/es/estadistica/temas/estadisticas-agrarias/agricultura/avances-superficies-producciones-agricolas/2012-2015.aspx (accessed on 10 July 2020).
- López-Escudero, F.J.; Mercado-Blanco, J.; Roca, J.M.; Valverde-Corredor, A.; Blanco-López, M.A. Verticillium wilt of olive in the Guadalquivir valley (southern Spain): Relations with some agronomical factors and spread of Verticillium dahliae. Phytopathol. Mediterr. 2010, 49, 370–380. [Google Scholar] [CrossRef]
- Prieto, P.; Navarro-Raya, C.; Valverde-Corredor, A.; Amyotte, S.G.; Dobinson, K.F.; Mercado-Blanco, J. Colonization process of olive tissues by Verticillium dahliae and its in planta interaction with the biocontrol root endophyte Pseudomonas fluorescens PICF7. Microb. Biotechnol. 2009, 2, 499–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fradin, E.; Thomma, B. Physiology and molecular aspects of Verticillium wilt diseases caused by V. dahliae and V. albo-atrum. Mol. Plant Pathol. 2006, 7, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Tjamos, E.; Botseas, D. Occurrence of Verticillium dahliae in leaves of Verticillium-wilted olive trees. Can. J. Plant Pathol. 1987, 9, 86. [Google Scholar]
- Hiemstra, J. Some general features of Verticillium wilts in trees. In A Compendium of Verticillium Wilts in Tree Species.; Hiemstra, J., Harris, D., Eds.; Ponsen and Looijen: Wageningen, The Netherlands, 1998; pp. 5–11. [Google Scholar]
- Tjamos, E. Prospects and strategies in controlling Verticillium wilt of olive. EPPO Bull. 1993, 23, 505–512. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Rodrıíguez-Jurado, D.; Hervás, A.; Jiménez-Dıíaz, R.M. Suppression of Verticillium wilt in olive planting stocks by root-associated fluorescent Pseudomonas spp. Biol. Control 2004, 30, 474–486. [Google Scholar] [CrossRef]
- Colla, P.; Gilardi, G.; Gullino, M.L. A review and critical analysis of the European situation of soilborne disease management in the vegetable sector. Phytoparasitica 2012, 40, 515–523. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Borriss, R. Use of Plant-Associated Bacillus Strains as Biofertilizers and Biocontrol Agents in Agriculture. In Bacteria in Agrobiology: Plant Growth Responses; Springer: Berlin, Germany, 2011; pp. 41–76. [Google Scholar]
- Ryu, C.; Farag, M.A.; Hu, C.; Reddy, M.S.; Kloepper, J.W.; Pare, P.W. Bacterial volatiles induced resistance in Arabidobsis. Plant Physiol. 2004, 134, 1017–1026. [Google Scholar] [CrossRef] [Green Version]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Wei, G. Induction of systemic resistance of cucumber to Colletotrichum orbiculare by select strains of plant growth-promoting rhizobacteria. Phytopathology 1991, 81, 1508. [Google Scholar] [CrossRef]
- Deketelaere, S.; Tyvaert, L.; França, S.C.; Hofte, M. Desirable traits of a good biocontrol agent against Verticillium wilt. Front. Microbiol. 2017, 8, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Carrero-Carrón, I.; Trapero-Casas, J.L.; Olivares-García, C.; Monte, E.; Hermosa, R.; Jiménez-Díaz, R.M. Trichoderma asperellum is effective for biocontrol of Verticillium wilt in olive caused by the defoliating pathotype of Verticillium dahliae. Crop Prot. 2016, 88, 45–52. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Agustí-Brisach, C.; Varo, Á.; López-Escudero, F.J.; Trapero, A. A non-pathogenic strain of Fusarium oxysporum as a potential biocontrol agent against Verticillium wilt of olive. Biol. Control 2019, 139, 104045. [Google Scholar] [CrossRef]
- Müller, H.; Westendorf, C.; Leitner, E.; Chernin, L.; Riedel, K.; Schmidt, S.; Eberl, L.; Berg, G. Quorum-sensing effects in the antagonistic rhizosphere bacterium Serratia plymuthica HRO-C48. FEMS Microbiol. Ecol. 2009, 67, 468–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markakis, E.A.; Tjamos, S.E.; Antoniou, P.P.; Paplomatas, E.J.; Tjamos, E.C. Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. BioControl 2016, 61, 293–303. [Google Scholar] [CrossRef]
- Maldonado-González, M.M.; Bakker, P.A.H.M.; Prieto, P.; Mercado-Blanco, J. Arabidopsis thaliana as a tool to identify traits involved in Verticillium dahliae biocontrol by the olive root endophyte Pseudomonas fluorescens PICF7. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef]
- Li, S.; Zhang, N.; Zhang, Z.; Luo, J.; Shen, B.; Zhang, R.; Shen, Q. Antagonist Bacillus subtilis HJ5 controls Verticillium wilt of cotton by root colonization and biofilm formation. Biol. Fertil. Soils 2013, 49, 295–303. [Google Scholar] [CrossRef]
- Azabou, M.C.; Gharbi, Y.; Medhioub, I.; Ennouri, K.; Barham, H.; Tounsi, S.; Triki, M.A. The endophytic strain Bacillus velezensis OEE1: An efficient biocontrol agent against Verticillium wilt of olive and a potential plant growth promoting bacteria. Biol. Control 2020, 142, 104168. [Google Scholar] [CrossRef]
- Levin, A.G.; Lavee, S.; Tsror (Lahkim), L. The influence of salinity on Verticillium dahliae in stem cuttings of five olive cultivars. J. Phytopathol. 2007, 155, 587–592. [Google Scholar] [CrossRef]
- Saadatmand, A.R.; Banihashemi, Z.; Sepaskhah, A.R.; Maftoun, M. Soil salinity and water stress and their effect on susceptibility to Verticillium wilt disease, ion composition and growth of pistachio. J. Phytopathol. 2008, 156, 287–292. [Google Scholar] [CrossRef]
- Regragui, A.; Lahlou, H. Effect of salinity on in vitro Trichoderma harzianum antagonism against Verticillium dahliae. Pakistan J. Biol. Sci. 2005, 8, 872–876. [Google Scholar] [CrossRef] [Green Version]
- Torres, M.; Llamas, I.; Torres, B.; Toral, L.; Sampedro, I.; Béjar, V. Growth promotion on horticultural crops and antifungal activity of Bacillus velezensis XT1. Appl. Soil Ecol. 2020, 150, 103453. [Google Scholar] [CrossRef]
- Varo, A.; Moral, J.; Lozano-Tóvar, M.D.; Trapero, A. Development and validation of an inoculation method to assess the efficacy of biological treatments against Verticillium wilt in olive trees. BioControl 2016, 61, 283–292. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of Caenorhabditis elegans. In Wormbook: The Online Review of C. elegans Biology; WormBook: Pasadena, CA, USA, 2006; pp. 1–11. [Google Scholar]
- Vílchez, J.I.; Navas, A.; González-lópez, J.; Arcos, S.C.; Gutierrez, F.J. Biosafety test for plant growth-promoting bacteria: Proposed environmental and human safety index (EHSI) protocol. Front. Microbiol. 2016, 6, 1514. [Google Scholar] [CrossRef]
- Johnson, B. Microtox® acute toxicity test. In Small-Scale Freshwater Toxicity Investigations: Toxicity Test Methods; Blaise, C., Férard, J., Eds.; Springer: Dordrecht, The Netherlands, 2005; Volume 1, pp. 69–105. ISBN 140203119X. [Google Scholar]
- Cooper, D.; MacDonald, C.; Duff, S.; Kosaric, N. Enhanced production of surfactin from Bacillus subtilis by continuous product removal and metal cations addition. Appli. Environ. Microbiol. 1981, 54, 224–229. [Google Scholar] [CrossRef] [Green Version]
- Onorati, F.; Mecozzi, M. Effects of two diluents in the Microtox® toxicity bioassay with marine sediments. Chemosphere 2004, 54, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Darby, C.; Cosma, C.L.; Thomas, J.H.; Manoil, C. Lethal paralysis of Caenorhabditis elegans by Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 1999, 96, 15202–15207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navas, A.; Cobas, G.; Talavera, M.; Ayala, J.A.; Lopez, J.A.; Martinez, J.L. Experimental validation of Haldane’s hypothesis on the role of infection as an evolutionary force for Metazoans. Proc. Natl. Acad. Sci. USA 2007, 104, 13728–13731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, M.; Mahajan-Miklos, S.; Ausubel, F. Killing of Caenorhabditis elegans by Pseudomonas aeruginosa used to model mammalian bacterial pathogenesis. Proc. Natl. Acad. Sci. USA 1999, 96, 715–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar]
- Williams, P.L.; Dusenbery, D.B. Using the nematode Caenorhabditis elegans to predict mammalian acute lethality to metallic salts. Toxicol. Ind. Health 1988, 4, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Boyd, W.A.; Smith, M.V.; Freedman, J.H. Caenorhabditis elegans as a model in developmental toxicology. Methods Mol. Biol. 2012, 889, 15–24. [Google Scholar] [CrossRef] [Green Version]
- Schaeffer, P.; Millet, J.; Aubert, J.P. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 1965, 54, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Frikha-Gargouri, O.; Ben Abdallah, D.; Ghorbel, I.; Charfeddine, I.; Jlaiel, L.; Triki, M.; Tounsi, S. Lipopeptides from a novel Bacillus methylotrophicus 39b strain supress Agrobacterium crown gall tumours on tomato plants. Pest Manag. Sci. 2017, 73, 568–574. [Google Scholar] [CrossRef]
- Toral, L.; Rodríguez, M.; Béjar, V.; Sampedro, I. Antifungal activity of lipopeptides from Bacillus XT1 CECT 8661 against Botrytis cinerea. Front. Microbiol. 2018, 9, 1315. [Google Scholar] [CrossRef]
- Ji, S.H.; Paul, N.C.; Deng, J.X.; Kim, Y.S.; Yun, B.-S.; Yu, S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 2013, 41, 234–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahimou, F.; Jacques, P.; Deleu, M. Surfactin and iturin A effects on Bacillus subtilis surface hydrophobicity. Enzyme Microb. Technol. 2000, 27, 749–754. [Google Scholar] [CrossRef]
- Schneider, C.; Rasband, W.; Eliceiri, K. NIH Image to ImageJ: 25 years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Yazgan, A.; Özcengiz, G.; Marahiel, M.A. Tn10 insertional mutations of Bacillus subtilis that block the biosynthesis of bacilysin. Biochim. Biophys. Acta Gene Struct. Expr. 2001, 1518, 87–94. [Google Scholar] [CrossRef]
- Colella, C.; Miacola, C.; Amenduni, M.; D’Amico, M.; Bubici, G.; Cirulli, M. Sources of Verticillium wilt resistance in wild olive germplasm from the Mediterranean region. Plant Pathol. 2008, 57, 533–539. [Google Scholar] [CrossRef]
- Trapero, C.; Díez, C.M.; Rallo, L.; Barranco, D.; López-Escudero, F.J. Effective inoculation methods to screen for resistance to Verticillium wilt in olive. Sci. Hortic. 2013, 162, 252–259. [Google Scholar] [CrossRef]
- Doyle, J.; Doyle, J. A rapid total DNA preparation procedure for fresh plant tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Carder, J.; Morton, A.; Tabrett, A.; Barbara, D. Detection and differentiation by PCR of subspecific groups within two Verticillium species causing vascular wilts in herbaceous hosts. In Modern Assays for plant pathogenic Fungi: Identification, detection and quantification; Schots, A., Dewey, F., Oliver, R., Eds.; CAB International: Oxford, UK, 1994; pp. 91–97. [Google Scholar]
- Aquino-Bolaños, E.N.; Mercado-Silva, E. Effects of polyphenol oxidase and peroxidase activity, phenolics and lignin content on the browning of cut jicama. Postharvest Biol. Technol. 2004, 33, 275–283. [Google Scholar] [CrossRef]
- López-Escudero, F.J.; Del Río, C.; Caballero, J.M.; Blanco-López, M.A. Evaluation of olive cultivars for resistance to Verticillium dahliae. Eur. J. Plant Pathol. 2004, 110, 79–85. [Google Scholar] [CrossRef]
- Kabir, Z.; Bhat, R.G.; Subbarao, K.V. Comparison of media for recovery of Verticillium dahliae from soil. Plant Dis. 2004, 88, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Kapulnik, E.; Quick, J.; DeVay, J. Germination of propagules of Verticillium dahliae in soil treated with methionine and other substances affecting ethylene production. Phytopathology 1975, 75, 1348. [Google Scholar]
- Bandoni, R. Safranin O as a rapid nuclear stain for fungi. Mycologia 1979, 11, 873–874. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Soil Map of the World. Revised Legend, by FAO–UNESCO–ISRIC. World Soil Resour. Rep. 1988, 84, 21–22. [Google Scholar]
- Team R.C., R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2007. [Google Scholar]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Robison, M.M.; Shah, S.; Tamot, B.; Pauls, K.P.; Moffatt, B.A.; Glick, B.R. Reduced symptoms of Verticillium wilt in transgenic tomato expressing a bacterial ACC deaminase. Mol. Plant Pathol. 2001, 2, 135–145. [Google Scholar] [CrossRef]
- Hamidov, A.; Helming, K.; Bellocchi, G.; Bojar, W.; Dalgaard, T.; Ghaley, B.B.; Hoffmann, C.; Holman, I.; Holzkämper, A.; Krzeminska, D.; et al. Impacts of climate change adaptation options on soil functions: A review of European case-studies. L. Degrad. Dev. 2018, 29, 2378–2389. [Google Scholar] [CrossRef]
- Kaurichev, I. Prácticas de Edafologia, 1st ed.; Mir Publishers: Moscow, Russia, 1980. [Google Scholar]
- Maas, E.; Hoffman, G. Crop salt tolerance-current assessment. J. Irrig. Drain. Div. 1977, 103, 115–134. [Google Scholar]
- Hollensteiner, J.; Wemheuer, F.; Harting, R.; Kolarzyk, A.M.; Diaz Valerio, S.M.; Poehlein, A.; Brzuszkiewicz, E.B.; Nesemann, K.; Braus-Stromeyer, S.A.; Braus, G.H.; et al. Bacillus thuringiensis and Bacillus weihenstephanensis inhibit the growth of phytopathogenic Verticillium species. Front. Microbiol. 2017, 7, 2171. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Fang, Y.; Tang, C.; Klosterman, S.J.; Tian, C.; Wang, Y. Genomewide transcriptome profiles reveal how Bacillus subtilis lipopeptides inhibit microsclerotia formation in Verticillium dahliae. Mol. Plant-Microbe Interact. 2019, 32, 622–634. [Google Scholar] [CrossRef]
- Cao, Y.; Pi, H.; Chandrangsu, P.; Li, Y.; Wang, Y.; Zhou, H.; Xiong, H.; Helmann, J.D.; Cai, Y. Antagonism of two plant-growth promoting Bacillus velezensis isolates against Ralstonia solanacearum and Fusarium oxysporum. Sci. Rep. 2018, 8, 4360. [Google Scholar] [CrossRef]
- Audrain, B.; Farag, M.A.; Ryu, C.-M.; Ghigo, J.-M. Role of bacterial volatile compounds in bacterial biology. FEMS Microbiol. Rev. 2015, 39, 222–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernando, W.G.D.; Ramarathnam, R.; Krishnamoorthy, A.S.; Savchuk, S.C. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biol. Biochem. 2005, 37, 955–964. [Google Scholar] [CrossRef]
- Mulero-Aparicio, A.; Cernava, T.; Turrà, D.; Schaefer, A.; Di Pietro, A.; López-Escudero, F.J.; Trapero, A.; Berg, G. The role of volatile organic compounds and rhizosphere competence in mode of action of the non-pathogenic Fusarium oxysporum FO12 toward Verticillium wilt. Front. Microbiol. 2019, 10, 1808. [Google Scholar] [CrossRef] [Green Version]
- Keswani, C.; Prakash, O.; Bharti, N.; Vílchez, J.I.; Sansinenea, E.; Lally, R.D.; Borriss, R.; Singh, S.P.; Gupta, V.K.; Fraceto, L.F.; et al. Re-addressing the biosafety issues of plant growth promoting rhizobacteria. Sci. Total Environ. 2019, 690, 841–852. [Google Scholar] [CrossRef]
- Checinska, A.; Paszczynski, A.; Burbank, M. Bacillus and other spore-forming genera: Variations in responses and mechanisms for survival. Annu. Rev. Food Sci. Technol. 2015, 6, 351–369. [Google Scholar] [CrossRef]
- Young, C. Survival of inoculated Bacillus cereus spores and vegetative cells in non-planted and rhizosphere soil. Soil Biol. Biochem. 1995, 27, 1017–1026. [Google Scholar] [CrossRef]
- Prieto, P.; Mercado-Blanco, J. Endophytic colonization of olive roots by the biocontrol strain Pseudomonas fluorescens PICF7. FEMS Microbiol. Ecol. 2008, 64, 297–306. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Reddy, M.S.; Kloepper, J.W. Survival and colonization of rhizobacteria in a tomato transplant system. Can. J. Microbiol. 2003, 49, 383–389. [Google Scholar] [CrossRef] [Green Version]
- Coy, R.M.; Held, D.W.; Kloepper, J.W. Rhizobacterial colonization of bermudagrass by Bacillus spp. in a Marvyn loamy sand soil. Appl. Soil Ecol. 2019, 141, 10–17. [Google Scholar] [CrossRef]
- Mendis, H.C.; Thomas, V.P.; Schwientek, P.; Salamzade, R.; Chien, J.-T.; Waidyarathne, P.; Kloepper, J.; De La Fuente, L. Strain-specific quantification of root colonization by plant growth promoting rhizobacteria Bacillus firmus I-1582 and Bacillus amyloliquefaciens QST713 in non-sterile soil and field conditions. PLoS ONE 2018, 13, e0193119. [Google Scholar] [CrossRef] [PubMed]
- Glandorf, D.C.M.; Brand, I.; Bakker, P.A.H.M.; Schippers, B. Stability of rifampicin resistance as a marker for root colonization studies of Pseudomonas putida in the field. Plant Soil 1992, 147, 135–142. [Google Scholar] [CrossRef]
- Xu, J.-X.; Li, Z.-Y.; Lv, X.; Yan, H.; Zhou, G.-Y.; Cao, L.-X.; Yang, Q.; He, Y.-H. Isolation and characterization of Bacillus subtilis strain 1-L-29, an endophytic bacteria from Camellia oleifera with antimicrobial activity and efficient plant-root colonization. PLoS ONE 2020, 15, e0232096. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.-C.; Xi, H.; Liang, L.; Liu, J.-D.; Liu, C.-H.; Xue, Y.-R.; Yu, X.-Y. Rifampicin-resistance mutations in the rpoB gene in Bacillus velezensis CC09 have pleiotropic effects. Front. Microbiol. 2017, 8, 178. [Google Scholar] [CrossRef] [Green Version]
- Martos-Moreno, C.; López-Escudero, F.J.; Blanco-López, M.A. Resistance of olive cultivars to the defoliating pathotype of Verticillium dahliae. HortScience 2006, 41, 1313–1316. [Google Scholar] [CrossRef] [Green Version]
- Trapero, C.; Rallo, L.; López-Escudero, F.J.; Barranco, D.; Díez, C.M. Variability and selection of Verticillium wilt resistant genotypes in cultivated olive and in the Olea genus. Plant Pathol. 2015, 64, 890–900. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Cabanás, C.G.L.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas spp. strains from the olive (Olea europaea L.) rhizosphere as effective biocontrol agents against Verticillium dahliae: From the host roots to the bacterial genomes. Front. Microbiol. 2018, 9, 227. [Google Scholar] [CrossRef] [Green Version]
- Mulero-Aparicio, A.; Varo, A.; Agustí-Brisach, C.; López-Escudero, F.J.; Trapero, A. Biological control of Verticillium wilt of olive in the field. Crop Prot. 2020, 128, 104993. [Google Scholar] [CrossRef]
- Varo, A.; Raya-Ortega, M.C.; Trapero, A. Selection and evaluation of micro-organisms for biocontrol of Verticillium dahliae in olive. J. Appl. Microbiol. 2016, 121, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Short, D.P.G.; Sandoya, G.; Vallad, G.E.; Koike, S.T.; Xiao, C.-L.; Wu, B.-M.; Gurung, S.; Hayes, R.J.; Subbarao, K.V. Dynamics of Verticillium species microsclerotia in field soils in response to fumigation, cropping patterns and flooding. Phytopathology 2015, 105, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Jurado, D. Interacciones huesped-parasito en la Verticilosis del olivo (Olea europaea L.) inducida por Verticillium dahliae Kleb. Ph.D., Thesis, University of Cordoba, Cordoba, Spain, 1993. [Google Scholar]
- Mercado-Blanco, J.; Rodriguez-Jurado, D.; Perez-Artes, E.; Jimenez-Diaz, R. Detection of the defoliating pathotype of Verticillium dahliae in infected olive plants by nested PCR. Eur. J. Plant Pathol. 2002, 108, 1–13. [Google Scholar] [CrossRef]
- Mercado-Blanco, J.; Collado-Romero, M.; Parrilla-Araujo, S.; Rodríguez-Jurado, D.; Jiménez-Díaz, R.M. Quantitative monitoring of colonization of olive genotypes by Verticillium dahliae pathotypes with real-time polymerase chain reaction. Physiol. Mol. Plant Pathol. 2003, 63, 91–105. [Google Scholar] [CrossRef]
- Tzelepis, G.; Bejai, S.; Sattar, M.N.; Schwelm, A.; Ilbäck, J.; Fogelqvist, J.; Dixelius, C. Detection of Verticillium species in Swedish soils using real-time PCR. Arch. Microbiol. 2017, 199, 1383–1389. [Google Scholar] [CrossRef] [Green Version]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.; Cardinali, A. Role of phenolics in the resistance mechanisms of plants against fungal pathogens and insects. Phytochemistry 2006, 661, 23–67. [Google Scholar]
- Agrios, G. Plant Pathology; Elsevier Academic Press: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Xue, L.; Gu, M.-Y.; Xu, W.-L.; Lu, J.-J.; Xue, Q.-H. Antagonistic Streptomyces enhances defense-related responses in cotton for biocontrol of wilt caused by phytotoxin of Verticillium dahliae. Phytoparasitica 2016, 44, 225–237. [Google Scholar] [CrossRef]




| Treatment | Before the Treatment | After 14 Months |
|---|---|---|
| Control | 1.6 ± 0.6a | 2.7 ± 1.2 a |
| B. velezensis XT1 | 1.6 ± 0.6a | 1.0 ± 0.9 b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, D.; Torres, M.; Sampedro, I.; Martínez-Checa, F.; Torres, B.; Béjar, V. Biological Control of Verticillium Wilt on Olive Trees by the Salt-Tolerant Strain Bacillus velezensis XT1. Microorganisms 2020, 8, 1080. https://doi.org/10.3390/microorganisms8071080
Castro D, Torres M, Sampedro I, Martínez-Checa F, Torres B, Béjar V. Biological Control of Verticillium Wilt on Olive Trees by the Salt-Tolerant Strain Bacillus velezensis XT1. Microorganisms. 2020; 8(7):1080. https://doi.org/10.3390/microorganisms8071080
Chicago/Turabian StyleCastro, David, Marta Torres, Inmaculada Sampedro, Fernando Martínez-Checa, Borja Torres, and Victoria Béjar. 2020. "Biological Control of Verticillium Wilt on Olive Trees by the Salt-Tolerant Strain Bacillus velezensis XT1" Microorganisms 8, no. 7: 1080. https://doi.org/10.3390/microorganisms8071080