Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation
2.2. Characterization and Identification: Biochemical Assay and 16S rDNA Amplification
2.3. Genomic DNA Isolation
2.4. Agarose Gel Electrophoreses
2.5. Whole-Genome Sequencing, Assembly and Annotation
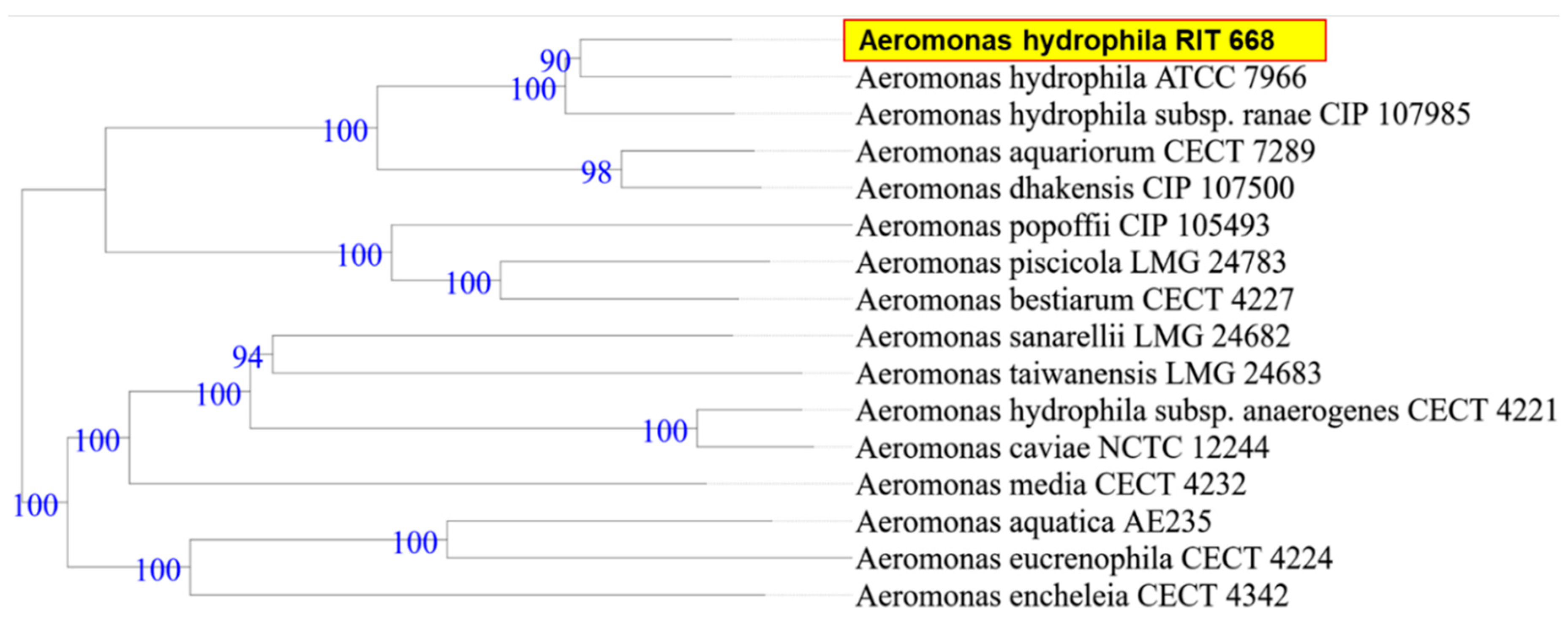
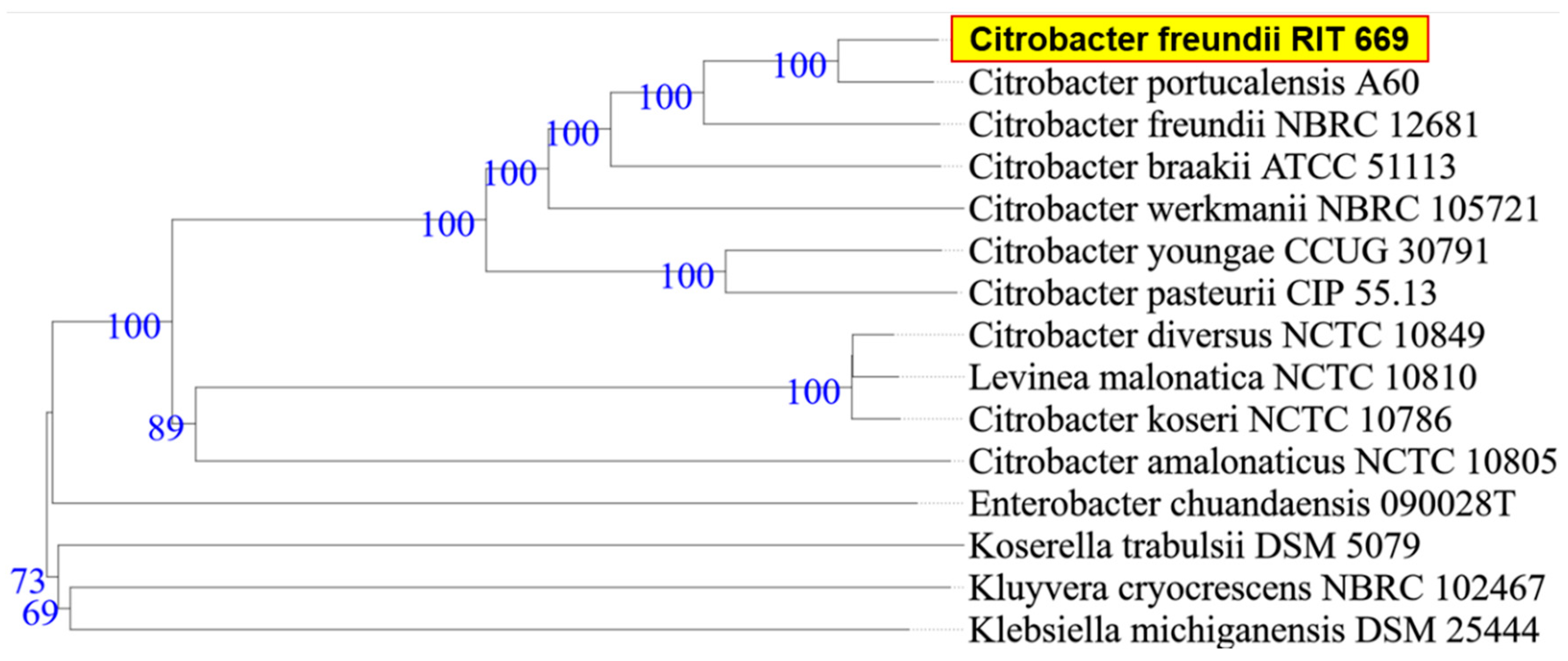
2.6. Phylogenetic Analysis
2.7. Resistance Gene Identifier (RGI)
2.8. Predictions of Secondary Metabolite Production
2.9. Colonization of Planktonic and Biofilm Forms on Plastics
2.9.1. Classical Biofilm Forms
2.9.2. Planktonic and Adherent Forms
2.9.3. RNA Isolation
2.10. Detection of Biofilm-Related Genes and Virulence Factors by RT-PCR
2.11. Estimation of Gene Expression
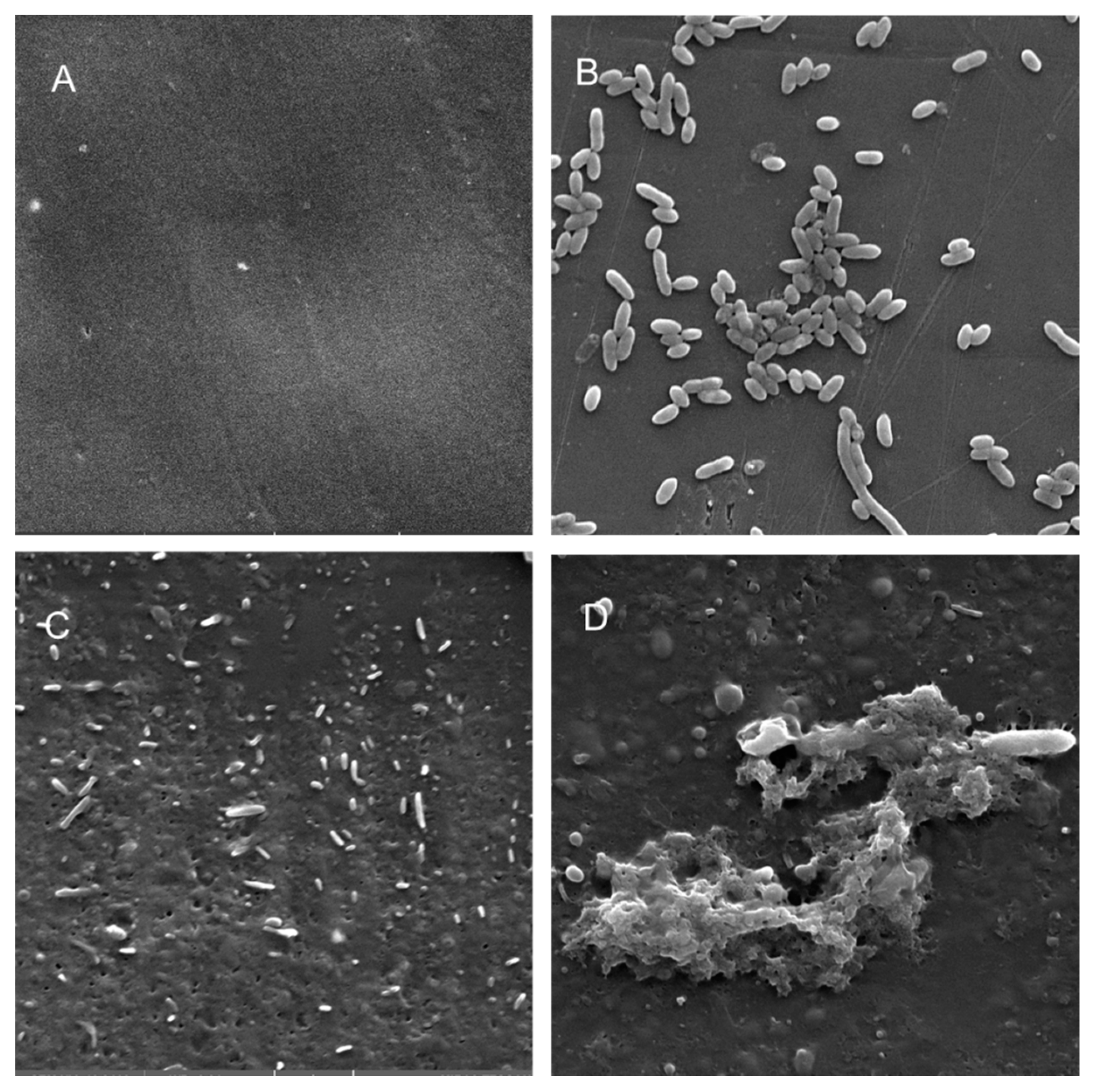
2.12. Scanning Electron Microscopy Analysis
3. Results
3.1. Biochemical Characterization and Taxonomy
3.2. Resistome Analysis and Secondary Metabolite Analysis
3.3. Polymer Adhesion and Gene Expression
3.4. Secondary Metabolite Production via antiSMASH
3.5. Electron Microscopy Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cooper, M.; Shlaes, D. Fix the antibiotics pipeline. Nature 2011, 472, 32. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.D. The antibiotic resistome. Expert Opin. Drug Discov. 2010, 5, 779–788. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Ehlers, L.J.; Bouwer, E.J. RP4 Plasmid transfer among species of Pseudomonas in a biofilm reactor. Water Sci. Technol. 1999, 39, 163. [Google Scholar] [CrossRef]
- Roberts, A.P.; Pratten, J.; Wilson, M.; Mullany, P. Transfer of a Conjugative Transposon, Tn5397 in a Model Oral Biofilm. FEMS Microbiol. Lett. 1999, 177, 63–66. [Google Scholar] [CrossRef]
- Hausner, M.; Wuertz, S. High rates of conjugation in bacterial biofilms as determined by quantitative in situ analysis. Appl. Environ. Microbiol. 1999, 65, 3710–3713. [Google Scholar] [CrossRef] [Green Version]
- Hancock, V.; Ferrières, L.; Klemm, P. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 2007, 267, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Shunmugaperumal, T. Biofilm Eradication and Prevention: A Pharmaceutical Approach to Medical Device Infections; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 978-0-470-47996-4. [Google Scholar]
- Jaff, M.R. Advances in the management of patients with vascular disease. Expert Rev. Cardiovasc. Ther. 2012, 10, 151–153. [Google Scholar] [CrossRef]
- Mani, G.; Feldman, M.; Patel, D.; Agrawal, C. Coronary stents: A materials perspective. Biomaterials 2002, 28, 1689–1710. [Google Scholar] [CrossRef] [PubMed]
- Engelsman, A.; Saldarriaga-Fernandez, I.; Nejadnik, M.; van Dam, G.; Francis, K.; Ploeg, R.; Busscher, H.; van der Mei, H. The risk of biomaterial-associated infection after revision surgery due to an experimental primary implant infection. Biofouling 2010, 26, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Ibekwe, A.M.; Murinda, S.E. Linking microbial community composition in treated wastewater with water quality in distribution systems and subsequent health effects. Microorganisms 2019, 7, 660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Tender, C.; Devriese, L.I.; Haegeman, A.; Maes, S.; Vangeyte, J.; Cattrijsse, A.; Dawyndt, P.; Ruttink, T. Temporal Dynamics of Bacterial and Fungal Colonization on Plastic Debris in the North Sea. Environ. Sci. Technol. 2017, 51, 7350–7360. [Google Scholar] [CrossRef] [PubMed]
- Kettner, M.T.; Rojas-Jimenez, K.; Oberbeckmann, S.; Labrenz, M.; Grossart, H.-P. Microplastics alter composition of fungal communities in aquatic ecosystems. Environ. Microbiol. 2017, 19, 4447–4459. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Wang, P.; Hou, J.; Yao, Y.; Liu, Z.; Liu, S.; Li, T. Distinct Community structure and microbial functions of biofilms colonizing microplastics. Sci. Total Environ. 2019, 650, 2395–2402. [Google Scholar] [CrossRef]
- Kirstein, I.V.; Wichels, A.; Krohne, G.; Gerdts, G. Mature biofilm communities on synthetic polymers in seawater-specific or general? Mar. Environ. Res. 2018, 142, 147–154. [Google Scholar] [CrossRef]
- Hoellein, T.J.; McCormick, A.R.; Hittie, J.; London, M.G.; Scott, J.W.; Kelly, J.J. Longitudinal patterns of microplastic concentration and bacterial assemblages in surface and benthic habitats of an urban river. Freshw. Sci. 2017, 36, 491–507. [Google Scholar] [CrossRef]
- McCormick, A.; Hoellein, T.J.; Mason, S.A.; Schluep, J.; Kelly, J.J. Microplastic is an abundant and distinct microbial habitat in an urban river. Environ. Sci. Technol. 2014, 48, 11863–11871. [Google Scholar] [CrossRef]
- Harrison, J.H.; Hoellein, T.J.; Sapp, M.; Tagg, A.S.; Ju-Nam, Y.; Ojeda, J.J. Microplastic-associated biofilms: A comparison of freshwater and marine environments. In Freshwater Microplastics; Wagner, M., Lambert, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 58, pp. 181–201. [Google Scholar] [CrossRef] [Green Version]
- Characklis, W.; McFeters, G.; Marshall, K. Physiological ecology in biofilm systems. In Biofilms; Wiley Series in Ecological and Applied Microbiology; Characklis, W., Marshall, K., Eds.; John Wiley & Sons: New York, NY, USA, 1990; pp. 341–394. ISBN 9780471826637. [Google Scholar]
- Fletcher, M.; Loeb, G.I. Influence of substratum characteristics on the attachment of a marine pseudomonad to solid surfaces. Appl. Environ. Microbiol. 1979, 37, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Pringle, J.H.; Fletcher, M. Influence of substratum wettability on attachment of freshwater bacteria to solid surfaces. Appl. Environ. Microbiol. 1983, 45, 811–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendinger, B.; Rijnaarts, H.H.; Altendorf, K.; Zehnder, A.J. Physicochemical cell surface and adhesive properties of coryneform bacteria related to the presence and chain length of mycolic acids. Appl. Environ. Microbiol. 1993, 59, 3973–3977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin-Based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef] [PubMed]
- Mogosanu, G.D.; Grumezescu, A.M.; Chifiriuc, M.C. Keratin-Based biomaterials for biomedical applications. Curr. Drug Targets 2014, 15, 518–530. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C 2020, 110, 110612. [Google Scholar] [CrossRef]
- McKnight, D.T.; Zenger, K.R.; Alford, R.A.; Huerlimann, R. Microbiome Diversity and Composition Varies Across Body Areas in a Freshwater Turtle. Microbiology 2020, 166, 440–452. [Google Scholar] [CrossRef]
- Costerton, J.; Stewart, P. Battling biofilms. Sci. Am. 2001, 285, 74–81. [Google Scholar] [CrossRef]
- Busscher, H.J.; van der Mei, H.C.; Subbiahdoss, G.; Jutte, P.C.; van den Dungen, J.J.; Zaat, S.A.; Schultz, M.J.; Grainger, D.W. Biomaterial-associated infection: Locating the finish line in the race for the surface. Sci. Transl. Med. 2012, 4, 153rv10. [Google Scholar] [CrossRef] [Green Version]
- Janda, J.M.; Abbott, S.L. The genus aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Tena, D.; Aspiroz, C.; Figueras, M.J.; Gonzalez-Praetorius, A.; Aldea, M.J.; Alperi, A.; Bisquert, J. Surgical site infection due to Aeromonas species: Report of nine cases and literature review. Scand. J. Infect. Dis. 2009, 41, 164–170. [Google Scholar] [CrossRef]
- Minnaganti, V.R.; Patel, P.J.; Iancu, D.; Schoch, P.E.; Cunha, B.A. Necrotizing fasciitis caused by Aeromonas hydrophila. Heart Lung 2000, 29, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Ugarte-Torres, A.; Perry, S.; Franko, A.; Church, D.L. Multidrug-resistant Aeromonas hydrophila causing fatal bilateral necrotizing fasciitis in an immunocompromised patient: A case report. J. Med. Case Rep. 2018, 12, 326. [Google Scholar] [CrossRef] [PubMed]
- Hochedez, P.; Hope-Rapp, E.; Olive, C.; Nicolas, M.; Beaucaire, G.; Cabié, A. Bacteremia caused by aeromonas species [corrected] complex in the Caribbean islands of Martinique and Guadeloupe. Am. J. Trop. Med. Hyg. 2010, 83, 1123–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rangrez, A.Y.; Dayananda, K.M.; Atanur, S.; Joshi, R.; Patole, M.S.; Shouche, Y.S. Detection of conjugation related type four secretion machinery in Aeromonas culicicola. PLoS ONE 2006, 1, e115. [Google Scholar] [CrossRef]
- Alperi, A.; Figueras, M.J. Human isolates of Aeromonas possess Shiga toxin genes (stx1 and stx2) highly similar to the most virulent gene variants of Escherichia coli. Clin. Microbiol. Infect. 2010, 16, 1563–1567. [Google Scholar] [CrossRef] [Green Version]
- Haque, Q.M.; Sugiyama, A.; Iwade, Y.; Midorikawa, Y.; Yamauchi, T. Diarrheal and environmental isolates of Aeromonas spp. Produce a toxin similar to Shiga-like toxin 1. Curr. Microbiol. 1996, 32, 239–245. [Google Scholar] [CrossRef]
- Huddleston, J.R.; Brokaw, J.M.; Zak, J.C.; Jeter, R.M. Natural transformation as a mechanism of horizontal gene transfer among environmental Aeromonas species. Syst. Appl. Microbiol. 2013, 36, 224–234. [Google Scholar] [CrossRef]
- Esteve, C.; Alcaide, E.; Giménez, M.J. Multidrug-resistant (MDR) Aeromonas Recovered from the metropolitan area of Valencia (Spain): Diseases spectrum and prevalence in the environment. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 137–145. [Google Scholar] [CrossRef]
- Marchandin, H.; Godreuil, S.; Darbas, H.; Jean-Pierre, H.; Jumas-Bilak, E.; Chanal, C.; Bonnet, R. Extended-spectrum beta-lactamase TEM-24 in an Aeromonas clinical strain: Acquisition from the prevalent Enterobacter aerogenes clone in France. Antimicrob. Agents Chemother. 2003, 47, 3994–3995. [Google Scholar] [CrossRef] [Green Version]
- Piotrowska, M.; Popowska, M. Insight into the mobilome of Aeromonas strains. Front. Microbiol. 2015, 6, 494. [Google Scholar] [CrossRef] [Green Version]
- Igbinosa, I.H.; Igumbor, E.U.; Aghdasi, F.; Tom, M.; Okoh, A.I. Emerging Aeromonas species infections and their significance in public health. Sci. World J. 2012, 2012, 625023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Bravo, A.; Figueras, M.J. An update on the genus Aeromonas: Taxonomy, epidemiology, and pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahid, I.K.; Mizan, M.F.R.; Myoung, J.; Ha, S.-D. Aeromonas hydrophila biofilm, exoprotease, and quorum sensing responses to co-cultivation with diverse foodborne pathogens and food spoilage bacteria on crab surfaces. Biofouling 2018, 34, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Mizan, M.F.; Jahid, I.K.; Ha, S.D. Microbial biofilms in seafood: A food-hygiene challenge. Food Microbiol. 2015, 49, 41–55. [Google Scholar] [CrossRef]
- Zalmum, A.A.; Marialegite, K.; Ghenghesh, K.S. Bacterial composition of the biofilm on the surface of course sediment of the danube: With special reference to the clinically important bacteria. Arch. Inst. Pasteur Tunis 1998, 75, 205–209. [Google Scholar]
- Balasubramanian, V.; Palanichamy, S.; Subramanian, G.; Rajaram, R. Development of polyvinyl chloride biofilms for succession of selected marine bacterial populations. J. Environ. Biol. 2012, 33, 57–60. [Google Scholar]
- Béchet, M.; Blondeau, R. Factors Associated with the Adherence and Biofilm Formation by Aeromonas caviae on Glass Surfaces. J. Appl. Microbiol. 2003, 94, 1072–1078. [Google Scholar] [CrossRef] [Green Version]
- Doğruöz, N.; Göksay, D.; Ilhan-Sungur, E.; Cotuk, A. Pioneer colonizer microorganisms in biofilm formation on galvanized steel in a simulated recirculating cooling-water system. J. Basic Microbiol. 2009, 49, S5–S12. [Google Scholar] [CrossRef]
- Kühn, I.; Allestam, G.; Huys, G.; Janssen, P.; Kersters, K.; Krovacek, K.; Stenström, T.A. Diversity, Persistence, and virulence of Aeromonas strains isolated from drinking water distribution systems in Sweden. Appl. Environ. Microbiol. 1997, 63, 2708–2715. [Google Scholar] [CrossRef] [Green Version]
- Villari, P.; Crispino, M.; Montuori, P.; Boccia, S. Molecular typing of Aeromonas isolates in natural mineral waters. Appl. Environ. Microbiol. 2003, 69, 697–701. [Google Scholar] [CrossRef] [Green Version]
- John, A.K.; Schmaler, M.; Khanna, N.; Landmann, R. Reversible daptomycin tolerance of adherent staphylococci in an implant infection model. Antimicrob. Agents Chemother. 2011, 55, 3510–3516. [Google Scholar] [CrossRef] [Green Version]
- Qu, Y.; Daley, A.J.; Istivan, T.S.; Rouch, D.A.; Deighton, M.A. Densely adherent growth mode, rather than extracellular polymer substance matrix build-up ability, contributes to high resistance of staphylococcus epidermidis biofilms to antibiotics. J. Antimicrob. Chemother. 2010, 65, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J. The genus citrobacter. In The Prokaryotes; Starr, M., Stolp, H., Truper, H., Balows, A., Schlegel, H., Eds.; Springer: Heidelberg, Germany, 1981; pp. 1140–1147. ISBN 978-3-662-13187-9. [Google Scholar]
- Sedlak, J. Citrobacter. In Enterobacteriaceae-Infektionen, Epidemiologie und Laboratoriumsdiagnostik; Sedlak, J., Rische, H., Eds.; VEB Georg Thieme Verlag: Leipzig, Germany, 1968; pp. 528–530. [Google Scholar]
- Sedlák, J. Present Knowledge and Aspects of Citrobacter. Curr Top. Microbiol. Immunol. 1973, 62, 41–59. [Google Scholar] [PubMed]
- Bai, L.; Xia, S.; Lan, R.; Liu, L.; Ye, C.; Wang, Y.; Jin, D.; Cui, Z.; Jing, H.; Xiong, Y.; et al. Isolation and characterization of cytotoxic, aggregative Citrobacter freundii. PLoS ONE 2012, 7, e33054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, M.T.; Mitchell, L.A.; Zhao, L.; Mobley, H.L.T. Citrobacter freundii fitness during bloodstream Infection. Sci. Rep. 2018, 8, 11792. [Google Scholar] [CrossRef]
- Ranjan, K.P.; Ranjan, N. Citrobacter: An emerging health care associated urinary pathogen. Urol. Ann. 2013, 5, 313–314. [Google Scholar]
- Liu, L.; Chen, D.; Lan, R.; Hao, S.; Jin, W.; Sun, H.; Wang, Y.; Liang, Y.; Xu, J. Genetic diversity, multidrug resistance, and virulence of Citrobacter freundii from diarrheal patients and healthy individuals. Front. Cell. Infect. Microbiol. 2018, 8, 233. [Google Scholar] [CrossRef] [Green Version]
- Mohanty, S.; Singhal, R.; Sood, S.; Dhawan, B.; Kapil, A.; Das, B.K. Citrobacter infections in a tertiary care hospital in northern India. J. Infect. 2007, 54, 58–64. [Google Scholar] [CrossRef]
- Samonis, G.; Karageorgopoulos, D.E.; Kofteridis, D.P.; Matthaiou, D.K.; Sidiropoulou, V.; Maraki, S.; Falagas, M.E. Citrobacter infections in a general hospital: Characteristics and outcomes. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 61–68. [Google Scholar] [CrossRef]
- Chen, Y.S.; Wong, W.W.; Fung, C.P.; Yu, K.W.; Liu, C.Y. Clinical features and antimicrobial susceptibility trends in Citrobacter freundii bacteremia. J. Microbiol. Immunol. Infect. 2002, 35, 109–114. [Google Scholar]
- Liu, L.H.; Wang, N.Y.; Wu, A.Y.; Lin, C.C.; Lee, C.M.; Liu, C.P. Citrobacter freundii bacteremia: Risk factors of mortality and prevalence of resistance genes. J. Microbiol. Immunol. Infect. 2018, 51, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Brook, T.C.; Alcon-Giner, C.; Clarke, P.; Hall, L.J.; Hoyles, L. Draft genome sequences of Citrobacter freundii and Citrobacter murliniae strains isolated from the feces of preterm infants. Microbiol. Resour. Announc. 2019, 8, e00494-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Y.; Xiong, Z.; Li, X.; Hu, L.; Shen, J.; Li, T.; Hu, F.; Chen, S. Prevalence of plasmid-mediated quinolone resistance determinants in Citrobacter freundii isolates from Anhui Province, PR China. J. Med. Microbiol. 2011, 60, 1801–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, Y.J.; Yu, J.K.; Lee, S.; Oh, E.J.; Woo, G.J. Prevalence and diversity of qnr alleles in AmpC-producing Enterobacter cloacae, Enterobacter aerogenes, Citrobacter freundii and Serratia marcescens: A multicentre study from Korea. J. Antimicrob. Chemother. 2007, 60, 868–871. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Park, S.Y.; Oh, E.J.; Park, J.J.; Lee, K.Y.; Woo, G.J.; Lee, K. Occurrence of extended-spectrum beta-Lactamases among chromosomal AmpC-producing Enterobacter cloacae, Citrobacter freundii, and Serratia marcescens in Korea and investigation of screening criteria. Diagn. Microbiol. Infect. Dis. 2005, 51, 265–269. [Google Scholar] [CrossRef]
- Moland, E.S.; Hanson, N.D.; Black, J.A.; Hossain, A.; Song, W.; Thomson, K.S. Prevalence of newer beta-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J. Clin. Microbiol. 2006, 44, 3318–3324. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Lee, J.E.; Park, S.J.; Kim, M.N.; Choo, E.J.; Kwak, Y.G.; Jeong, J.Y.; Woo, J.H.; Kim, N.J.; Kim, Y.S. Prevalence, microbiology, and clinical characteristics of extended-spectrum beta-lactamase-producing Enterobacter spp., Serratia marcescens, Citrobacter freundii, and Morganella morganii in Korea. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 557–561. [Google Scholar] [CrossRef]
- Kregiel, D.; Rygala, A.; Kolesinska, B.; Nowacka, M.; Herc, A.S.; Kowalewska, A. Antimicrobial and antibiofilm N-acetyl-L-cysteine Grafted siloxane polymers with potential for use in water systems. Int. J. Mol. Sci. 2019, 20, 2011. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Chen, L.; Zhou, S.; Li, H.; Long, J.; Yao, F.; Zhuang, Y.; Zhang, Z.; Huang, Y.; Duan, K. Co-existence of Citrobacter freundii exacerbated Pseudomonas aeruginosa infection in vivo. Int. J. Med. Microbiol. 2020, 310, 151379. [Google Scholar] [CrossRef]
- Pereira, A.L.; Silva, T.N.; Gomes, A.C.; Araújo, A.C.; Giugliano, L.G. Diarrhea-associated biofilm formed by Enteroaggregative Escherichia coli and aggregative Citrobacter freundii: A Consortium mediated by putative F pili. BMC Microbiol. 2010, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Aminharati, F.; Ehrampoush, M.H.; Soltan Dallal, M.M.; Yaseri, M.; Dehghani Tafti, A.A.; Rajabi, Z. Citrobacter freundii foodborne disease outbreaks related to environmental conditions in Yazd Province, Iran. Iran. J. Public Health 2019, 48, 1099–1105. [Google Scholar] [CrossRef] [PubMed]
- Tschape, H.; Prager, R.; Streckel, W.; Fruth, A.; Tietze, E.; Böhme, G. Verotoxinogenic Citrobacter freundii associated with severe gastroenteritis and cases of haemolytic uraemic syndrome in a nursery school: Green butter as the infection source. Epidemiol. Infect. 1995, 114, 441–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parida, S.N.; Verma, I.C.; Deb, M.; Bhujwala, R.A. An outbreak of diarrhea due to Citrobacter freundii in a neonatal special care nursery. Indian J. Pediatr. 1980, 47, 81–84. [Google Scholar] [CrossRef] [PubMed]
- Guarino, A.; Capano, G.; Malamisura, B.; Alessio, M.; Guandalini, S.; Rubino, A. Production of Escherichia coli STa-like heat-stable enterotoxin by Citrobacter freundii isolated from humans. J. Clin. Microbiol. 1987, 25, 110–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guarino, A.; Giannella, R.; Thompson, M.R. Citrobacter freundii produces an 18-Amino-acid heat-stable enterotoxin identical to the 18-amino-acid Escherichia coli heat-stable enterotoxin (ST Ia). Infect. Immun. 1989, 57, 649–652. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, H.; Montag, M.; Bockemühl, J.; Heesemann, J.; Karch, H. Shiga-like toxin II-related cytotoxins in Citrobacter freundii strains from humans and beef samples. Infect. Immun. 1993, 61, 534–543. [Google Scholar] [CrossRef] [Green Version]
- Karasawa, T.; Ito, H.; Tsukamoto, T.; Yamasaki, S.; Kurazono, H.; Faruque, S.M.; Nair, G.B.; Nishibuchi, M.; Takeda, Y. Cloning and Characterization of genes encoding homologues of the B subunit of cholera toxin and the Escherichia coli heat-labile enterotoxin from clinical isolates of Citrobacter freundii and E. coli. Infect. Immun. 2002, 70, 7153–7155. [Google Scholar] [CrossRef] [Green Version]
- McCoy, R.H.; Seidler, R.J. Potential pathogens in the environment: Isolation, enumeration, and identification of seven genera of intestinal bacteria associated with small green pet turtles. Appl. Microbiol. 1973, 25, 534–538. [Google Scholar] [CrossRef] [Green Version]
- Murdoch, D.R.; French, N.P. COVID-19: Another infectious disease emerging at the animal-human interface. N. Z. Med. J. 2020, 133, 12–15. [Google Scholar]
- Hossain, S.; Wimalasena, S.H.M.P.; Heo, G.J. Virulence factors and antimicrobial resistance pattern of Citrobacter Freundii isolated from healthy pet turtles and their environment. Asian J. Anim. Vet. Adv. 2016, 12, 10–16. [Google Scholar] [CrossRef]
- Chung, T.; Yi, S.; Kim, B.; Kim, W.; Shin, G. Identification and antibiotic resistance profiling of bacterial isolates from septicaemic soft-shelled turtles (Pelodiscus sinensis). Vet. Med. 2017, 62, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Wimalasena, S.H.M.P.; Shin, G.W.; Hossain, S.; Heo, G.J. Potential enterotoxicity and antimicrobial resistance pattern of Aeromonas species isolated from pet turtles and their environment. J. Vet. Med. Sci. 2017, 79, 921–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blasi, M.F.; Migliore, L.; Mattei, D.; Rotini, A.; Thaller, M.C.; Alduina, R. Antibiotic resistance of gram-negative bacteria from wild captured loggerhead sea turtles. Antibiotics 2020, 9, 162. [Google Scholar] [CrossRef] [Green Version]
- Al-Bahry, S.; Mahmoud, I.; Al-Zadjali, M.; Elshafie, A.; Al-Harthy, A.; Al-Alawi, W. Antibiotic resistant bacteria as bio-indicator of polluted effluent in the green turtles, Chelonia mydas in Oman. Mar. Environ. Res. 2011, 71, 139–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pace, A.; Dipineto, L.; Fioretti, A.; Hochscheid, S. Loggerhead sea turtles as sentinels in the western mediterranean: Antibiotic resistance and environment-related modifications of gram-negative bacteria. Mar. Pollut. Bull. 2019, 149, 110575. [Google Scholar] [CrossRef]
- Al-Bahry, S.N.; Al-Zadjali, M.A.; Mahmoud, I.Y.; Elshafie, A.E. Biomonitoring marine habitats in reference to antibiotic resistant bacteria and ampicillin resistance determinants from oviductal fluid of the nesting green sea turtle, Chelonia mydas. Chemosphere 2012, 87, 1308–1315. [Google Scholar] [CrossRef]
- Rabinowitz, P.; Conti, L. Links among human health, animal health, and ecosystem health. Annu. Rev. Public Health 2013, 34, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Rosen, G.E.; Smith, K.F. Summarizing the evidence on the international trade in illegal wildlife. Ecohealth 2010, 7, 24–32. [Google Scholar] [CrossRef]
- Pace, A.; Rinaldi, L.; Ianniello, D.; Borrelli, L.; Cringoli, G.; Fioretti, A.; Hochscheid, S.; Dipineto, L. Gastrointestinal investigation of parasites and enterobacteriaceae in loggerhead sea turtles from italian coasts. BMC Vet. Res. 2019, 15, 370. [Google Scholar] [CrossRef]
- Alduina, R.; Gambino, D.; Presentato, A.; Gentile, A.; Sucato, A.; Savoca, D.; Filippello, S.; Visconti, G.; Caracappa, G.; Vicari, D.; et al. Is Caretta caretta a carrier of antibiotic resistance in the Mediterranean Sea? Antibiotics 2020, 9, 116. [Google Scholar] [CrossRef] [Green Version]
- Duncan, E.M.; Broderick, A.C.; Fuller, W.J.; Galloway, T.S.; Godfrey, M.H.; Hamann, M.; Limpus, C.J.; Lindeque, P.K.; Mayes, A.G.; Omeyer, L.C.M.; et al. Microplastic ingestion ubiquitous in marine turtles. Glob. Chang. Biol. 2019, 25, 744–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias-Andres, M.; Klümper, U.; Rojas-Jimenez, K.; Grossart, H.P. Microplastic pollution increases gene exchange in aquatic ecosystems. Environ. Pollut. 2018, 237, 253–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckert, E.M.; Di Cesare, A.; Kettner, M.T.; Arias-Andres, M.; Fontaneto, D.; Grossart, H.P.; Corno, G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018, 234, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Enneson, J.; Litzgus, J. Using long-term data and stage-classified matrix to assess conservation strategies for an endangered turtle (Clemmys guttata). Biol. Conserv. 2018, 141, 1560–1568. [Google Scholar] [CrossRef]
- Howell, J.; McKnight, D.; Seigel, R. A novel method of collecting spotted turtles (Clemmys guttata). Herpetol. Rev. 2016, 47, 28–31. [Google Scholar]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Cech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The galaxy platform for accessible, reproducible and collaborative biomedical analyses: 2018 update. Nucleic Acids Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef] [Green Version]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef] [Green Version]
- Meier-Kolthoff, J.P.; Göker, M. TYGS is an automated high-throughput platform for state-of-the-art genome-based taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef]
- Ondov, B.D.; Treangen, T.J.; Melsted, P.; Mallonee, A.B.; Bergman, N.H.; Koren, S.; Phillippy, A.M. Mash: Fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016, 17, 132. [Google Scholar] [CrossRef] [Green Version]
- Camacho, C.; Coulouris, G.; Avagyan, V.; Ma, N.; Papadopoulos, J.; Bealer, K.; Madden, T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009, 10, 421. [Google Scholar] [CrossRef] [Green Version]
- Lagesen, K.; Hallin, P.; Rødland, E.A.; Staerfeldt, H.H.; Rognes, T.; Ussery, D.W. RNAmmer: Consistent and rapid annotation of ribosomal RNA genes. Nucleic Acids Res. 2007, 35, 3100–3108. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, B.; Raphenya, A.R.; Alcock, B.; Waglechner, N.; Guo, P.; Tsang, K.K.; Lago, B.A.; Dave, B.M.; Pereira, S.; Sharma, A.N.; et al. CARD 2017: Expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res. 2017, 45, D566–D573. [Google Scholar] [CrossRef] [PubMed]
- Blin, K.; Shaw, S.; Steinke, K.; Villebro, R.; Ziemert, N.; Lee, S.Y.; Medema, M.H.; Weber, T. antiSMASH 5.0: Updates to the secondary metabolite genome mining pipeline. Nucleic Acids Res. 2019, 47, W81–W87. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.L.; Chiu, C.H.; Chu, C.; Huang, Y.C.; Lin, T.Y.; Ou, J.T. A Multiplex polymerase chain reaction method for rapid identification of Citrobacter freundii and Salmonella species, including Salmonella Typhi. J. Microbiol. Immunol. Infect. 2007, 40, 222–226. [Google Scholar]
- Schmitt, C.K.; McKee, M.L.; O’Brien, A.D. Two copies of shiga-like toxin ii-related genes common in enterohemorrhagic Escherichia coli strains are responsible for the antigenic heterogeneity of the O157: H- strain E32511. Infect. Immun. 1991, 59, 1065–1073. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The imagej ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.G.; Gonçalves, B.R.; da Silva, M.S.; Novais, Â.; Machado, E.; Carriço, J.A.; Peixe, L. Citrobacter portucalensis Sp. Nov., isolated from an aquatic sample. Int. J. Syst. Evol. Microbiol. 2017, 67, 3513–3517. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.J.; Shi, Q.S.; Ouyang, Y.S.; Chen, Y.B.; Hu, W.F. Effects of nutritional and environmental conditions on planktonic growth and biofilm formation of Citrobacter werkmanii BF-6. J. Microbiol. Biotechnol. 2013, 23, 1673–1682. [Google Scholar] [CrossRef]
- Zhou, G.; Peng, H.; Wang, Y.-S.; Huang, X.-M.; Xie, X.-B.; Shi, Q.-S. Complete genome sequence of Citrobacter werkmanii strain BF-6 isolated from industrial putrefaction. BMC Genom. 2017, 18, 765. [Google Scholar] [CrossRef] [Green Version]
- Weber, M.M.; French, C.L.; Barnes, M.B.; Siegele, D.A.; McLean, R.J. A previously uncharacterized gene, YjfO (BsmA), influences Escherichia coli biofilm formation and stress response. Microbiology 2010, 156, 139–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labbate, M.; Queck, S.Y.; Koh, K.S.; Rice, S.A.; Givskov, M.; Kjelleberg, S. Quorum sensing-controlled biofilm development in Serratia liquefaciens MG1. J. Bacteriol. 2004, 186, 692–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domka, J.; Lee, J.; Wood, T.K. YliH (BssR) and YceP (BssS) regulate escherichia coli K-12 biofilm formation by influencing cell signaling. Appl. Environ. Microbiol. 2006, 72, 2449–2459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kirillina, O.; Fetherston, J.D.; Bobrov, A.G.; Abney, J.; Perry, R.D. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol. Microbiol. 2004, 54, 75–88. [Google Scholar] [CrossRef]
- Bobrov, A.G.; Kirillina, O.; Perry, R.D. The phosphodiesterase activity of the HmsP EAL domain is required for negative regulation of biofilm formation in Yersinia pestis. FEMS Microbiol. Lett. 2005, 247, 123–130. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Wang, X.; Ma, Q.; Zhang, X.S.; Wood, T.K. Toxin-antitoxin systems in Escherichia coli influence biofilm formation through YjgK (TabA) and fimbriae. J. Bacteriol. 2009, 191, 1258–1267. [Google Scholar] [CrossRef] [Green Version]
- Ogasawara, H.; Yamamoto, K.; Ishihama, A. Role of the biofilm master regulator Csgd in cross-regulation between biofilm formation and flagellar synthesis. J. Bacteriol. 2011, 193, 2587–2597. [Google Scholar] [CrossRef] [Green Version]
- Labrie, J.; Pelletier-Jacques, G.; Deslandes, V.; Ramjeet, M.; Auger, E.; Nash, J.H.; Jacques, M. Effects of growth conditions on biofilm formation by Actinobacillus Pleuropneumoniae. Vet. Res. 2010, 41, 3. [Google Scholar] [CrossRef] [Green Version]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic Di-GMP: The first 25 Years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [Green Version]
- Gerstel, U.; Park, C.; Römling, U. Complex regulation of Csgd promoter activity by global regulatory proteins. Mol. Microbiol. 2003, 49, 639–654. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.; Mylona, E.; Frankel, G. Typhoidal salmonella: Distinctive virulence factors and pathogenesis. Cell. Microbiol. 2018, 20, e12939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keitel, W.A.; Bond, N.L.; Zahradnik, J.M.; Cramton, T.A.; Robbins, J.B. Clinical and serological responses following primary and booster immunization with salmonella typhi vi capsular polysaccharide vaccines. Vaccine 1994, 12, 195–199. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Z.; Xiong, K.; Wang, J.; Rao, X.; Cong, Y. Vi Capsular polysaccharide: Synthesis, virulence, and application. Crit. Rev. Microbiol. 2017, 43, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.M.; Baron, L.S. Genetic transfer of the Vi antigen from Salmonella typhosa to Escherichia coli. J. Bacteriol. 1969, 99, 358–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetter, M.; Goulding, D.; Pickard, D.; Kowarik, M.; Waechter, C.J.; Dougan, G.; Wacker, M. Molecular characterization of the ViaB locus encoding the biosynthetic machinery for Vi capsule formation in Salmonella Typhi. PLoS ONE 2012, 7, e45609. [Google Scholar] [CrossRef]
- Al Safadi, R.; Abu-Ali, G.S.; Sloup, R.E.; Rudrik, J.T.; Waters, C.M.; Eaton, K.A.; Manning, S.D. Correlation between in vivo biofilm formation and virulence gene expression in Escherichia coli O104:H4. PLoS ONE 2012, 7, e41628. [Google Scholar] [CrossRef]
- Bassler, B.L. How bacteria talk to each other: Regulation of gene expression by quorum sensing. Curr. Opin. Microbiol. 1999, 2, 582–587. [Google Scholar] [CrossRef]
- Waters, C.M.; Bassler, B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005, 21, 319–346. [Google Scholar] [CrossRef] [Green Version]
- Nealson, K.H.; Platt, T.; Hastings, J.W. Cellular control of the synthesis and activity of the bacterial luminescent system. J. Bacteriol. 1970, 104, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, R.; Joseph, S.W.; Chopra, A.K.; Sha, J.; Shaw, J.; Graf, J.; Haft, D.; Wu, M.; Ren, Q.; Rosovitz, M.J.; et al. Genome sequence of Aeromonas hydrophila ATCC 7966T: Jack of all trades. J. Bacteriol. 2006, 188, 8272–8282. [Google Scholar] [CrossRef] [Green Version]
- Igbinosa, E.O.; Rathje, J.; Habermann, D.; Brinks, E.; Cho, G.S.; Franz, C.M.A.P. Draft genome sequence of multidrug-resistant strain Citrobacter portucalensis MBTC-1222, isolated from uziza (Piper guineense) leaves in Nigeria. Genome Announc. 2018, 6, e00123-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, M.S.; Sultana, M.; Hossain, M.A. Complete genome arrangement revealed the emergence of a poultry origin superbug Citrobacter portucalensis strain NR-12. J. Glob. Antimicrob. Resist. 2019, 18, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Holmes, P.; Niccolls, L.M.; Sartory, D.P. The ecology of mesophilic Aeromonas in the aquatic environment. In The Genus: Aeromonas, 1st ed.; Austin, B., Altwegg, M., Gosling, P.J., Joseph, S.W., Eds.; John Wiley & Sons: Chicester, UK, 1996; p. 127. [Google Scholar]
- Martin-Carnahan, A.; Joseph, S.W. Aeromonas. In Bergey’s Manual of Systematic Bacteriology, 2nd ed.; Brenner, D.J., Krieg, N.R., Staley, J.T., Garrity, G.M., Eds.; Williams and Wilkins: New York, NY, USA, 2005; Volume 2. [Google Scholar]
- Pasquale, V.; Baloda, S.B.; Dumontet, S.; Krovacek, K. An outbreak of Aeromonas hydrophila infection in turtles (Pseudemis scripta). Appl. Environ. Microbiol. 1994, 60, 1678–1680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xianle, Y.; Fuen, K.; Jianguang, Z.; Xiaohui, A. Virulence of Aeromonas hydrophila isolated from diseased soft-shelled turtle, Trionyx sinensis. J. Fish. Sci. China 1999, 6, 107–121. [Google Scholar]
- Gao, G.; Shi, Q.; Zhang, Y.; Gao, G.; Chen, C. Separation and identification of pathogens causing soft-shelled turtle fulminant infectious disease and preparation of anti-aeromonas serum. Agric. Sci. Technol. 2012, 13, 2155–2158. [Google Scholar]
- Shan, Q.; Zheng, G.; Liu, S.; Bai, Y.; Li, L.; Yin, Y.; Ma, L.; Zhu, X. Pharmacokinetic/pharmacodynamic relationship of marbofloxacin against Aeromonas hydrophila in Chinese soft-shelled turtles (Trionyx sinensis). J. Vet. Pharmacol. Ther. 2015, 38, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Delli Paoli Carini, A.; Ariel, E.; Picard, J.; Elliott, L. Antibiotic resistant bacterial isolates from captive green turtles and in vitro sensitivity to bacteriophages. Int. J. Microbiol. 2017, 2017, 5798161. [Google Scholar] [CrossRef] [Green Version]
- Palma-Martínez, I.; Guerrero-Mandujano, A.; Ruiz-Ruiz, M.J.; Hernández-Cortez, C.; Molina-López, J.; Bocanegra-García, V.; Castro-Escarpulli, G. Active Shiga-like toxin produced by some Aeromonas spp., isolated in Mexico City. Front. Microbiol. 2016, 7, 1522. [Google Scholar] [CrossRef] [Green Version]
- Grotiuz, G.; Sirok, A.; Gadea, P.; Varela, G.; Schelotto, F. Shiga toxin 2-producing Acinetobacter haemolyticus associated with a case of bloody diarrhea. J. Clin. Microbiol. 2006, 44, 3838–3841. [Google Scholar] [CrossRef] [Green Version]
- Torres, A.G.; Jeter, C.; Langley, W.; Matthysse, A.G. Differential binding of Escherichia coli O157:H7 to alfalfa, human epithelial cells, and plastic is mediated by a variety of surface structures. Appl. Environ. Microbiol. 2005, 71, 8008–8015. [Google Scholar] [CrossRef] [Green Version]
- Raad, I.; Costerton, W.; Sabharwal, U.; Sacilowski, M.; Anaissie, E.; Bodey, G.P. Ultrastructural analysis of indwelling vascular catheters: A Quantitative relationship between luminal colonization and duration of placement. J. Infect. Dis. 1993, 168, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Stickler, D.; Morris, N.; Moreno, M.C.; Sabbuba, N. Studies on the formation of crystalline bacterial biofilms on urethral catheters. Eur. J. Clin. Microbiol. Infect. Dis. 1998, 17, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Norton, T.; Thompson, R.; Pope, J.; Veltkamp, C.; Banks, B.; Howard, C.; Hawkins, S. Using confocal laser scanning microscopy, scanning electron microscopy and phase contrast light microscopy to examine marine biofilms. Aquat. Microb. Ecol. 1998, 16, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Hannig, C.; Follo, M.; Hellwig, E.; Al-Ahmad, A. Visualization of adherent micro-organisms using different techniques. J. Med. Microbiol. 2010, 59, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gagné-Thivierge, C.; Barbeau, J.; Levesque, R.C.; Charette, S.J. A new approach to study attached biofilms and floating communities from Pseudomonas aeruginosa strains of various origins reveals diverse effects of divalent ions. FEMS Microbiol. Lett. 2018, 365, fny155. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, C.R.; Parsek, M.R. New insight into the early stages of biofilm formation. Proc. Natl. Acad. Sci. USA 2018, 115, 4317–4319. [Google Scholar] [CrossRef] [Green Version]





| Organism | Accession No. | Genome Size (bp) | %GC Content | Genome Coverage | No. of Contigs | No. of ORFs | No. of tRNAs | No. of rRNAs |
|---|---|---|---|---|---|---|---|---|
| Aeromonas hydrophila RIT668 | JABAJN000000000 | 4,773,422 | 61.52 | 82X | 90 | 4341 | 99 | 4 |
| Citrobacter freundii RIT669 | JABAJM000000000 | 4,900,040 | 51.97 | 80X | 76 | 4645 | 72 | 4 |
| Category | Read Count | 16S-Normalized Read Count | RGI Criteria | Antimicrobial Resistance Gene Family | Drug Class | % Identity of Matching Region | % Length of Reference Sequence | |
|---|---|---|---|---|---|---|---|---|
| multidrug | 72 | 2.65 | Strict | TRU beta-lactamase | Carbapenem | 96.46 | 100 | |
| unclassified | 4 | 0.35 | Strict | CphA beta-lactamase | Penem, Cephalosporin | 93.56 | 100 | |
| beta-lactam | 5 | 0.26 | Strict | resistance-nodulation-cell division (RND) antibiotic efflux pump | Fluroquinolone, Tetracycline | 49.32 | 98.39 | |
| MLS (macrolide, lincosamide, streptogramin) | 4 | 0.2 | Strict | elfamycin-resistant EF-Tu | Fluroquinolone, Tetracycline | 43.71 | 99.06 | |
| aminoglycoside | 3 | 0.16 | Strict | elfamycin-resistant EF-Tu | Elfamycin | 90.84 | 96.33 | |
| bacitracin | 3 | 0.16 | Strict | OXA beta-lactamase | Elfamycin | 90.84 | 96.33 | |
| glycopeptide | 1 | 0.06 | Strict | resistance-nodulation-cell division (RND) antibiotic efflux pump | Cephalosporin | 99.74 | 110.73 |
| Category | Read Count | 16S-Normalized Read Count | RGI Criteria | Antimicrobial Resistance Gene Family | Drug Class | % Identity of Matching Region | % Length of Reference Sequence | |
|---|---|---|---|---|---|---|---|---|
| multidrug | 72 | 2.6504884 | Perfect | CMY beta-lactamase | Cephamycin Cephalosporin | 100 | 100 | |
| unclassified | 4 | 0.351411 | Strict | penicillin-binding protein mutation conferring resistance to beta-lactam antibiotics | Carbapenem, Cephamycin Penem, Monobactam, Cephalosporin | 52.75 | 96.39 | |
| beta-lactam | 5 | 0.2594203 | Strict | kdpDE | Aminoglycoside | 90.62 | 100 | |
| MLS (macrolide, lincosamide, streptogramin) | 4 | 0.1995819 | Strict | MFS, RND antibiotic efflux pump | Cephamycin; Cephalosporin; Fluoroquinolone, Macrolide, Penem | 95.62 | 100 | |
| aminoglycoside | 3 | 0.1573626 | Strict | ATP-binding cassette antibiotic efflux pump | Nitroimidazole | 94.33 | 100 | |
| bacitracin | 3 | 0.1573626 | Strict | RND antibiotic efflux pump | Macrolide, Fluoroquinolone, Penem | 99.05 | 100 | |
| glycopeptide | 1 | 0.0625328 | Strict | antibiotic resistance nfsA | Nitrofuran | 85.8 | 100 | |
| Strict | GlpT | Fosfomycin | 94.91 | 100 | ||||
| Strict | MFS antibiotic efflux pump | Fluoroquinolone | 94.29 | 100 | ||||
| Strict | RND, antibiotic efflux pump | Aminoglycoside, Aminocoumarin | 97.07 | 100 | ||||
| Strict | pmr phosphoethanolamine transferase | Peptide | 87.85 | 101.55 | ||||
| Strict | quinolone resistance protein (qnr) | Fluoroquinolone | 99.56 | 100 | ||||
| Strict | elfamycin-resistant EF-Tu | Elfamycin | 98.75 | 78.24 | ||||
| Strict | major facilitator superfamily (MFS) antibiotic efflux pump | Fluoroquinolone | 95.12 | 100 | ||||
| Strict | resistance-nodulation-cell division (RND) antibiotic efflux pump | Aminocoumarin | 93.27 | 100 | ||||
| Strict | resistance-nodulation-cell division (RND) antibiotic efflux pump | Cephalosporin, Fluoroquinolone Phenicol Tetracycline Glycylcycline, Penem, Rifamycin Triclosan | 94.57 | 100 | ||||
| Strict | resistance-nodulation-cell division (RND) antibiotic efflux pump | Cephalosporin, Fluoroquinolone Penem, Phenicol, Glycylcycline, Tetracycline, Rifamycin Triclosan | 90.97 | 100 | ||||
| Strict | major facilitator superfamily (MFS) antibiotic efflux pump | Rhodamine, Tetracycline, Benzalkonium chloride | 87.8 | 100 | ||||
| Strict | general bacterial porin with reduced permeability to beta-lactams, RND, antibiotic efflux pump, ATP-binding cassette (ABC) antibiotic efflux pump, MFS antibiotic efflux pump | Cephamycin, Cephalosporin, Fluoroquinolone Penem, Glycylcycline, Monobactam, Triclosan, Phenicol Tetracycline Carbapenem, Rifamycin | 94.36 | 100 | ||||
| Strict | UhpT | Fosfomycin | 94.82 | 100 | ||||
| Strict | RND, antibiotic efflux pump | Cephalosporin, Fluoroquinolone Phenicol Tetracycline Glycylcycline, Penem, Rrifamycin Triclosan | 90.43 | 100 | ||||
| Strict | major facilitator superfamily (MFS) antibiotic efflux pump | Fosfomycin | 90.89 | 99.51 | ||||
| Strict | general bacterial porin with reduced permeability to beta-lactams, resistance-nodulation-cell division (RND) antibiotic efflux pump | Cephamycin, Cephalosporin, Fluoroquinolone, Glycylcycline, Penem, Monobactam, Triclosan, Phenicol, Tetracycline, Carbapenem, Rifamycin | 94.44 | 100 |
| Accession Number or Locus Tag | Genes Names | Annotation | Primer Sequence (5′–3′) | Tm °C |
|---|---|---|---|---|
| KC489166 | 16S RNA | 16S ribosomal RNA (house-keeping gene) | TTACCTACTCTTGACATC | 55.0 |
| GACTTAACCCAACATTTC | ||||
| B2G73_RS15900 | bsmA | biofilm peroxide resistance protein | TAATGGGTTACAGCGAATAG | 53.1 |
| ATAAGACCACATAATAATCAGC | 50.6 | |||
| B2G73_RS10300 | bssR | biofilm formation regulatory protein | CGCTTATCTGCTGTTGAG | 52.9 |
| ATACCGTGAAGTTGTGATTG | 53.5 | |||
| B2G73_RS09175 | bssS | biofilm formation regulatory protein | GGACTGAAGTTGGACAAA | 51.5 |
| CGCTGATACTCATTTACCT | 50.3 | |||
| B2G73_RS19460 | hmsP | biofilm formation regulator | GTTAATACTCACGGTAGC | 45.1 |
| GGTAATGCCAGTTGATAG | 48.5 | |||
| B2G73_RS15490 | tabA | Toxin-antitoxin biofilm protein | GTCGGCAATATTCACAAC | 52.0 |
| TCATATCTTCGGCAATCA | 53.3 | |||
| B2G73_RS09280 | csgD | transcriptional activator of curli operon | GCGTTATTACAGCACTTA | 47.1 |
| TTATCTGCCTCCATCATAT | 50.3 | |||
| - | viaB | Virulence (Vi polysaccharide antigen) | TGTCGAGCAGATGGATGAGCAT (VIAB-1) | 65.4 |
| ACGGCTGAAGGTTACGGACCGA (VIAB-2) | 69.1 | |||
| - | slt-ii | Virulence (SLT-II enterotoxin) | CCGGATCCATGAAGTGTATATTATTTAAATGG (GK1) | 62.0 |
| CCCGAATTCTCAGTCATTATTAAACTGCAC (GK4) | 67.2 |
| Organism | Cluster Number | Location within the Cluster | Predicted Gene Product | Percent Similarity to Known Cluster (Name of Cluster, Type) |
|---|---|---|---|---|
| A. hydrophila | 6 | 36,891–91,228 | NRPS (non-ribosomal peptide synthase) | 100 (Amonabactin, NRPS) |
| A. hydrophila | 27 | 34,062–70,902 | arylpolyene | 90 (Aeromonas sp. arylpolyene) |
| A. hydrophila | 15 | 59,435–80,058 | homoserine lactone | 100 (Aeromonas sp. homoserine lactone) |
| A. hydrophila | 37 | 8,045–18,305 | bacteriocin | 80 (A. hydrophila Strain TN-97-08, bacteriocin) |
| C. freundii | 2 | 283,708–327,304 | arylpolyene | 94 (APE Ec biosynthetic gene cluster from E. coli CFT037, arylpolyene) |
| C. freundii | 8 | 1–40,280 | NRPS | 30 (Turnerbactin biosynthetic gene cluster from Teredinibacter turnerae T7901, NRPS) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomas, S.G.; Glover, M.A.; Parthasarathy, A.; Wong, N.H.; Shipman, P.A.; Hudson, A.O. Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata). Microorganisms 2020, 8, 1172. https://doi.org/10.3390/microorganisms8081172
Thomas SG, Glover MA, Parthasarathy A, Wong NH, Shipman PA, Hudson AO. Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata). Microorganisms. 2020; 8(8):1172. https://doi.org/10.3390/microorganisms8081172
Chicago/Turabian StyleThomas, Seema G., Maryah A. Glover, Anutthaman Parthasarathy, Narayan H. Wong, Paul A. Shipman, and André O. Hudson. 2020. "Expression of a Shiga-Like Toxin during Plastic Colonization by Two Multidrug-Resistant Bacteria, Aeromonas hydrophila RIT668 and Citrobacter freundii RIT669, Isolated from Endangered Turtles (Clemmys guttata)" Microorganisms 8, no. 8: 1172. https://doi.org/10.3390/microorganisms8081172






