Teff Type-I Sourdough to Produce Gluten-Free Muffin
Abstract
1. Introduction
2. Materials and Methods
2.1. Teff Sourdough
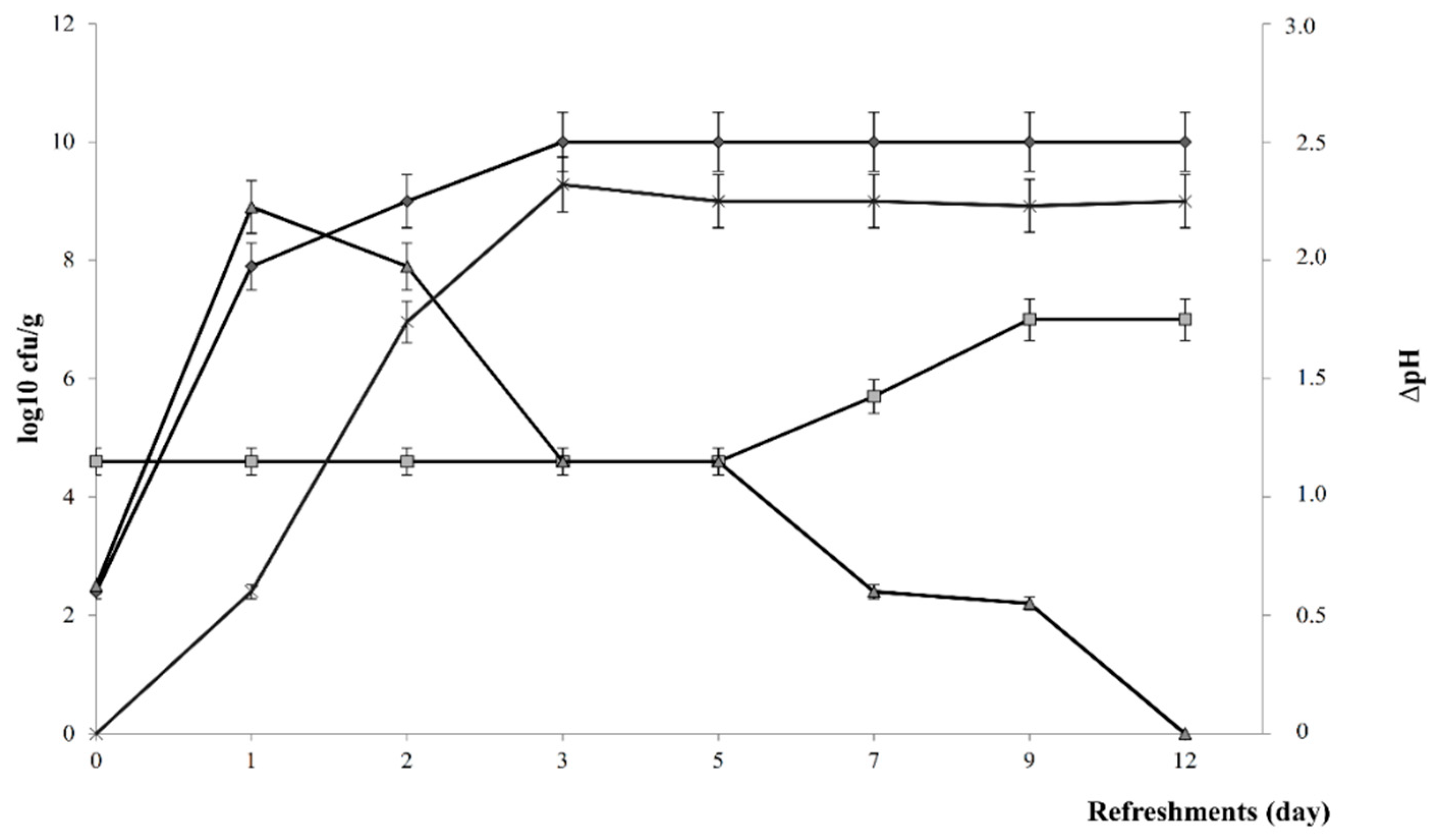
2.1.1. Propagation
2.1.2. Chemical and Microbiological Characterization
2.1.3. Isolation, Genotypic Characterization, and Identification of Lactic Acid Bacteria and Yeasts
2.1.4. Antioxidant Activity
2.2. Muffin Preparation
2.3. Muffin Characterization
2.3.1. Chemical and Nutritional Characteristics
2.3.2. Fatty Acid (FA) Composition
2.3.3. Phytic Acid, Protein, and Starch Digestibility
2.3.4. Structure and Color Parameter
2.3.5. Sensory Flash Profiling
2.3.6. Volatile Compounds
2.4. Bio-Preservation Effect of Teff Sourdough
2.5. Statistical Analysis
3. Results
3.1. Teff Type-I Sourdough: Microbiological and Biochemical Characterization
3.2. Teff Muffins
3.2.1. Biochemical and Nutritional Characteristics
3.2.2. Total Phenols and Antioxidant Activity
3.2.3. Phytic Acid, IVPD and Starch Hydrolysis
3.2.4. Volatile Components
3.3. Structural Properties and Sensory Profile of the Teff Muffins
3.4. Bio-Preservation Effect
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cabrera-Chavez, F.; Islas-Rubio, A.; Rouzaud-Sandez, O.; Sotelo-Cruz, N.; de la Barca, A.M.C. Modification of gluten by methionine binding to prepare wheat bread with reduced reactivity to serum IgA of celiac disease patients. J. Cereal Sci. 2010, 52, 310–313. [Google Scholar] [CrossRef]
- Do Nascimento, A.B.; Fiates, G.M.R.; Dos Anjos, A.; Teixeira, E. Gluten-free is not enough—Perception and suggestions of celiac consumers. Int. J. Food Sci. Nutr. 2014, 65, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.E.; Rosell, C.M. Understanding gluten-free dough for reaching breads with physical quality and nutritional balance. J. Sci. Food Agric. 2015, 95, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T. Folate, iron and dietary fiber contents of the gluten-free diet. J. Am. Diet. Assoc. 2000, 100, 1389–1396. [Google Scholar] [CrossRef]
- Thompson, T.; Dennis, M.; Higgins, L.A.; Lee, A.R.; Sharrett, M.K. Gluten-free diet survey: Are Americans with coeliac disease consuming recommended amounts of fibre, iron, calcium and grain foods? J. Hum. Nutr. Diet. 2005, 18, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Matthan, N.R.; Ausman, L.M.; Lichtenstein, A.H. Effect of macronutrients and fiber on postprandial glycemic responses and meal glycemic index and glycemic load value determinations. Am. J. Clin. Nutr. 2017, 105, 842–853. [Google Scholar] [CrossRef] [PubMed]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Teff (Eragrostis tef) as a raw material for malting, brewing and manufacturing of gluten-free foods and beverages: A review. J. Food Sci. Technol. 2014, 51, 2881–2895. [Google Scholar] [CrossRef]
- Shumoy, H.; Raes, K. Antioxidant potentials and phenolic composition of tef varieties: An indigenous Ethiopian cereal. Cereal Chem. 2016, 93, 465–470. [Google Scholar] [CrossRef]
- Salawu, S.O.; Bester, M.J.; Duodu, K.G. Phenolic composition and bioactive properties of cell wall preparations and whole grains of selected cereals and legumes. J. Food Biochem. 2014, 38, 62–72. [Google Scholar] [CrossRef]
- Pontonio, E.; Rizzello, C.G. Minor and ancient cereals: Exploitation of the nutritional potential through the use of selected starters and sourdough fermentation. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V., Watson, R., Patel, V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands; Academic Press: London, UK, 2019; pp. 443–452. [Google Scholar]
- Coda, R.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Sourdough lactic acid bacteria: Exploration of non-wheat cereal-based fermentation. Food Microbiol. 2014, 37, 51–58. [Google Scholar] [CrossRef]
- Ercolini, D.; Pontonio, E.; De Filippis, F.; Minervini, F.; La Storia, A.; Gobbetti, M.; Di Cagno, R. Microbial ecology dynamics during rye and wheat sourdough preparation. Appl. Environ. Microbiol. 2013, 79, 7827–7836. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Dingeo, C.; Gobbetti, M.; Rizzello, C.G. Maize milling by-products: From food wastes to functional ingredients through lactic acid bacteria fermentation. Front. Microbiol. 2019, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Pontonio, E.; Nionelli, L.; Curiel, J.A.; Sadeghi, A.; Di Cagno, R.; Gobbetti, M.; Rizzello, C.G. Iranian wheat flours from rural and industrial mills: Exploitation of the chemical and technology features and selection of autochthonous sourdough starters for making breads. Food Microbiol. 2015, 47, 99–110. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Siragusa, S.; Berloco, M.; Caputo, L.; Settanni, L.; Alfonsi, G.; Amerio, M.; Grandi, A.; Ragni, A.; Gobbetti, M. Selection of potential probiotic lactobacilli from pig feces to be used as additives in pelleted feeding. Res. Microbiol. 2006, 157, 792–801. [Google Scholar] [CrossRef]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Env. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- O’Donnell, K. Fusarium and its near relatives. In The Fungal Holomorph: Mitotic, Meiotic and Pleomorphic Speciation in Fungal Systematics; Reynolds, D.R., Taylor, J.W., Eds.; CAB International: Wallingford, UK, 1993; pp. 225–233. [Google Scholar]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Goebel, B.M.; Stackebrandt, E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl. Environ. Microbiol. 1994, 60, 1614–1621. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Nionelli, L.; Coda, R.; De Angelis, M.; Gobbetti, M. Effect of sourdough fermentation on stabilization and chemical and nutritional characteristics of wheat germ. Food Chem. 2010, 119, 1079–1089. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1977, 28, 49–55. [Google Scholar] [CrossRef]
- AACC. Approved Methods of the American Association of Cereal Chemists, 11th ed.; American Association of Cereal Chemists, Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- AOAC. International Official Methods of Analysis of AOAC International, 18th ed.; AOAC: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Commission Regulation (EEC). Official Journal of the European Communities; Commission Regulation No. 2568/91; European Commission: Brussels, Belgium, 1991; pp. 1–83. [Google Scholar]
- Caponio, F.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Gomes, T. Evolution of the oxidative and hydrolytic degradation of biscuits’ fatty fraction during storage. J. Sci. Food Agric. 2009, 89, 1392–1396. [Google Scholar] [CrossRef]
- Akeson, W.R.; Stahmann, M.A. A pepsin pancreatin digest index of protein quality evaluation. J. Nutr. 1964, 83, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Curiel, J.; Nionelli, L.; Vincentini, O.; Di Cagno, R.; Silano, M.; Gobbetti, M.; Coda, R. Use of fungal proteases and selected sourdough lactic acid bacteria for making wheat bread with an intermediate content of gluten. Food Microbiol. 2014, 37, 59–68. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- De Angelis, M.; Damiano, N.; Rizzello, C.G.; Cassone, A.; Di Cagno, R.; Gobbetti, M. Sourdough fermentation as a tool for the manufacture of low-glycemic index white wheat bread enriched in dietary fibre. Eur. Food Res. Technol. 2009, 229, 593–601. [Google Scholar] [CrossRef]
- Capriles, V.D.; Areas, J.A.G. Effects of prebiotic inulin-type fructans on structure, quality, sensory acceptance and glycemic response of gluten-free breads. Food Funct. 2013, 4, 4–10. [Google Scholar] [CrossRef]
- Pasqualone, A.; Caponio, F.; Pagani, M.A.; Summo, C.; Paradiso, V.M. Effect of salt reduction on quality and acceptability of durum wheat bread. Food Chem. 2019, 298, 575–581. [Google Scholar] [CrossRef]
- Scheuer, P.M.; Ferreira, J.A.S.; Mattioni, B.; De Miranda, M.Z.; De Francisco, A. Optimization of image analysis techniques for quality assessment of whole-wheat breads made with fat replacer. Food Sci. Technol. 2015, 35, 133–142. [Google Scholar] [CrossRef]
- Liu, J.; Bredie, W.L.P.; Sharman, E.; Harbertson, J.F.; Heymann, H. Comparison of rapid descriptive sensory methodologies: Free-choice profiling, flash profile and modified flash profile. Food Res. Int. 2018, 106, 892–900. [Google Scholar] [CrossRef]
- Caponio, F.; Giarnetti, M.; Summo, C.; Paradiso, V.M.; Cosmai, L.; Gomes, T. A comparative study on oxidative and hydrolytic stability of monovarietal extra virgin olive oil in bakery products. Food Res. Int. 2013, 54, 1995–2000. [Google Scholar] [CrossRef]
- Difonzo, G.; Pasqualone, A.; Silletti, R.; Cosmai, L.; Summo, C.; Paradiso, V.M.; Caponio, F. Use of olive leaf extract to reduce lipid oxidation of baked snacks panel. Food Res. Int. 2018, 108, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Coda, R.; Rizzello, C.G.; Nigro, F.; De Angelis, M.; Arnault, P.; Gobbetti, M. Long-term fungal inhibitory activity of water-soluble extracts of Phaseolus vulgaris cv. Pinto and sourdough lactic acid bacteria during bread storage. Appl. Environ. Microb. 2008, 74, 7391–7398. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harris, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901 and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef]
- Gobbetti, M.; Pontonio, E.; Filannino, P.; Rizzello, C.G.; De Angelis, M.; Di Cagno, R. How to improve the gluten-free diet: The state of the art from a food science perspective. Food Res. Int. 2018, 110, 22–32. [Google Scholar] [CrossRef]
- Naqash, F.; Gani, A.; Gani, A.; Masoodi, F.A. Gluten-free baking: Combating the challenges—A review. Trends Food Sci. Technol. 2017, 66, 98–107. [Google Scholar] [CrossRef]
- Ashenafi, M. A review on the microbiology of indigenous fermented foods and beverages of Ethiopia. Ethiop. J. Biol. Sci. 2006, 5, 89–245. [Google Scholar]
- Cheng, A.; Mayes, S.; Dalle, G.; Demissew, S.; Massawe, F. Diversifying crops for food and nutrition security—A case of teff. Biol. Rev. 2017, 92, 188–198. [Google Scholar] [CrossRef]
- Campo, E.; del Arco, L.; Urtasun, L.; Oria, R.; Ferrer-Mairal, A. Impact of sourdough on sensory properties and consumers’ preference of gluten-free breads enriched with teff flour. J. Cereal Sci. 2016, 67, 75–82. [Google Scholar] [CrossRef]
- Moroni, A.V.; Arendt, E.K.; Morrissey, J.P.; Dal Bello, F. Development of buckwheat and teff sourdoughs with the use of commercial starters. Int. J. Food Microbiol. 2010, 142, 142–148. [Google Scholar] [CrossRef]
- Moroni, A.; Arendt, E.K.; Dal Bello, F. Biodiversity of lactic acid bacteria and yeasts in spontaneously-fermented buckwheat and teff sourdoughs. Food Microbiol. 2011, 28, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Harth, H.; Kerrebroeck, S.V.; De Vuyst, L. Impact of process conditions on the microbial community dynamics and metabolite production kinetics of teff sourdough fermentations under bakery and laboratory conditions. Food Sci. Nutr. 2018, 6, 1438–1455. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, A. Technology of sourdough fermentation and sourdough applications. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; SSBM: New York, NY, USA, 2012; pp. 85–103. [Google Scholar]
- Hammes, W.P.; Ganzle, M.G. Sourdough breads and related products. In Microbiology of Fermented Foods; Woods, B.J.B., Ed.; Blackie Academic and Professional: London, UK, 1998; pp. 199–216. [Google Scholar]
- Pico, J.; Tapia, J.; Bernal, J.; Gómez, M. Comparison of different extraction methodologies for the analysis of volatile compounds in gluten-free flours and corn starch by GC/QTOF. Food Chem. 2018, 267, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. Recent advances in lipid oxidation. J. Sci. Food Agric. 1991, 54, 495–511. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Cerny, C. Origin of carbons in sulfur-containing aroma compounds from the Maillard reaction of xylose, cysteine and thiamine. LWT Food Sci. Technol. 2007, 40, 1309–1315. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, Y.; Soladoye, O.P.; Aluko, R.E. Maillard reaction products derived from food protein-derived peptides: Insights into flavor and bioactivity. Crit. Rev. Food Sci. Nutr. 2019, 1–14. [Google Scholar] [CrossRef]
- Hannon, J.A.; Kilcawley, K.N.; Wilkinson, M.G.; Delahunty, C.M.; Beresford, T.P. Flavour precursor development in Cheddar cheese due to lactococcal starters and the presence and lysis of Lactobacillus helveticus. Int. Dairy J. 2007, 17, 316–327. [Google Scholar] [CrossRef]
- Li, L.; Ma, Y. Effect of fatty acids on the β-oxidation system and thioesterase of Lactococcus lactis subspecies lactis. J. Dairy Sci. 2013, 96, 2003–2010. [Google Scholar] [CrossRef]
- Goepfert, S.; Poirier, Y. β-Oxidation in fatty acid degradation and beyond. Curr. Opin. Plant. Biol. 2007, 10, 245–251. [Google Scholar] [CrossRef]
- Arendt, E.K.; O’Brien, C.M.; Schober, T.; Gormley, T.R.; Gallagher, E. Development of gluten-free cereal products. Farm. Food. 2002, 12, 21–27. [Google Scholar]
- Wolter, A.; Hager, A.S.; Zannini, E.; Czerny, M.; Arendt, E.K. Impact of sourdough fermented with Lactobacillus plantarum FST 1.7 on baking and sensory properties of gluten-free breads. Eur. Food Res. Technol. 2014, 239, 1–12. [Google Scholar] [CrossRef]
- Hager, A.S.; Wolter, A.; Czerny, M.; Bez, J.; Zannini, E.; Arendt, E.; Czerny, M. Investigation of product quality, sensory profile and ultrastructure of breads made from a range of commercial gluten-free flours compared to their wheat counterparts. Eur. Food Res. Technol. 2012, 235, 333–344. [Google Scholar] [CrossRef]
- Polaki, A.; Xasapis, P.; Fasseas, C.; Yanniotis, S.; Mandala, I. Fiber and hydrocolloid content affect the microstructural and sensory characteristics of fresh and frozen stored bread. J. Food Eng. 2010, 97, 1–7. [Google Scholar] [CrossRef]
- Esteller, M.S.; Zancanaro, O.; Palmeira, C.N.S.; da Silva Lannes, S.C. The effect of kefir addition on microstructure parameters and physical properties of porous white bread. Eur. Food Res. Technol. 2006, 222, 26–31. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotech. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Official Journal of the European Union. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on Nutrition and Health Claims Made on Foods; Official Journal of the European Union—L404: Brussels, Beligum, 2006; pp. 9–25. [Google Scholar]
- Shahidi, F.; Zhong, Y. Lipid oxidation and improving the oxidative stability. Chem. Soc. Rev 2010, 39, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Cockell, K.A.; Estatira, S. Effects of antinutritional factors on protein digestibility and amino acid availability in foods. J. AOAC Int. 2005, 88, 967–987. [Google Scholar] [CrossRef]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53, S330–S375. [Google Scholar] [CrossRef]
- Zhu, F. Chemical composition and food uses of teff (Eragrostis tef). Food Chem. 2018, 239, 402–415. [Google Scholar] [CrossRef]
- Handa, V.; Sharma, D.; Kaur, A.; Arya, S.K. Biotechnological applications of microbial phytase and phytic acid in food and feed industries. Biocatal. Agric. Biotechnol. 2020, 25, 101600. [Google Scholar] [CrossRef]
- Omoba, O.S.; Isah, L.R. Influence of sourdough fermentation on amino acids composition, phenolic profile and antioxidant properties of sorghum biscuits. Prev. Nutr. Food Sci. 2018, 23, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Dallagnol, A.M.; Pescuma, M.; Font De Valedez, G.; Rollàn, G. Fermentation of quinoa and wheat slurries by Lactobacillus plantarum CRL 778: Proteolytic activity. Applied Microbial. Cell Physiology. 2013, 97, 3129–3140. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, C.G.; Lorusso, A.; Montemurro, M.; Gobbetti, M. Use of sourdough made with quinoa (Chenopodium quinoa) flour and autochthonous selected lactic acid bacteria for enhancing the nutritional, textural and sensory features of white bread. Food Microbiol. 2016, 56, 1–13. [Google Scholar] [CrossRef]
- Gobbetti, M.; Rizzello, C.G.; Di Cagno, R.; De Angelis, M. How the sourdough may affect the functional features of leavened baked goods. Food Microbiol. 2014, 37, 30–40. [Google Scholar] [CrossRef]
- Brandt, M.J. Sourdough products for convenient use in baking. Food Microbiol. 2007, 24, 161–164. [Google Scholar] [CrossRef]
- Reis, J.A.; Paula, A.T.; Casarotti, S.N.; Penna, A.L.B. Lactic acid bacteria antimicrobial compounds: Characteristics and applications. Food Eng. Rev. 2012, 4, 124–140. [Google Scholar] [CrossRef]


| M5% | M10% | M15% | MCT | |
|---|---|---|---|---|
| Proximate composition * | ||||
| Moisture | 21.18 ± 1.86 b | 25.43 ± 1.08 a | 26.92 ± 1.08 a | 21.33 ± 1.53 b |
| Protein | 7.67 ± 0.16 a | 7.78 ± 0.13 a | 7.62 ± 0.10 a | 7.75 ± 0.16 a |
| Fat | 22.52 ± 0.49 a | 22.67 ± 0.60 a | 23.03 ± 0.24 a | 22.96 ± 2.64 a |
| SFA | 2.14 ± 0.95 a | 2.76 ± 0.11 a | 2.79 ± 0.01 a | 2.75 ± 0.26 a |
| MUFA | 8.02 ± 0.18 a | 8.27 ± 0.21 a | 8.37 ± 0.11 a | 8.24 ± 0.94 a |
| PUFA | 11.81 ± 0.28 a | 11.65 ± 0.35 a | 11.88 ± 0.36 a | 11.91 ± 1.45 a |
| Carbohydrates | 43.46 ± 1.21 a | 37.49 ± 1.44 b | 36.28 ± 1.37 b | 42.42 ± 1.51 a |
| Total dietary fibers | 3.88 ± 0.77 a | 4.98 ± 0.89 a | 4.55 ± 1.22 a | 4.09 ± 1.09 a |
| Ash | 1.29 ± 0.26 b | 1.65 ± 0.17 a | 1.6 ± 0.09 a | 1.45 ± 0.50 ab |
| M5% | M10% | M15% | MCT | |
|---|---|---|---|---|
| pH | 6.04 ± 0.05 b | 5.98 ± 0.04 b | 5.72 ± 0.05 c | 6.51 ± 0.05 a |
| TTA (ml NaOH 0.1 M) | 2.0 ± 0.4 c | 3.2 ± 0.6 b | 5.4 ± 0.5 a | 1.4 ± 0.3 c |
| Lactic acid (mmol/kg) | 25.9 ± 0.4 b | 30.4 ± 0.2 b | 48.4 ± 0.6 a | n.d. |
| Acetic acid (mmol/kg) | 9.8 ± 0.3 b | 10.5 ± 0.6 b | 20.8 ± 0.4 a | n.d. |
| QF | 2.6 | 2.9 | 2.3 | n.d. |
| TFAA (mg/Kg) | 824 ± 15 c | 987 ± 16 b | 1090 ± 18 a | 389 ± 14 d |
| Peptide concentration (mg/100 g) | 144 ± 20 a | 143 ± 15 a | 167 ± 15 a | 95 ± 15 b |
| Total phenols (mmol/kg) | 2.37 ± 0.05 b | 2.40 ± 0.04 b | 3.30 ± 0.05 a | 2.02 ± 0.03 c |
| Radical scavenging (%) on ME | 49.5 ± 0.5 b | 50.2 ± 0.3 b | 55.7 ± 0.4 a | 34.6 ± 0.5 c |
| Radical scavenging (%) on WSE | 44.9 ± 0.7 c | 46.6 ± 0.3 b | 50.6 ± 0.4 a | 40.6 ± 0.4 d |
| M5% | M10% | M15% | MCT | |
|---|---|---|---|---|
| Phytic acid (mg/100 g) | 116 ± 4 b | 116 ± 2 b | 95 ± 4 c | 223 ± 3 a |
| IVPD (%) | 70 ± 5 a | 75 ± 3 a | 78 ± 4 a | 50 ± 6 b |
| HI (%) | 62 ± 2 ab | 59 ± 1 b | 52 ± 3 c | 65 ± 3 a |
| pGI | 74 ± 2 a | 72 ± 1 a,b | 68 ± 3 b | 75 ± 3 a |
| Compounds | M5% | M10% | M15% | MCT |
|---|---|---|---|---|
| Aldehydes | ||||
| Hexanal | 18.48 ± 2.67 b | 66.40 ± 4.20 a | 66.63 ± 5.21 a | 9.24 ± 3.37 c |
| Octanal | 5.23 ± 1.44 b | 14.29 ± 0.28 a | 15.67 ± 1.38 a | 14.45 ± 2.94 a |
| 2-Heptenal, (E)- | 9.56 ± 1.67 b | 31.39 ± 6.45 a | 41.30 ± 15.32 a | 3.80 ± 0.47 c |
| Nonanal | 45.13 ± 3.18 b | 109.14 ± 13.70 a | 106.18 ± 13.62 a | 16.96 ± 3.33 c |
| 2-Octenal, (E)- | 3.86 ± 1.31 b | 11.88 ± 3.32 a | 14.24 ± 4.25 a | 1.63 ± 0.42 c |
| Benzaldehyde | 2.57 ± 0.12 c | 5.33 ± 1.03 a | 7.06 ± 1.43 a | 3.00 ± 0.26 b |
| 2-Nonenal, (E)- | 6.17 ± 0.57 b | 16.75 ± 5.33 a | 17.78 ± 3.33 a | 1.77 ± 0.31 c |
| 2,4-Decadienal, (E,E)- | n.d. | n.d. | 7.35 ± 3.72 a | n.d. |
| Phenylacetaldehyde | 2.82 ± 0.30 b | 7.46 ± 0.83 a | 6.79 ± 1.11 a | 3.80 ± 1.87 b |
| Ketones and esters | ||||
| 2-Pentanone | 83.99 ± 4.98 a | 21.14 ± 3.97 c | 21.89 ± 13.30 b,c | 34.33 ± 3.54 b |
| 2-Hexanone | 36.96 ± 2.60 a | 10.80 ± 0.86 b | 11.27 ± 4.12 b,c | 13.98 ± 0.38 c |
| 2-Heptanone | 232.58 ± 45.85 a | 66.73 ± 8.62 b | 62.87 ± 6.88 b | 46.39 ± 4.01 c |
| 3-Octanone | 4.19 ± 0.43 b | 1.76 ± 0.07 c | 2.41 ± 0.80 c | 11.76 ± 4.08 a |
| 2-Octanone | 101.68 ± 14.02 a | 19.53 ± 1.49 c | 15.15 ± 4.46 c | 28.64 ± 3.10 b |
| 6-methyl-5-hepten-2-one | n.d. | 4.32 ± 1.22 a | 4.10 ± 1.04 a | 5.45 ± 1.61 a |
| 2-Nonanone | 9.89 ± 0.72 a | 2.08 ± 0.18 b | 1.87 ± 0.35 b | 9.13 ± 1.10 a |
| 3-Octen-2-one, (E)- | n.d. | 3.52 ± 1.21 a | 3.73 ± 0.81 a | n.d. |
| Acetophenone | n.d. | 3.87 ± 1.26 a | 5.17 ± 1.77 a | n.d. |
| Ethyl octanoate | 0.89 ± 0.20 c | 1.39 ± 0.21 b | 2.24 ± 0.55 a | n.d. |
| Alcohols | ||||
| 1-Hexanol | 83.54 ± 4.71 c | 104.29 ± 7.19 b | 125.72 ± 10.65 a | 13.09 ± 2.50 d |
| 1-Octen-3-ol | 37.07 ± 4.32 b | 20.02 ± 0.45 c | 21.29 ± 5.92 c | 94.17 ± 16.81 a |
| 1-Octanol | 6.34 ± 1.85 b | 12.09 ± 0.84 a | 13.63 ± 1.38 a | n.d. |
| Benzenemethanol | n.d. | 3.36 ± 1.28 a | 2.76 ± 0.41 a | n.d. |
| Phenylethyl alcohol | 3.98 ± 0.33 b | 4.63 ± 0.43 b | 6.57 ± 0.63 a | n.d. |
| Acids | ||||
| Propanoic acid | 3.78 ± 0.28 a | 3.77 ± 0.19 a | 4.30 ± 0.33 a | 4.34 ± 0.89 a |
| Hexanoic acid | 4.62 ± 1.33 b | 17.74 ± 2.68 a | 20.59 ± 7.13 a | 2.84 ± 0.71 b |
| Nonanoic acid | n.d. | n.d. | 4.79 ± 1.59 a | 4.41 ± 0.85 a |
| Furans | ||||
| Furan, 2-pentyl- | 12.11 ± 0.66 b | 14.58 ± 0.25 a | 16.65 ± 2.10 a | 14.97 ± 1.13 ab |
| 2-Furanmethanol | n.d. | 4.65 ± 0.71 a | 5.56 ± 0.74 a | n.d. |
| 2(3H)-Furanone, dihydro-5-pentyl- | 9.34 ± 0.38 b | 7.77 ± 1.54 c | 12.01 ± 1.63 a | 3.18 ± 1.05 d |
| Sulfurs | ||||
| Carbon sulfide | 0.55 ± 0.28 b | 8.04 ± 1.37 a | 8.72 ± 1.38 a | n.d. |
| Others | ||||
| dl-limonene | 15.54 ± 0.80 b | 16.12 ± 1.37 b | 16.86 ± 1.95 b | 27.88 ± 6.72 a |
| Phenol, 2,6-bis(1,1-dimethylethyl)-4-methyl | n.d. | n.d. | n.d. | 4.48 ± 1.30 a |
| M5% | M10% | M15% | MCT | |
|---|---|---|---|---|
| Structural properties | ||||
| Firmness (N) | 29.6 ± 3.5 a | 19.5 ± 2.3 c | 22.4 ± 2.5 bc | 24.7 ± 1.1 b |
| Springiness | 0.89 ± 0.1 a | 0.89 ± 0.1 a | 0.88 ± 0.1 a | 0.88 ± 0.1 a |
| Chewiness (N) | 12.4 ± 1.4 a | 12.1 ± 3.6 a | 9.6 ± 1.2 a | 11.1 ± 0.4 a |
| Cohesiveness | 0.41 ± 0.01 b | 0.74 ± 0.01 a | 0.42 ± 0.01 b | 0.41 ± 0.01 b |
| Image analysis | ||||
| Mean area (mm2) | 0.72 ± 0.01 b | 0.94 ±0.08 a | 1.06 ±0.21 a | 0.66 ± 0.01 c |
| Gas cells (n. cells/mm2) | 220.4 ± 1.9 b | 161.4 ± 25.2 c | 141.3 ±44.2 c | 260.0 ± 21.2 a |
| Day | Non-Inoculated | Inoculated | ||||||
|---|---|---|---|---|---|---|---|---|
| M5% | M10% | M15% | MCT | M5% | M10% | M15% | MCT | |
| 7 | ++ | ± | − | ++ | + | − | − | + |
| 14 | ++++ | ++ | ± | ++++ | ++ | ± | − | ++ |
| 21 | ++++ | +++ | ++ | ++++ | +++ | ++ | + | +++ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dingeo, C.; Difonzo, G.; Paradiso, V.M.; Rizzello, C.G.; Pontonio, E. Teff Type-I Sourdough to Produce Gluten-Free Muffin. Microorganisms 2020, 8, 1149. https://doi.org/10.3390/microorganisms8081149
Dingeo C, Difonzo G, Paradiso VM, Rizzello CG, Pontonio E. Teff Type-I Sourdough to Produce Gluten-Free Muffin. Microorganisms. 2020; 8(8):1149. https://doi.org/10.3390/microorganisms8081149
Chicago/Turabian StyleDingeo, Cinzia, Graziana Difonzo, Vito Michele Paradiso, Carlo Giuseppe Rizzello, and Erica Pontonio. 2020. "Teff Type-I Sourdough to Produce Gluten-Free Muffin" Microorganisms 8, no. 8: 1149. https://doi.org/10.3390/microorganisms8081149
APA StyleDingeo, C., Difonzo, G., Paradiso, V. M., Rizzello, C. G., & Pontonio, E. (2020). Teff Type-I Sourdough to Produce Gluten-Free Muffin. Microorganisms, 8(8), 1149. https://doi.org/10.3390/microorganisms8081149








