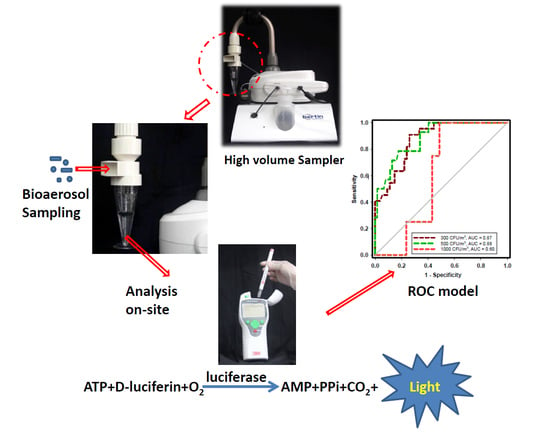
Optimization of a Portable Adenosine Triphosphate Bioluminescence Assay Coupled with a Receiver Operating Characteristic Model to Assess Bioaerosol Concentrations on Site
Abstract
1. Introduction
2. Material and Methods
2.1. Phase One: Optimal Conditions for the Coriolis Sampler Coupled with the ATP Assay
2.1.1. Test Microorganisms and Culture
2.1.2. ATP and Bacterial Standard Curves
2.1.3. Preparation of Viable and Dead Microbes
2.1.4. Aerosol Preparation and Test System
2.1.5. Test Sampler and Sample Processing
2.1.6. Calculation of VR and CR Values for Air Sampling of Bioaerosols
2.1.7. Statistical Analysis
2.2. Phase Two: Field Sampling in Hospitals, Libraries and Long-Term Care Institutions
2.2.1. Sampling Locations
2.2.2. Field Aerosol Sampling
2.2.3. Statistical Analysis
3. Results
3.1. ATP Standard Curves; Correlation of ATP Contents and RLU Values
3.2. Culture Standard Curves; Correlation of RLU Values and Culture Results
3.3. Comparison of Culturable and Viable Ratios as Measured by Culture and ATP Assays
3.4. The Lost and Replenished Medium Volume during Air Sampling with the Coriolis Sampler
3.5. VR and CR for Different Bioaerosol Species Collected by the BioSampler and the Coriolis Sampler
3.6. Applying the Coriolis Sampler Coupled with the ATP Assay in the Field
3.7. The ATP Criterion Indicates Airborne Bacterial and Fungal Levels by ROC Curves
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Stockwell, R.E.; Ballard, E.L.; O’Rourke, P.; Knibbs, L.D.; Morawska, L.; Bell, S.C. Indoor hospital air and the impact of ventilation on bioaerosols: A systematic review. J. Hosp. Infect. 2019, 103, 175–184. [Google Scholar] [CrossRef]
- Seshadri, S.; Han, T.; Krumins, V.; Fennell, D.E.; Mainelis, G. Application of ATP bioluminescence method to characterize performance of bioaerosol sampling devices. J. Aerosol Sci. 2009, 40, 113–121. [Google Scholar] [CrossRef]
- Park, C.W.; Park, J.W.; Lee, S.H.; Hwang, J. Real-time monitoring of bioaerosols via cell-lysis by air ion and ATP bioluminescence detection. Biosens. Bioelectron. 2014, 52, 379–383. [Google Scholar] [CrossRef]
- Oppliger, A.; Masclaux, F.G.; Niculita-Hirzel, H. Assessment of airborne microorganisms by real-time PCR: Optimistic findings and research challenges. Front. Biosci. (Schol. Ed.) 2011, 3, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Chua, B.; Son, A. Detection of airborne bacteria with disposable bio-precipitator and NanoGene assay. Biosens. Bioelectron. 2016, 83, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, K.A.; Chua, B.; Son, A. Development of first generation in-situ pathogen detection system (Gen1-IPDS) based on NanoGene assay for near real time E. coli O157:H7 detection. Biosens. Bioelectron. 2014, 54, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.I.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. (China) 2017, 51, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, B.; Lal, H.; Srivastava, A. Review of bioaerosols in indoor environment with special reference to sampling, analysis and control mechanisms. Environ. Int. 2015, 85, 254–272. [Google Scholar] [CrossRef]
- Tseng, C.C.; Hsiao, P.K.; Chang, K.C.; Cheng, C.C.; Yiin, L.M.; Hsieh, C.J. Detection of viable antibiotic-resistant/sensitive Acinetobacter baumannii in indoor air by propidium monoazide quantitative polymerase chain reaction. Indoor Air 2015, 25, 475–487. [Google Scholar] [CrossRef]
- Karl, D.M. Cellular nucleotide measurements and applications in microbial ecology. Microbiol. Rev. 1980, 44, 739–796. [Google Scholar] [CrossRef]
- Lee, S.J.; Park, J.S.; Im, H.T.; Jung, H.-I. A microfluidic ATP-bioluminescence sensor for the detection of airborne microbes. J. Sens. Actuators B Chem. 2008, 132, 443–448. [Google Scholar] [CrossRef]
- Park, J.W.; Kim, H.R.; Hwang, J. Continuous and real-time bioaerosol monitoring by combined aerosol-to-hydrosol sampling and ATP bioluminescence assay. Anal. Chim. Acta 2016, 941, 101–107. [Google Scholar] [CrossRef]
- Park, J.W.; Park, C.W.; Lee, S.H.; Hwang, J. Fast monitoring of indoor bioaerosol concentrations with ATP bioluminescence assay using an electrostatic rod-type sampler. PLoS ONE 2015, 10, e0125251. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Park, C.W.; Byeon, J.H.; Hwang, J. Design and application of an inertial impactor in combination with an ATP bioluminescence detector for in situ rapid estimation of the efficacies of air controlling devices on removal of bioaerosols. Environ. Sci. Technol. 2010, 44, 1742–1746. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; An, S.; Hwang, J.; Park, J.H.; Byeon, J.H. In situ lysis droplet supply to efficiently extract ATP from dust particles for near-real-time bioaerosol monitoring. J. Hazard. Mater. 2019, 369, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kim, Z.Y.; Lee, S.; Ko, G. Comparison of molecular and total ATP-based analytical methods with culture for the analysis of bioaerosols. J. Sci. Total Environ. 2011, 409, 1732–1737. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Dino, C. Use of ATP bioluminescence for assessing the cleanliness of hospital surfaces: A review of the published literature (1990–2012). J. Infect. Public Health 2014, 7, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Han, T.; Wren, M.; DuBois, K.; Therkorn, J.; Mainelis, G. Application of ATP-based bioluminescence for bioaerosol quantification: Effect of sampling method. J. Aerosol Sci. 2015, 90, 114–123. [Google Scholar] [CrossRef]
- Whiteley, G.S.; Derry, C.; Glasbey, T. The comparative performance of three brands of portable ATP-bioluminometer intended for use in hospital infection control. J. Healthc. Infect. 2012, 17, 91–97. [Google Scholar] [CrossRef]
- Bottari, B.; Santarelli, M.; Neviani, E. Determination of microbial load for different beverages and foodstuff by assessment of intracellular ATP. Trends Food Sci. Technol. 2015, 44, 36–48. [Google Scholar] [CrossRef]
- Tseng, C.C.; Chang, D.C.; Chang, K.C. Development of a Biocontrol Method Applying Bacteriophage-Containing Aerosol against Mycobacterium tuberculosis Using the Bacteriophage BTCU-1 and M. smegmatis as Models. Microorganisms 2019, 7, 237. [Google Scholar] [CrossRef] [PubMed]
- Goyer, N.; Lavoie, J.; Lazure, L.; Marchand, G.; Allard, R.; Bhérer, L. Bioaerosols in the Workplace: Evaluation, Control and Prevention Guide. Direction des communications 505, boul. de Maisonneuve Ouest Montréal (Québec), Canada. 2001. Available online: https://www.irsst.qc.ca/media/documents/PubIRSST/T-24.pdf?v=2020-06-25 (accessed on 25 June 2020).
- Davies, R.; Summerbell, R.; Haldane, D.; Dufour, A.; Yu, K.; Broder, I.; Dales, R.; Kirkbride, J.; Kauri, T.; Robertson, W. Fungal Contamination in Public Buildings: A Guide to Recognition and Management; Environmental Health Directorate, Health Canada, Ottawa: Ottawa, ON, Canada, 1995.
- US Environmental Protection Agency. A Standardized EPA Protocol for Characterizing Indoor Air Quality in Large Office Buildings; US Environmental Protection Agency: Washington, DC, USA, 2003.
- Venkateswaran, K.; Hattori, N.; La Duc, M.T.; Kern, R. ATP as a biomarker of viable microorganisms in clean-room facilities. J. Microbiol. Methods 2003, 52, 367–377. [Google Scholar] [CrossRef]
- Rao, C.Y.; Burge, H.A.; Chang, J.C. Review of quantitative standards and guidelines for fungi in indoor air. J. Air Waste Manag. Assoc. 1996, 46, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Hsiao, P.-K.; Chang, K.-C.; Chen, W.-T.; Yiin, L.-M.; Hsieh, C.-J. Optimization of propidium monoazide quantitative PCR for evaluating performances of bioaerosol samplers for sampling airborne Staphylococcus aureus. Aerosol Sci. Technol. 2014, 48, 1308–1319. [Google Scholar] [CrossRef]
- Carvalho, E.; Sindt, C.; Verdier, A.; Galan, C.; O’Donoghue, L.; Parks, S.; Thibaudon, M. Performance of the Coriolis air sampler, a high-volume aerosol-collection system for quantification of airborne spores and pollen grains. Aerobiologia 2008, 24, 191–201. [Google Scholar] [CrossRef]
- Cho, Y.S.; Hong, S.C.; Choi, J.; Jung, J.H. Development of an automated wet-cyclone system for rapid, continuous and enriched bioaerosol sampling and its application to real-time detection. Sens. Actuators B Chem. 2019, 284, 525–533. [Google Scholar] [CrossRef]
- Sigaev, G.I.; Tolchinsky, A.D.; Sigaev, V.I.; Soloviev, K.G.; Varfolomeev, A.N.; Chen, B.T.J.A. Development of a cyclone-based aerosol sampler with recirculating liquid film: Theory and experiment. Aerosol Sci. Technol. 2006, 40, 293–308. [Google Scholar] [CrossRef]
- Gómez-Domenech, M.; García-Mozo, H.; Alcázar, P.; Brandao, R.; Caeiro, E.; Munhoz, V.; Galán, C. Evaluation of the efficiency of the Coriolis air sampler for pollen detection in South Europe. Aerobiologia 2010, 26, 149–155. [Google Scholar] [CrossRef]
- Willeke, K.; Lin, X.; Grinshpun, S.A. Improved aerosol collection by combined impaction and centrifugal motion. Aerosol Sci. Technol. 1998, 28, 439–456. [Google Scholar] [CrossRef]
- Lin, X.; Reponen, T.; Willeke, K.; Wang, Z.; Grinshpun, S.A.; Trunov, M. Survival of airborne microorganisms during swirling aerosol collection. Aerosol Sci. Technol. 2000, 32, 184–196. [Google Scholar] [CrossRef]
- Dybwad, M.; Skogan, G.; Blatny, J.M. Comparative testing and evaluation of nine different air samplers: End-to-end sampling efficiencies as specific performance measurements for bioaerosol applications. Aerosol Sci. Technol. 2014, 48, 282–295. [Google Scholar] [CrossRef]
- Verreault, D.; Gendron, L.; Rousseau, G.M.; Veillette, M.; Masse, D.; Lindsley, W.G.; Moineau, S.; Duchaine, C. Detection of airborne lactococcal bacteriophages in cheese manufacturing plants. Appl. Environ. Microbiol. 2011, 77, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, A.P.; Reboux, G.; Scherer, E.; Vacheyrou, M.; Millon, L. Contribution of a cyclonic-based liquid air collector for detecting Aspergillus fumigatus by QPCR in air samples. J. Occup. Environ. Hyg. 2012, 9, D7–D11. [Google Scholar] [CrossRef]
- King, M.D.; McFarland, A.R. Bioaerosol sampling with a wetted wall cyclone: Cell culturability and DNA integrity of Escherichia coli bacteria. Aerosol Sci. Technol. 2012, 46, 82–93. [Google Scholar] [CrossRef]
- Nelson, W.H. Physical Methods for Microorganisms Detection; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Langer, V.; Hartmann, G.; Niessner, R.; Seidel, M. Rapid quantification of bioaerosols containing L. pneumophila by Coriolis® μ air sampler and chemiluminescence antibody microarrays. J. Aerosol Sci. 2012, 48, 46–55. [Google Scholar] [CrossRef]
- Zorman, T.; Jeršek, B. Assessment of bioaerosol concentrations in different indoor environments. Indoor Built Environ. 2008, 17, 155–163. [Google Scholar] [CrossRef]
- Madureira, J.; Paciência, I.; Rufo, J.C.; Pereira, C.; Teixeira, J.P.; de Oliveira Fernandes, E. Assessment and determinants of airborne bacterial and fungal concentrations in different indoor environments: Homes, child day-care centres, primary schools and elderly care centres. Atmos. Environ. 2015, 109, 139–146. [Google Scholar] [CrossRef]
- Harkawy, A.; Gorny, R.L.; Ogierman, L.; Wlazlo, A.; Lawniczek-Walczyk, A.; Niesler, A. Bioaerosol assessment in naturally ventilated historical library building with restricted personnel access. Ann. Agric. Environ. Med. 2011, 18, 323–329. [Google Scholar]
- Lee, B.U.; Hong, I.G.; Lee, D.H.; Chong, E.-S.; Jung, J.H.; Lee, J.H.; Kim, H.J.; Lee, I.-S. Bacterial bioaerosol concentrations in public restroom environments. Aerosol Air Qual. Res. 2012, 12, 251–255. [Google Scholar] [CrossRef]
- Hsu, C.S.; Lu, M.C.; Huang, D.J. Disinfection of indoor air microorganisms in stack room of university library using gaseous chlorine dioxide. Environ. Monit. Assess. 2015, 187, 17. [Google Scholar] [CrossRef]
- The Government of the Hong Kong Special Administrative Region. Indoor Air Quality Management Group. A Guide on Indoor Air Quality Certification Scheme for Offices and Public Places. 2003. Available online: http://www.zyaura.com/tutorial/regulation/A%20Guide%20on%20Indoor%20Air%20Quality%20Scheme%20for%20Offices%20and%20Public%20Places.pdf (accessed on 25 June 2020).
- Rafał, L. Górny Bioaerosols and OSH. Available online: https://oshwiki.eu/wiki/Bioaerosols_and_OSH (accessed on 25 June 2020).









© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, C.-C.; Lu, Y.-C.; Chang, K.-C.; Hung, C.-C. Optimization of a Portable Adenosine Triphosphate Bioluminescence Assay Coupled with a Receiver Operating Characteristic Model to Assess Bioaerosol Concentrations on Site. Microorganisms 2020, 8, 975. https://doi.org/10.3390/microorganisms8070975
Tseng C-C, Lu Y-C, Chang K-C, Hung C-C. Optimization of a Portable Adenosine Triphosphate Bioluminescence Assay Coupled with a Receiver Operating Characteristic Model to Assess Bioaerosol Concentrations on Site. Microorganisms. 2020; 8(7):975. https://doi.org/10.3390/microorganisms8070975
Chicago/Turabian StyleTseng, Chun-Chieh, Yi-Chian Lu, Kai-Chih Chang, and Chien-Che Hung. 2020. "Optimization of a Portable Adenosine Triphosphate Bioluminescence Assay Coupled with a Receiver Operating Characteristic Model to Assess Bioaerosol Concentrations on Site" Microorganisms 8, no. 7: 975. https://doi.org/10.3390/microorganisms8070975
APA StyleTseng, C.-C., Lu, Y.-C., Chang, K.-C., & Hung, C.-C. (2020). Optimization of a Portable Adenosine Triphosphate Bioluminescence Assay Coupled with a Receiver Operating Characteristic Model to Assess Bioaerosol Concentrations on Site. Microorganisms, 8(7), 975. https://doi.org/10.3390/microorganisms8070975