Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil
Abstract
1. Introduction
2. Materials and Methods
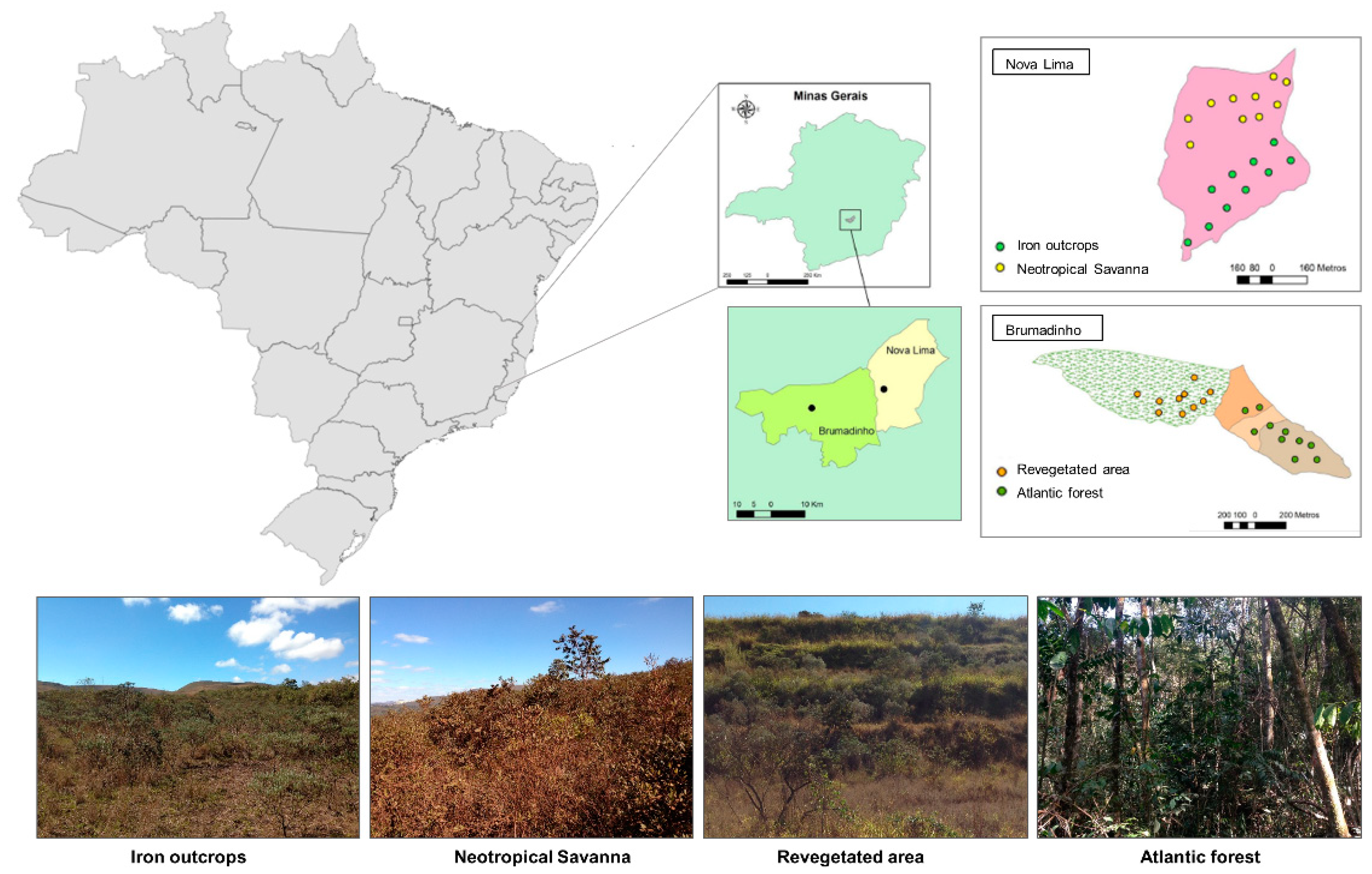
2.1. Study Area and Sampling Design
2.2. Yeast Isolation and Identification
2.3. Community Structure and Multivariate Statistics
3. Results
3.1. Soil Characterization
3.2. Yeast Diversity
3.3. Yeast Communities by Phytocenoses
3.4. Yeast Communities by Season
3.5. Multivariate Approach
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Péter, G.; Takashima, M.; Cadez, N. Yeast Habitats: Different but Global. In Yeasts in Natural Ecosystems: Ecology; Buzzini, P., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 39–71. [Google Scholar]
- Botha, A. The importance and ecology of yeasts in soil. Soil Biol. Biochem. 2011, 43, 1–8. [Google Scholar] [CrossRef]
- Golubtsova, Y.V.; Glushakova, A.M.; Chernov, I.Y. The Seasonal Dynamics of Yeast Communities in the Rhizosphere of Soddy-Podzolic Soils. Eurasian Soil Sci. 2007, 40, 875–879. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Kemler, M.; Begerow, D. Assessment of yeast diversity in soils under different management regimes. Fungal Ecol. 2012, 5, 24–35. [Google Scholar] [CrossRef]
- Mestre, M.C.; Fontenla, S.; Rosa, C.A. Ecology of cultivable yeasts in pristine forests in northern Patagonia (Argentina) influenced by different environmental factors. Can. J. Microbiol. 2014, 60, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, A.M.; Kachalkin, A.V.; Tiunov, A.V.; Chernov, I.Y. Distribution of Yeast Complexes in the Profiles of Different Soil Types. Eurasian Soil Sci. 2017, 50, 820–825. [Google Scholar] [CrossRef]
- Mok, W.Y.; Luizão, R.C.C.; Silva, M.S.B.; Teixeira, M.F.S.; Muniz, E.G. Ecology of Pathogenic Yeasts in Amazonian Soil. Appl. Environ. Microbiol. 1984, 47, 390–394. [Google Scholar] [CrossRef]
- De Azeredo, L.A.I.; Gomes, E.A.T.; Mendonca-Hagler, L.C.; Hagler, A.N. Yeast communities associated with sugarcane in Campos, Rio de Janeiro, Brazil. Int. Microbiol. 1998, 1, 205–208. [Google Scholar]
- Vital, M.; Abranches, J.; Hagler, A.; Mendonça-Hagler, L. Mycocinogenic yeasts isolated from amazon soils of the Maracá ecological station, Roraima-Brazil. Brazilian J. Microbiol. 2002, 33, 230–235. [Google Scholar] [CrossRef]
- Gomes, N.C.M.; Fagbola, O.; Costa, R.; Rumjanek, N.G.; Buchner, A.; Mendona-Hagler, L.; Smalla, K. Dynamics of Fungal Communities in Bulk and Maize Rhizosphere Soil in the Tropics. Appl. Environ. Microbiol. 2003, 69, 3758–3766. [Google Scholar] [CrossRef]
- Carvalho, F.P.; de Souza, A.C.; Magalhães-Guedes, K.T.; Dias, D.R.; Silva, C.F.; Schwan, R.F. Yeasts diversity in Brazilian Cerrado soils: Study of the enzymatic activities. Afr. J. Microbiol. Res. 2013, 7, 4176–4190. [Google Scholar] [CrossRef]
- Skirycz, A.; Castilho, A.; Chaparro, C.; Carvalho, N.; Tzotzos, G.; Siqueira, J.O. Canga biodiversity, a matter of mining. Front. Plant. Sci. 2014, 5, 653. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.K.; Marascalchi, M.N.; Rodrigues, A.V.; de Armas, R.D.; Stürmer, S.L. Morphological and molecular diversity of arbuscular mycorrhizal fungi in revegetated iron-mining site has the same magnitude of adjacent pristine ecosystems. J. Environ. Sci. 2018, 67, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, Ú.R.; Machado, M.M.M.; Castro, P.T.A.; Renger, F.E.; Trevisol, A.; Beato, D.A.C. Geoparque Quadrilátero Ferrífero (MG); Geoparques do Brasil /Propostas—Vol 1; CPRM: Recife/Fortaleza, Brazil, 2011; pp. 183–219. [Google Scholar]
- Mendes Filho, P.F.; Vasconcellos, R.L.F.; De Paula, A.M.; Cardoso, E.J.B.N. Evaluating the potential of forest species under “microbial management” for the restoration of degraded mining areas. Water Air Soil Pollut. 2010, 208, 79–89. [Google Scholar] [CrossRef]
- Gastauer, M.; Souza Filho, P.W.M.; Ramos, S.J.; Caldeira, C.F.; Silva, J.R.; Siqueira, J.O.; Neto, A.E.F. Mine land rehabilitation in Brazil: Goals and techniques in the context of legal requirements. Ambio 2018, 48, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Klauber-Filho, O.; Siqueira, J.O.; Moreira, F.M.S. Fungos micorrízicos arbusculares em solos de área poluída com metais pesados. Rev. Bras. de Ciênc. do Solo 2002, 26, 125–134. [Google Scholar] [CrossRef]
- Silva, K.D.A.; Martins, S.V.; Neto, A.M.; Demolinari, R.D.A.; Lopes, A.T. Restauração Florestal de uma Mina de Bauxita: Avaliação do Desenvolvimento das Espécies Arbóreas Plantadas. Floresta Ambient. 2016, 23, 309–319. [Google Scholar] [CrossRef]
- Gastauer, M.; Silva, J.R.; Caldeira Junior, C.F.; Ramos, S.J.; Souza Filho, P.W.M.; Neto, A.E.F.; Siqueira, J.O. Mine land rehabilitation: Modern ecological approaches for more sustainable mining. J. Clean Prod. 2018, 172, 1409–1422. [Google Scholar] [CrossRef]
- Fernandes, C.C.; Kishi, L.T.; Lopes, E.M.; Omori, W.P.; Souza, J.A.M.; Alves, L.M.C.; Lemos, E.G.M. Bacterial communities in mining soils and surrounding areas under regeneration process in a former ore mine. Braz. J. Microbiol. 2018, 49, 489–502. [Google Scholar] [CrossRef]
- Castro, J.L.; Souza, M.G.; Rufini, M.; Azarias, A.; Rodrigues, T.L.; Moreira, F.M.D.S. Diversity and Efficiency of Rhizobia Communities from Iron Mining Areas Using Cowpea as a Trap Plant. Rev. Bras. Cienc. Solo 2017, 41, 1–20. [Google Scholar] [CrossRef]
- Teixeira, A.F.S.; Kemmelmeier, K.; Marascalchi, M.N.; Sturmer, S.L.; Carneiro, M.A.C.; Moreira, F.M.D.S. Arbuscular mycorrhizal fungal communities in an iron mining area and its surroundings: Inoculum potential, density, and diversity of spores related to soil properties. Ciênc. Agrotecnologia 2017, 41, 511–525. [Google Scholar] [CrossRef]
- Vieira, C.K.; Borges, L.G.A.; Marconatto, L.; Giongo, A.; Stürmer, S.L. Microbiome of a revegetated iron-mining site and pristine ecosystems from the Brazilian Cerrado. Appl. Soil Ecol. 2018, 131, 55–65. [Google Scholar] [CrossRef]
- Moreira, G.A.M.; Vale, H.M.M. Occurrence of Yeast Species in Soils under Native and Modified Vegetation in an Iron Mining Area. Rev. Bras. de Cienc. do Solo 2018, 42, 1–15. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources (WRB), 2015. Available online: http://www.fao.org/3/a-i3794e.pdf (accessed on 9 July 2020).
- Coelho, M.R.; Vasques, G.M.; Tassinari, D.; Souza, Z.R.; Oliveira, A.P.; Moreira, F.M.S. Solos do Quadrilátero Ferrífero sob Diferentes Coberturas Vegetais e Materiais de Origem. Embrapa Solos Bol. de Pesqui. e Desenvolv. 2017, 130. [Google Scholar]
- Donagema, G.K.; Campos, D.V.B.; Calderano, S.B.; Teixeira, W.G.; Viana, J.H.M. Manual de Métodos de Análise do solo, 2nd ed.; Centro Nacional de Pesquisa de Solos: Rio de Janeiro, Brazil, 2011. [Google Scholar]
- Dias, D.R.; Schwan, R.F. Isolamento e identificação de leveduras. In Manual de Biologia dos Solos Tropicais: Amostragem e Caracterização da Biodiversidade; Moreira, F.M.S., Huising, E.J., Bignell, D.E., Eds.; UFLA: Lavras, Brail, 2010; pp. 227–277. [Google Scholar]
- Kurtzman, C.P.; Robnett, C.J. Identification and phylogeny of ascomycetous yeasts from analysis of nuclear large subunit (26S) ribosomal DNA partial sequences. Antonie van Leeuwenhoek 1998, 73, 331–371. [Google Scholar] [CrossRef]
- Fell, J.W.; Boekhout, T.; Fonseca, A.; Scorzetti, G.; Statzell-Tallman, A. Biodiversity and systematics of basidiomycetous yeasts as determined by large-subunit rDNA D1/D2 domain sequence analysis. Int. J. Syst. Evol. Micrbiol. 2000, 50, 1351–1371. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Colwell, R.K.; Mao, C.X.; Chang, J.; Chang, X.M.; Chang, J. Interpolating, Extrapolating, and Comparing Incidence-Based Species Accumulation Curves. Ecology 2004, 85, 2717–2727. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Kemler, M.; Begerow, D. Species accumulation curves and incidence-based species richness estimators to appraise the diversity of cultivable yeasts from beech forest soils. PLoS ONE 2011, 6. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Yergeau, E.; Wong, L.C.; Pijl, A.S.; Van Veen, J.A.; Kowalchuk, G.A. Soil characteristics more strongly influence soil bacterial communities than land-use type. FEMS Microbiol. Ecol. 2012, 79, 12–24. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; Mcglinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community ecology package, R Package version 24-1; 2016. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 20 June 2020).
- The R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2017. [Google Scholar]
- Alvarez, V.V.H.; Novais, R.F.; Barros, N.F.; Cantarutti, R.B.; Lopes, A.S. Interpretação dos resultados das análises de solos. In Recomendações Para o uso de Corretivos e Fertilizantes em Minas Gerais—5a Aproximação; Ribeiro, A.C., Guimarães, P.T.G., Alvarez, V.V.H., Eds.; Viçosa, MG: Comissão de Fertilidade do Solo Do Estado de Minas Gerais; CFSEMG: Viçosa, Brazil, 1999; pp. 25–32. [Google Scholar]
- Yurkov, A. Yeasts in Forest Soils. In Yeasts in Natural Ecosystems: Diversity; Buzzini, P., Lachance, M., Yurkov, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 87–116. [Google Scholar] [CrossRef]
- Mestre, M.C.; Rosa, C.A.; Safar, S.V.B.; Libkind, D.; Fontenla, S.B. Yeast communities associated with the bulk-soil, rhizosphere and ectomycorrhizosphere of a Nothofagus pumilio forest in northwestern Patagonia, Argentina. FEMS Microbiol. Ecol. 2011, 78, 531–541. [Google Scholar] [CrossRef]
- Rodrigues, J.L.M.; Pellizari, V.H.; Mueller, R.; Baek, K.; Jesus, E.C.; Paula, F.S.; Mirza, B.; Hamaoui, G.S.; Tsai, S.M.; Feigl, B.; et al. Conversion of the Amazon rainforest to agriculture results in biotic homogenization of soil bacterial communities. Proc. Natl. Acad. Sci. USA 2013, 110, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Maksimova, I.A.; Chernov, I.Y. Community structure of yeast fungi in forest biogeocenoses. Microbiology 2004, 73, 474–481. [Google Scholar] [CrossRef]
- Masínová, T.; Bahnmann, B.D.; Vetrovský, T.; Tomsovský, M.; Merunková, K.; Baldrian, P. Drivers of yeast community composition in the litter and soil of a temperate forest. FEMS Microbiol. Ecol. 2017, 93, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Harantová, L.; Mudrák, O.; Kohout, P.; Elhottová, D.; Frouz, J.; Baldrian, P. Development of microbial community during primary succession in areas degraded by mining activities. Land Degrad. Dev. 2017, 28, 2574–2584. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Chernov, I.Y.; Tiunov, A.V. Influence of Lumbricus terrestris earthworms on the structure of the yeast community of forest litter. Microbiology 2008, 77, 107–111. [Google Scholar] [CrossRef]
- Dunthorn, M.; Kauserud, H.; Bass, D.; Mayor, J.; Mahé, F. Yeasts dominate soil fungal communities in three lowland Neotropical rainforests. Environ. Microbiol. Rep. 2017, 9, 668–675. [Google Scholar] [CrossRef]
- Sláviková, E.; Vadkertiová, R. The diversity of yeasts in the agricultural soil. J. Basic Microbiol. 2003, 43, 430–436. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Sláviková, E. Metal tolerance of yeasts isolated from water, soil and plant environments. J. Basic Microbiol. 2006, 46, 145–152. [Google Scholar] [CrossRef]
- Chrzanowski, Ł.; Kaczorek, E.; Olszanowski, A. The Ability of Candida Maltosa for Hydrocarbon and Emulsified Hydrocarbon Degradation. Pol. J. Environ. Stud. 2006, 15, 47–51. [Google Scholar]
- Jaiboon, K.; Lertwattanasakul, N.; Limtong, P.; Limtong, S. Yeasts from peat in a tropical peat swamp forest in Thailand and their ability to produce ethanol, indole-3-acetic acid and extracellular enzymes. Mycol. Prog. 2016, 15, 755–770. [Google Scholar] [CrossRef]
- Yurkov, A.M.; Oliver, R.; Pontes, A.; Carvalho, C.; Maldonado, C.; Sampaio, P. Local climatic conditions constrain soil yeast diversity patterns in Mediterranean forests, woodlands and scrub biome. FEMS Yeast Res. 2016, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cadete, R.M.; Melo, M.A.; Dussán, K.J.; Rodrigues, R.C.L.B.; Silva, S.S.; Zilli, J.E.; Vital, M.J.S.; Gomes, F.C.O.; Lachance, M.A.; Rosa, C.A. Diversity and physiological characterization of D-xylose-fermenting yeasts isolated from the Brazilian Amazonian Forest. PLoS ONE. 2012, 7, e43135. [Google Scholar] [CrossRef]
- Morais, C.G.; Cadete, R.M.; Uetanabaro, A.P.T.; Rosa, L.H.; Lachance, M.-A.; Rosa, C.A. D-xylose-fermenting and xylanase-producing yeast species from rotting wood of two Atlantic Rainforest habitats in Brazil. Fungal Genet. Biol. 2013, 60, 19–28. [Google Scholar] [CrossRef]
- Yurkov, A.; Wehde, T.; Kahl, T.; Begerow, D. Aboveground deadwood deposition supports development of soil yeasts. Diversity 2012, 4, 453–474. [Google Scholar] [CrossRef]
- Mannazzu, I.; Landolfo, S.; Lopes, T.; Buzzini, P. Red yeasts and carotenoid production: Outlining a future for non-conventional yeasts of biotechnological interest. World J. Microbiol. Biotechnol. 2015, 31, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.F.; Morais, C.G.; Lopes, M.R.; Santos, R.O.; Uetanabaro, A.P.T.; Morais, P.B.; Vital, M.J.S.; De Morais, M.A.; Lachance, M.A.; Rosa, C.A. D-Xylose fermentation, xylitol production and xylanase activities by seven new species of Sugiyamaella. Antonie van Leeuwenhoek 2017, 110, 53–67. [Google Scholar] [CrossRef]
- Sláviková, E.; Vadkertiová, R. The occurrence of yeasts in the forest soils. J. Basic Microbiol. 2000, 40, 207–212. [Google Scholar] [CrossRef]
- Yurkov, A.M. Yeasts of the soil—obscure but precious. Yeast 2018, 35, 369–378. [Google Scholar] [CrossRef]
- Domsch, H.; Giebel, A. Estimation of Soil Textural Features from Soil Electrical Conductivity Recorded Using the EM38. Precis. Agric. 2004, 5, 389–409. [Google Scholar] [CrossRef]
- Vreulink, J.-M.; Esterhuyse, A.; Jacobs, K.; Botha, A. Soil properties that impact yeast and actinomycete numbers in sandy low nutrient soils. Can. J. Microbiol. 2007, 53, 1369–1374. [Google Scholar] [CrossRef]
- Richter, A.; Schöning, I.; Kahl, T.; Bauhus, J.; Ruess, L. Regional environmental conditions shape microbial community structure stronger than local forest management intensity. For. Ecol. Manage. 2018, 409, 250–259. [Google Scholar] [CrossRef]
- Vishniac, H.S. A multivariate analysis of soil yeasts isolated from a latitudinal gradient. Microb. Ecol. 2006, 52, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Fialová, A.; Boschke, E.; Bley, T. Rapid monitoring of the biodegradation of phenol-like compounds by the yeast Candida maltosa using BOD measurements. Int. Biodeter. Biodegr. 2004, 54, 69–76. [Google Scholar] [CrossRef]
- Kordowska-wiater, M.; Wagner, A.; Hetman, B. Efficacy of Candida melibiosica for control of post-harvest fungal diseases of carrot (Daucus carota L.). Acta Sci. Pol. 2012, 11, 55–65. [Google Scholar]
- Gárdonyi, M.; Osterberg, M.; Rodrigues, C.; Spencer-Martins, I.; Hahn-Hägerdal, B. High capacity xylose transport in Candida intermedia PYCC 4715. FEMS Yeast Res. 2003, 3, 45–52. [Google Scholar]
- Ikeda, R.; Sugita, T.; Jacobson, E.S.; Shinoda, T. Laccase and Melanization in Clinically Important Cryptococcus Species Other than Cryptococcus neoformans. J. Clin. Microbiol. 2002, 40, 1214–1218. [Google Scholar] [CrossRef]
- Eichlerová, I.; Homolka, L.; Zifcáková, L.; Lisá, L.; Dobiásová, P.; Baldrian, P. Enzymatic systems involved in decomposition reflects the ecology and taxonomy of saprotrophic fungi. Fungal Ecol. 2014, 3, 10–22. [Google Scholar] [CrossRef]
- Masinová, T.; Yurkov, A.; Baldrian, P. Forest soil yeasts: Decomposition potential and the utilization of carbon sources. Fungal Biol. 2018, 34, 10–19. [Google Scholar] [CrossRef]
- Vreulink, J.M.; Stone, W.; Botha, A. Effects of small increases in copper levels on culturable basidiomycetous yeasts in low-nutrient soils. J. Appl. Microbiol. 2010, 109, 1411–1421. [Google Scholar] [CrossRef]
- Nakayan, P.; Hameed, A.; Singh, S.; Young, L.S.; Hung, M.H.; Young, C.C. Phosphate-solubilizing soil yeast Meyerozyma guilliermondii CC1 improves maize (Zea mays L.) productivity and minimizes requisite chemical fertilization. Plant. Soil. 2013, 373, 301–315. [Google Scholar] [CrossRef]
- Sarabia, M.; Cazares, S.; González-Rodríguez, A.; Mora, F.; Carreón-Abud, Y.; Larsen, J. Plant growth promotion traits of rhizosphere yeasts and their response to soil characteristics and crop cycle in maize agroecosystems. Rhizosphere 2018, 6, 67–73. [Google Scholar] [CrossRef]
- Botha, A. Yeasts in soil. In Biodiversity and ecophysiology of yeasts; Rosa, C.A., Péter, G., Eds.; Springer: Heidelberg, Germany, 2006; pp. 221–240. [Google Scholar]
- Ramirez, C.; González, A. Five new filamentous, glucose-fermenting Candida isolated from decayed wood in the evergreen rainy Valdivian forest of southern Chile Carlos. Mycopathologia 1984, 92, 83–92. [Google Scholar] [CrossRef]
- González, A.E.; Martínez, A.T.; Almendros, G.; Grinbergs, J. A study of yeasts during the delignification and fungai transformation of wood into cattle feed in Chilean rain forest. Antonie van Leeuwenhoek 1989, 236, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Hagler, A.N. Yeasts as Indicators of Environmental Quality. In Biodiversity and Ecophysiology of Yeasts; Rosa, C.A., Péter, G., Eds.; Springer International Publishing: Cham, Switzeralnd, 2006; pp. 515–533. [Google Scholar]



| Geographical Characteristics | Phytocenoses | |||
|---|---|---|---|---|
| NS | IO | AF | RA | |
| Locality | Nova Lima | Nova Lima | Brumadinho | Brumadinho |
| Latitude | 20°6′42″S | 20°6′52″S | 20°9′4″S | 20°9′35″S |
| Longitude | 43°57′28″W | 43°57′27″W | 44°8′47″W | 44°9′5″W |
| Annual Rainfall (mm) | 1390 | 1390 | 1325 | 1325 |
| Mean Annual Temperature (°C) | 21.0 | 21.0 | 21.3 | 21.3 |
| Impact level | None to low | None to low | None to low | High |
| Soil Physicochemical Parameters a | Neotropical Savanna | Iron Outcrops | Atlantic Forest | Revegetated Area | ||||
|---|---|---|---|---|---|---|---|---|
| Winter | Summer | Winter | Summer | Winter | Summer | Winter | Summer | |
| pH (H2O) | 4.97 | 4.66 | 4.72 | 4.46 | 4.21 | 3.91 | 5.6 | 5.36 |
| K (mg dm−3) | 72.6 a | 83.8 a | 56.8 a | 64.0 a | 75.6 a | 78.0 a | 88.2 a | 85.2 a |
| P (mg dm−3) | 1.363 b | 1.623 b | 1.595 b | 2.275 b | 2.151 b | 2.125 b | 1.66 b | 3.626 a |
| Ca2+ (cmolc dm−3) | 0.91 a | 1.15 a | 1.28 a | 1.476 a | 0.99 a | 1.146 a | 0.75 a | 1.154 a |
| Mg2+ (cmolc dm−3) | 0.38 a | 0.461 a | 0.24 a | 0.269 a | 0.45 a | 0.506 a | 0.3 a | 0.364 a |
| Al3+ (cmolc dm−3) | 1.56 a | 1.65 a | 0.85 b | 0.79 b | 1.9 a | 1.86 a | 0.09 c | 0.12 c |
| H+Al (cmolc dm−3) | 15.46 b | 21.41 a | 12.64 b | 22.01 a | 12.26 b | 18.53 a | 1.94 c | 2.64 c |
| SB (cmolc dm−3) | 1.478 a | 1.826 a | 1.665 a | 1.909 a | 1.633 a | 1.852 a | 1.276 a | 1.737 a |
| t (cmolc dm−3) | 3.03 b | 3.47 a | 2.51 b | 2.69 b | 3.53 a | 3.71 a | 1.36 c | 1.85 c |
| T (cmolc dm−3) | 16.92 b | 23.24 a | 14.31 b | 23.92 a | 13.89 b | 20.38 a | 3.21 c | 4.38 c |
| V (%) | 12.72 b | 10.79 b | 13.56 b | 10.86 b | 13.64 b | 11.17 b | 40.73 a | 40.10 a |
| m (%) | 46.13 a | 46.42 a | 33.78 b | 29.15 b | 59.30 a | 56.81 a | 6.76 c | 7.27 c |
| OM (g kg−1) | 83.02 a | 82.35 a | 75.87 a | 80.8 a | 49.48 b | 48.04 b | 13.87 c | 12.44 c |
| P-rem (mg/L) | 4.56 d | 7.65 d | 12.61 c | 20.10 a | 11.02 c | 16.51 b | 11.03 c | 20.10 a |
| Zn (mg dm−3) | 3.136 a | 3.129 a | 3.292 a | 3.443 a | 1.927 b | 1.687 b | 1.608 b | 2.137 b |
| Fe (mg dm−3) | 134.53 b | 149.05 b | 403.73 a | 401.78 a | 124.79 b | 136.04 b | 150.81 b | 85.32 b |
| Mn (mg dm−3) | 112.29 a | 107.40 a | 88.88 a | 72.45 b | 40.76 b | 45.62 b | 103.99 a | 86.93 a |
| Cu (mg dm−3) | 0.799 b | 0.293 b | 0.57 b | 0.357 b | 0.809 b | 0.561 b | 2.14 a | 1.917 a |
| B (mg dm−3) | 0.202 a | 0.122 b | 0.266 a | 0.123 b | 0.201 a | 0.102 b | 0.154 b | 0.106 b |
| S (mg dm−3) | 36.29 b | 21.33 d | 26.58 c | 15.02 d | 29.06 b | 20.19 d | 45.14 a | 31.08 b |
| Clay (g kg−1) | 376 b | 362 b | 214 c | 193 c | 456 a | 478 a | 249 c | 240 c |
| Silt (g kg−1) | 243 a | 275 a | 174 b | 183 b | 188 b | 178 b | 262 a | 277 a |
| Sand (g kg−1) | 381 c | 363 c | 612 a | 624 a | 356 c | 344 c | 489 b | 483 b |
| EC (mS/cm) | 0.061 b | 0.21 a | 0.052 b | 0.057 b | 0.228 a | 0.182 a | 0.027 b | 0.088 b |
| Yeast Species | GenBank Reference | Frequency (%) | |||||
|---|---|---|---|---|---|---|---|
| Phytocenoses | Seasonality | ||||||
| NS | IO | AF | RA | Winter | Summer | ||
| Ascomycota | |||||||
| Saccharomycetales | |||||||
| Candida maltosa | KJ159031 | 31 | 60 | 43.70 | 40.00 | ||
| Candida melibiosica | KY106567 | 43 | 11.85 | ||||
| Candida parapsilosis | MG871743 | 11 | si | 2.4 | 5.63 | ||
| Candida pseudolambica | KY106707 | 5.3 | 2.96 | ||||
| Candida neerlandica | NG054776 | do | Do | ||||
| Candida sanyaensis | NG054829 | si | Si | ||||
| Candida intermedia | MG815863 | 20 | si | 2.96 | |||
| Diutina rugosa | HE716783 | do | do | ||||
| Candida edaphicus | AB247371 | si | si | Do | |||
| Candida insectorum | JN544058 | si | si | ||||
| Candida quercitrusa | KY996729 | si | si | ||||
| Candida glabrata | MG859667 | si | Si | ||||
| Candida sp. (Kurtzmaniella clade) | MG833304 | si | do | 1.88 | |||
| Schwanniomyces vanrijiae | NG054865 | si | 25 | si | do | 11.88 | |
| Schwanniomyces polymorphus | KY109623 | do | do | 2.96 | |||
| Schwanniomyces sp.1 | LN909492 | si | Si | ||||
| Debaryomyces sp.2 | NG055699 | si | si | do | |||
| Meyerozyma guilliermondii | KY952849 | 8 | do | 4.2 | si | 6.88 | |
| Meyerozyma sp.3 | KY952849 | si | 1.8 | 2.22 | Si | ||
| Barnettozyma californica | MG707647 | 6.7 | do | 1.88 | |||
| Hanseniaspora uvarum | KP990659 | 3 | 3.70 | ||||
| Pichia kudriavzevii | MF769603 | 2.4 | 2.22 | Si | |||
| Kodamaea ohmeri | KY684049 | si | do | si | Do | ||
| Wickerhamomyces anomalus | MF769591 | do | si | si | Do | ||
| Torulaspora delbrueckii | MH010872 | si | Si | ||||
| Torulaspora sp.4 | KY109871 | 1.8 | 1.88 | ||||
| Sugiyamaella xylolytica | KF889433 | do | Do | ||||
| Saturnispora silvae | MG707692 | si | si | Do | |||
| Lachancea kluyveri | KY108237 | si | si | ||||
| Basidiomycota | |||||||
| Tremellales | |||||||
| Papiliotrema laurentii | KY037816 | si | 11 | 11 | 12.59 | 4.38 | |
| Saitozyma podzolica | KX903051 | do | 8 | do | 3.75 | ||
| Apiotrichum laibachii | JQ672597 | si | si | ||||
| Apiotrichum loubieri | KY106137 | si | si | ||||
| Cutaneotrichosporon terricola | FJ527226 | si | si | do | |||
| Sporidiobolales | |||||||
| Rhodotorula mucilaginosa | KY744132 | do | 6 | 7.50 | |||
| Rhodotorula toruloides | KF971831 | si | do | 1.88 | |||
| Rhodotorula dairenensis | KY108994 | do | Do | ||||
| S (obs) | 9 | 13 | 17 | 20 | 23 | 24 | |
| Singletons (si) | 4 | 8 | 6 | 9 | 8 | 6 | |
| Doubletons (do) | 4 | 1 | 6 | 2 | 6 | 7 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro Moreira, G.A.; Martins do Vale, H.M. Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil. Microorganisms 2020, 8, 1116. https://doi.org/10.3390/microorganisms8081116
Monteiro Moreira GA, Martins do Vale HM. Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil. Microorganisms. 2020; 8(8):1116. https://doi.org/10.3390/microorganisms8081116
Chicago/Turabian StyleMonteiro Moreira, Geisianny Augusta, and Helson Mario Martins do Vale. 2020. "Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil" Microorganisms 8, no. 8: 1116. https://doi.org/10.3390/microorganisms8081116
APA StyleMonteiro Moreira, G. A., & Martins do Vale, H. M. (2020). Soil Yeast Communities in Revegetated Post-Mining and Adjacent Native Areas in Central Brazil. Microorganisms, 8(8), 1116. https://doi.org/10.3390/microorganisms8081116






