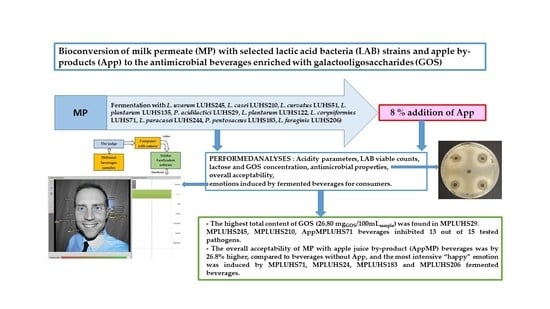
Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lactic Acid Bacteria Strains, Milk Permeate and Apple by-Products Used in the Preparation of Beverages
2.2. Fermentation of Milk Permeate (MP)
2.3. Determination of Acidity Parameters in the Beverages Throughout Fermentation Time
2.4. Determination of Lactic Acid Bacteria (LAB) Viable Counts in the Beverages throughout Fermentation Time
2.5. Determination of Lactose and Galactooligosaccharide (GOS) Concentration in the Initial Non-Fermented Milk Permeate (MP) and/or Final Fermented Beverages
2.6. Evaluation of the Antimicrobial Activity in the Milk Permeate (MP) and Final Fermented Beverages by the Agar Well-Diffusion and Liquid Culture Medium Methods
2.7. Evaluation of the Overall Acceptability and Emotions Induced by the Milk Permeate (MP) and Final Fermented Beverages
2.8. Statistical Analysis
3. Results and Discussion
3.1. Determination of Acidity Parameters in the Beverages throughout Fermentation Time
3.2. Determination of Lactic Acid Bacteria (LAB) Viable Counts in the Beverages throughout Fermentation Time
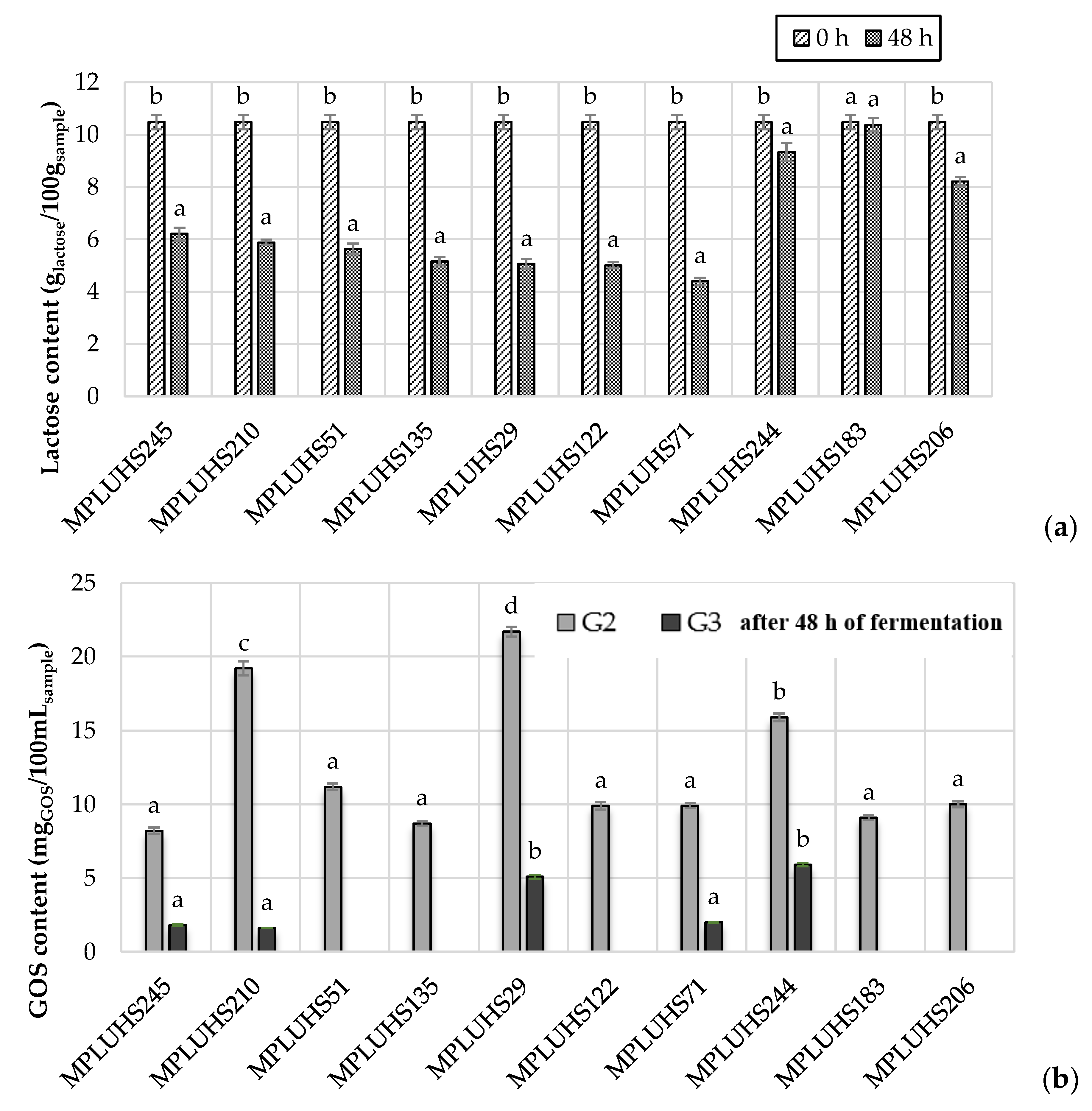
3.3. Determination of Lactose and Galactooligosaccharide (GOS) Concentration in the Initial Non-Fermented Milk Permeate (MP) and/or Final Fermented Beverages
3.4. Evaluation of the Antimicrobial Activity in the Milk Permeate (MP) and Final Fermented Beverages by the Agar Well-Diffusion and Liquid Culture Medium Methods
3.5. Evaluation of the Overall Acceptability and Emotions Induced by the Milk Permeate (MP) and Final Fermented Beverages
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| App | Apple by-products |
| CO2 | Carbon dioxide |
| log10(CFU/mL) | Colony-forming units per mL of sample |
| DIZ | Diameter of inhibition zones |
| EDTA-2Na | Ethylenediamine tetraacetic acid disodium salt |
| ELSD | Evaporative Light-Scattering Detector |
| GOS | Galactooligosaccharides |
| HPAEC-PAD | High-Performance Anion-Exchange Chromatography with Pulsed Amperometric Detection |
| HPLC | High-performance liquid chromatography |
| HPLC-ELSD | High-performance liquid chromatography with Evaporative Light-Scattering Detection |
| I.D. | Internal diameter |
| LAB | Lactic acid bacteria |
| MP | Milk permeate |
| AppMP | with Apple by-products |
| MH | Mueller–Hinton |
| °N | Neiman degrees |
| HNO3 | Nitric acid |
| NP | Normal-phase |
| nd | Not detected |
| 1-way ANOVA | One-way analysis of variance |
| OA | Overall acceptability |
| E | Potential |
| STDV | Standard deviation |
| The Man Rogosa and Sharpe | MRS |
| Time periods | t |
| Total titratable acidity | TTA |
| Water | H2O |
References
- Mirabella, N.; Castellani, V.; Sala, S. Current options for the valorization of food manufacturing waste: A review. J. Clean. Prod. 2014, 65, 28–41. [Google Scholar] [CrossRef] [Green Version]
- Zotta, T.; Solieri, L.; Iacumin, L.; Picozzi, C.; Gullo, M. Valorization of cheese whey using microbial fermentations. Appl. Microbiol. Biotechnol. 2020, 104, 2749–2764. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Guerra, M.A. On the valorisation of lactose and its derivatives from cheese whey as a dairy industry by-product: An overview. Eur. Food Res. Technol. 2020. In press. [Google Scholar] [CrossRef]
- Bartkiene, E.; Lele, V.; Sakiene, V.; Zavistanaviciute, P.; Ruzauskas, M.; Bernatoniene, J.; Jakstas, V.; Viskelis, P.; Zadeike, D.; Juodeikiene, G. Improvement of the antimicrobial activity of lactic acid bacteria in combination with berries/fruits and dairy industry by-products. J. Sci. Food Agric. 2019, 99, 3992–4002. [Google Scholar] [CrossRef]
- Bartkiene, E.; Bartkevics, V.; Starkute, V.; Zadeike, D.; Juodeikiene, G. The Nutritional and Safety Challenges Associated with Lupin Lacto-Fermentation. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novotni, D.; Gänzle, M.; Rocha, J.M. Chapter 15 Composition and activity of microbiota in sourdough and their effect on bread quality and safety. In Trends in Wheat and Bread Making; Galanakis, C.M., Ed.; Elsevier-Academic Press: Amsterdam, The Netherlands, 2020; In press. [Google Scholar]
- Bartkiene, E.; Bartkevics, V.; Lele, V.; Pugajeva, I.; Zavistanaviciute, P.; Mickiene, R.; Zadeike, D.; Juodeikiene, G. A concept of mould spoilage prevention and acrylamide reduction in wheat bread: Application of lactobacilli in combination with a cranberry coating. Food Control 2018, 91, 284–293. [Google Scholar] [CrossRef]
- Zotta, T.; Parente, E.; Ricciardi, A. Viability staining and detection of metabolic activity of sourdough lactic acid bacteria under stress conditions. World J. Microbiol. Biotechnol. 2009, 25, 1119–1124. [Google Scholar] [CrossRef]
- Aboukhalaf, A.; El Amraoui, B.; Tabatou, M.; Rocha, J.M.; Belahsen, R. Screening of the antimicrobial activity of some extracts of edible wild plants in Morocco. J. Funct. Food Health Dis. 2020, 6, 265–273. [Google Scholar] [CrossRef]
- Küley, E.; Özyurt, G.; Özogul, I.; Boga, M.; Akyol, I.; Rocha, J.M.; Özogul, F. The Role of Selected Lactic Acid Bacteria on Organic Acid Accumulation during Wet and Spray-Dried Fish-based Silages. Contributions to the Winning Combination of Microbial Food Safety and Environmental Sustainability. Microorganisms 2020, 8, 172. [Google Scholar] [CrossRef] [Green Version]
- Păcularu-Burada, B.; Georgescu, L.A.; Vasile, M.A.; Rocha, J.M.; Bahrim, G.-E. Selection of wild lactic acid bacteria strains as promoters of postbiotics in gluten-free sourdoughs. Microorganisms 2020, 8, 643. [Google Scholar] [CrossRef]
- Rocha, J.M. Microbiological and Lipid Profiles of Broa: Contributions for the Characterization of a Traditional Portuguese Bread. Ph.D. Thesis, University of Lisbon, Lisbon, Portugal, 2011. [Google Scholar]
- Rocha, J.M.; Malcata, F.X. Microbial ecology dynamics in Portuguese broa sourdough. J. Food Qual. 2016, 39, 634–648. [Google Scholar] [CrossRef]
- Rocha, J.M. Microencapsulation of probiotic bacteria. EC. Microb. 2016, 3, 480–481. [Google Scholar]
- Rocha, J.M.; Malcata, F.X. Behavior of the complex micro-ecology in maize and rye flour and mother-dough for broa throughout storage. J. Food Qual. 2016, 39, 218–233. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M.; Malcata, F.X. Microbiological profile of maize and rye flours, and sourdough used for the manufacture of traditional Portuguese bread. Food Microb. 2012, 31, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.M.; Malcata, F.X. On the microbiological profile of traditional Portuguese sourdough. J. Food Prot. 1999, 62, 1416–1429. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Liu, T.; Hou, J.; Pan, L.; Sadiq, F.A.; Yuan, L.; Yang, H.; He, G. Analysis of bacterial diversity and biogenic amines content during the fermentation processing of stinky tofu. Food Res. Int. Ott. Ont. 2018, 111, 689–698. [Google Scholar] [CrossRef] [PubMed]
- De Vos, W.M.; Vaughan, E.E. Genetics of lactose utilization in lactic acid bacteria. FEMS Microbiol. Rev. 1994, 15, 217–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaukat, A.; Levitt, M.D.; Taylor, B.C.; MacDonald, R.; Shamliyan, T.A.; Kane, R.L.; Wilt, T.J. Systematic review: Effective management strategies for lactose intolerance. Ann. Intern. Med. 2010, 152, 797–803. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Shoaf, K.; Mulvey, G.L.; Armstrong, G.D.; Hutkins, R.W. Prebiotic galactooligosaccharides reduce adherence of enteropathogenic Escherichia coli to tissue culture cells. Infect. Immun. 2006, 74, 6920–6928. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J.M. Composition and function of individual microbial biofilm matrix components: A key element to its control. EC. Chem. 2016, 2, 153–157. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic Acid Bacteria Isolation from Spontaneous Sourdough and Their Characterization Including Antimicrobial and Antifungal Properties Evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtin, C.M.; Swennen, K.; Verjans, P.; Delcour, J.A. Heat and pH stability of prebiotic arabinoxylooligosaccharides, xylooligosaccharides and fructooligosaccharides. Food Chem. 2009, 112, 831–837. [Google Scholar] [CrossRef]
- ISO 8586-1:1993. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/01/58/15875.html (accessed on 17 July 2020).
- Bartkiene, E.; Steibliene, V.; Adomaitiene, V.; Juodeikiene, G.; Cernauskas, D.; Lele, V.; Klupsaite, D.; Zadeike, D.; Jarutiene, L.; Guiné, R.P.F. Factors Affecting Consumer Food Preferences: Food Taste and Depression-Based Evoked Emotional Expressions with the Use of Face Reading Technology. BioMed. Res. Int. 2019, 2019, 2097415. [Google Scholar] [CrossRef]
- Reddy, A.; Norris, D.F.; Momeni, S.S.; Waldo, B.; Ruby, J.D. The pH of beverages in the United States. J. Am. Dent. Assoc. 1939 2016, 147, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Cais-Sokolińska, D.; Wójtowski, J.; Pikul, J.; Danków, R.; Majcher, M.; Teichert, J.; Bagnicka, E. Formation of volatile compounds in kefir made of goat and sheep milk with high polyunsaturated fatty acid content. J. Dairy Sci. 2015, 98, 6692–6705. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on lactic acid production from acidogenic fermentation of food waste with different types of inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Atallah, A.A. Development of new functional beverages from milk permeate using some probiotic bacteria and fruits pulp. Egypt. J. Dairy Sci. 2015, 43, 25–39. [Google Scholar]
- Hossein Marhamatizadeh, M.; Ehsandoost, E.; Gholami, P.; Moshiri, H.; Nazemi, M. Effect of Permeate on Growth and Survival of Lactobacillus acidophilus and Bifidobacterium bifidum for Production of Probiotic Nutritive Beverages. World Appl. Sci. J. 2012, 18, 1389–1393. [Google Scholar] [CrossRef]
- Hugo, A.A.; Bruno, F.; Golowczyc, M.A. Whey permeate containing galacto-oligosaccharides as a medium for biomass production and spray drying of Lactobacillus plantarum CIDCA 83114. LWT-Food Sci. Technol. 2016, 69, 185–190. [Google Scholar] [CrossRef]
- Papadimitriou, K.; Alegría, Á.; Bron, P.A.; de Angelis, M.; Gobbetti, M.; Kleerebezem, M.; Lemos, J.A.; Linares, D.M.; Ross, P.; Stanton, C.; et al. Stress Physiology of Lactic Acid Bacteria. Microbiol. Mol. Biol. Rev. MMBR 2016, 80, 837–890. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Cui, Y.; Qu, X. Mechanisms and improvement of acid resistance in lactic acid bacteria. Arch. Microbiol. 2018, 200, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Angmo, K.; Kumari, A.; Savitri; Bhalla, T.C. Probiotic characterization of lactic acid bacteria isolated from fermented foods and beverage of Ladakh. LWT-Food Sci. Technol. 2016, 66, 428–435. [Google Scholar] [CrossRef]
- Aamer, R.; El- Kholy, W.; Mailam, A. Production of Functional Beverages from Whey and Permeate Containing Kumquat Fruit. Alex. J. Food Sci. Technol. 2017, 14, 41–56. [Google Scholar] [CrossRef] [Green Version]
- Torres, D.P.M.; Gonçalves, M.d.P.F.; Teixeira, J.A.; Rodrigues, L.R. Galacto-Oligosaccharides: Production, Properties, Applications, and Significance as Prebiotics. Compr. Rev. Food Sci. Food Saf. 2010, 9, 438–454. [Google Scholar] [CrossRef] [Green Version]
- Vera, C.; Córdova, A.; Aburto, C.; Guerrero, C.; Suárez, S.; Illanes, A. Synthesis and purification of galacto-oligosaccharides: State of the art. World J. Microbiol. Biotechnol. 2016, 32, 197. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of Galactooligosaccharides in Milk and Whey: A Review. Compr. Rev. Food Sci. Food Saf. 2018, 17, 678–697. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kwak, M.-S.; Roh, J.-B.; Kim, K.; Sung, M.-H. Microbial β-Galactosidase of Pediococcus pentosaceus ID-7: Isolation, Cloning, and Molecular Characterization. J. Microbiol. Biotechnol. 2017, 27, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Chanalia, P.; Gandhi, D.; Attri, P.; Dhanda, S. Purification and characterization of β-galactosidase from probiotic Pediococcus acidilactici and its use in milk lactose hydrolysis and galactooligosaccharide synthesis. Bioorganic. Chem. 2018, 77, 176–189. [Google Scholar] [CrossRef]
- Hikmetoglu, M.; Sogut, E.; Sogut, O.; Gokirmakli, C.; Guzel-Seydim, Z.B. Changes in carbohydrate profile in kefir fermentation. Bioact. Carbohydr. Diet. Fibre 2020, 23, 100220. [Google Scholar] [CrossRef]
- Silva, J.; Carvalho, A.S.; Teixeira, P.; Gibbs, P.A. Bacteriocin production by spray-dried lactic acid bacteria. Lett. Appl. Microbiol. 2002, 34, 77–81. [Google Scholar] [CrossRef]
- Mokoena, M.P. Lactic Acid Bacteria and Their Bacteriocins: Classification, Biosynthesis and Applications against Uropathogens: A Mini-Review. Mol. Basel Switz. 2017, 22, 1255. [Google Scholar] [CrossRef] [PubMed]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fratianni, F.; Sada, A.; Cipriano, L.; Masucci, A.; Nazzaro, F. Biochemical Characteristics, Antimicrobial and Mutagenic Activity in Organically and Conventionally Produced Malus domestica, Annurca. Open Food Sci. J. 2007, 1, 10–16. [Google Scholar] [CrossRef]
- Barreca, D.; Bellocco, E.; Laganà, G.; Ginestra, G.; Bisignano, C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014, 160, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants Basel Switz. 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liya, S.J.; Siddique, R. Determination of antimicrobial activity of some commercial fruit (apple, papaya, lemon and strawberry) against bacteria causing urinary tract infection. Eur. J. Microbiol. Immunol. 2018, 8, 95–99. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Rodrigues, S. Chapter 15 Turning Fruit Juice Into Probiotic Beverages. In Fruit Juices; Gaurav, R., Brijesh, K.T., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 279–287. [Google Scholar] [CrossRef]
- Janiaski, D.R.; Pimentel, T.C.; Cruz, A.G.; Prudencio, S.H. Strawberry-flavored yogurts and whey beverages: What is the sensory profile of the ideal product? J. Dairy Sci. 2016, 48, 5273–5283. [Google Scholar] [CrossRef]
- Balthazar, C.F.; Santillo, A.; Figliola, L.; Silva, H.L.A.; Esmerino, E.A.; Freitas, M.Q.; Cruz, A.G.; Albenzio, M. Sensory evaluation of a novel prebiotic sheep milk strawberry beverage. LWT-Food Sci. Technol. 2018, 98, 94–98. [Google Scholar] [CrossRef]
- Juodeikiene, G.; Basinskiene, L.; Vidmantiene, D.; Klupsaite, D.; Bartkiene, E. The use of face reading technology to predict consumer acceptance of confectionery products. In Proceedings of the 9th Baltic Conference on Food Science and Technology “Food for Consumer Well-Being”, FOODBALT 2014, Jelgava, Latvia, 8–9 May 2014; pp. 276–279. [Google Scholar]
- Leitch, K.A.; Duncan, S.E.; O’Keefe, S.; Rudd, R.; Gallagher, D.L. Characterizing consumer emotional response to sweeteners using an emotion terminology questionnaire and facial expression analysis. Food Res. Int. 2015, 76, 283–292. [Google Scholar] [CrossRef] [Green Version]
- Danner, L.; Haindl, S.; Joechl, M.; Duerrschmid, K. Facial expressions and autonomous nervous system responses elicited by tasting different juices. Food Res. Int. 2014, 64, 81–90. [Google Scholar] [CrossRef]


| Milk Permeate Samples | Duration of Fermentation | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 h | 6 h | 12 h | 24 h | 48 h | |||||||||||
| pH | TTA (ºN) | LAB Viable Counts [(log10(CFU/mL)] | pH | TTA (ºN) | LAB Viable Counts [(log10(CFU/mL)] | pH | TTA (ºN) | LAB Viable Counts [(log10(CFU/mL)] | pH | TTA (ºN) | LAB Viable Counts [(log10(CFU/mL)] | pH | TTA (ºN) | LAB Viable Counts [(log10(CFU/mL)] | |
| MPNF | 5.88 ± 0.8 a | 3.0 ± 0.1 a | nd | 5.88 ± 0.8 a | 3.0 ± 0.14 a | nd | 5.88 ± 0.8 b | 3.0 ± 0.14 a | nd | 5.88 ± 0.8 b | 3.0 ± 0.14 a | nd | 5.88 ± 0.8 b | 3.0 ± 0.14 a | nd |
| MPLUHS245 | 5.58 ± 0.11 a | 3.6 ± 0.1 a | 6.85 ± 0.23 a | 5.57 ± 0.17 a | 3.6 ± 0.13 a | 6.79 ± 0.18 a | 5.48 ± 0.2 b | 3.5 ± 0.09 a | 6.89 ± 0.25 a | 4.25 ± 0.09 a | 6.4 ± 0.24 c | 7.06 ± 0.18 a | 3.97 ± 0.07 a | 9.5 ± 0.29 b | 8.68 ± 0.39 a |
| MPLUHS210 | 5.55 ± 0.14 a | 3.4 ± 0.14 a | 7.15 ± 0.26 a | 5.36 ± 0.11 a | 3.8 ± 0.15 a | 7.47 ± 0.27 a | 5.23 ± 0.1 b | 4.3 ± 0.1 b | 8.10 ± 0.28 b | 5.38 ± 0.12 b | 5.3 ± 0.23 b | 8.34 ± 0.32 a | 4.29 ± 0.16 a | 8.6 ± 0.17 b | 8.82 ± 0.23 a |
| MPLUHS51 | 5.57 ± 0.3 a | 2.9 ± 0.09 a | 6.83 ± 0.16 a | 5.60 ± 0.24 a | 3.2 ± 0.1 a | 6.91 ± 0.21 a | 5.65 ± 0.19 b | 3.6 ± 0.1 a | 7.38 ± 0.26 a | 4.19 ± 0.13 a | 6.2 ± 0.37 c | 7.49 ± 0.11 a | 3.88 ± 0.25a | 9.7 ± 0.41 b | 8.36 ± 0.28a |
| MPLUHS135 | 5.61 ± 0.3 a | 3.1 ± 0.9 a | 7.46 ± 0.21 a | 5.48 ± 0.19 a | 3.4 ± 0.07 a | 7.43 ± 0.17 a | 5.22 ± 0.21 b | 4.0 ± 0.1 b | 7.57 ± 0.14 a | 4.38 ± 0.09a | 7.5 ± 0.29 c | 7.98 ± 0.14 a | 4.03 ± 0.08 a | 9.4 ± 0.19 b | 8.58 ± 0.24 a |
| MPLUHS29 | 5.59 ± 0.15 a | 3.2 ± 0.03 a | 7.51 ± 0.26 a | 5.32 ± 0.27 a | 3.5 ± 0.07 a | 7.62 ± 0.21 a | 5.19 ± 0.13 b | 3.7 ± 0.07 a | 7.71 ± 0.28 a | 4.32 ± 0.11 a | 6.9 ± 0.26 c | 7.99 ± 0.22 a | 3.91 ± 0.23 a | 9.5 ± 0.19 b | 8.19 ± 0.23 a |
| MPLUHS122 | 5.62 ± 0.12 a | 3.5 ± 0.9 a | 7.08 ± 0.19 a | 5.36 ± 0.22 a | 3.8 ± 0.15 a | 7.16 ± 0.1 a | 5.22 ± 0.17 b | 4.1 ± 0.25 b | 7.25 ± 0.22 a | 4.33 ± 0.12 a | 5.8 ± 0.14 b | 7.68 ± 0.23 a | 4.04 ± 0.15 a | 8.7 ± 0.22 b | 8.64 ± 0.33 a |
| MPLUHS71 | 5.56 ± 0.17 a | 2.8 ± 0.12 a | 7.74 ± 0.15 a | 5.47 ± 0.14 a | 3.4 ± 0.13 a | 7.84 ± 0.19 a | 5.30 ± 0.19 b | 3.8 ± 0.08 a | 7.96 ± 0.29 a | 4.38 ± 0.12 a | 6.1 ± 0.12 c | 8.18 ± 0.31 a | 3.89 ± 0.12a | 9.6 ± 0.16 b | 8.78 ± 0.25 a |
| MPLUHS244 | 5.58 ± 0.17 a | 2.9 ± 0.06 a | 6.96 ± 0.23 a | 5.39 ± 0.2 a | 3.2 ± 0.09 a | 7.06 ± 0.21 a | 5.33 ± 0.16 b | 3.5 ± 0.25 a | 7.11 ± 0.17 a | 4.26 ± 0.09 a | 7.6 ± 0.28 c | 7.67 ± 0.26 a | 3.9 ± 0.13 a | 10.6 ± 0.21 c | 8.06 ± 0.31 a |
| MPLUHS183 | 5.61 ± 0.11 a | 3.5 ± 0.2 a | 7.16 ± 0.19 a | 5.35 ± 0.1 a | 3.7 ± 0.10 a | 6.91 ± 0.26 a | 5.25 ± 0.13 b | 4.2 ± 0.21 b | 7.01 ± 0.27 a | 4.32 ± 0.09 a | 7.9 ± 0.15 c | 7.52 ± 0.27 a | 3.8 ± 0.15 a | 11.0 ± 0.35 c | 8.10 ± 0.22 a |
| MPLUHS206 | 5.57 ± 0.12 a | 3.3 ± 0.2 a | 6.34 ± 0.17 a | 5.03 ± 0.18 a | 3.2 ± 0.12 a | 6.87 ± 0.24 a | 4.16 ± 0.08 a | 3.1 ± 0.18 a | 7.05 ± 0.15 a | 4.16 ± 0.12 a | 7.6 ± 0.23 c | 7.19 ± 0.32 a | 3.9 ± 0.08 a | 10.8 ± 0.41 c | 7.68 ± 0.14 a |
| Samples | Diameter of Inhibition Zones (DIZ) (mm) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic and Opportunistic Bacterial Strains | |||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Without apple by-products | |||||||||||||||
| MPLUHS245 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 15.4 ± 0.3 c | nd | nd | 13.0 ± 0.6 b | nd | 20.4 ± 0.6 d |
| MPLUHS210 | nd | 11.0 ± 0.2 | 12.5 ± 0.1 | nd | nd | nd | nd | nd | 12.6 ± 0.7 c | 15.9 ± 0.4 c | 11.7 ± 0.8 | nd | 15.4 ± 0.3 c | 12.9 ± 0.5 b | 14.7 ± 0.2 b |
| MPLUHS51 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 13.2 ± 0.9 b | nd | nd | 15.9 ± 0.6 c | 12.7 ± 0.3 b | 14.3 ± 0.4 b |
| MPLUHS135 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 13.5 ± 0.6 b | nd | nd | nd | 10.2 ± 0.6 a | 20.2 ± 0.9 d |
| MPLUHS29 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 12.7 ± 0.4 b | nd | nd | nd | nd | 15.0 ± 0.1 b |
| MPLUHS122 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 12.6 ± 0.8 b | nd | nd | 10.7 ± 0.6 a | nd | 20.4 ± 0.6 d |
| MPLUHS71 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 10.4 ± 0.3 a | nd | nd | 11.9 ± 0.4 a | nd | 23.3 ± 0.3 e |
| MPLUHS244 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 15.3 ± 0.2 c | nd | nd | 10.2 ± 0.1 a | nd | 20.7 ± 0.6 d |
| MPLUHS183 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 15.4 ± 0.9 c | nd | nd | 10.3 ± 0.2 a | nd | 20.6 ± 0.5 d |
| MPLUHS206 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 14.7 ± 0.7 c | nd | nd | 10.6 ± 0.6 a | nd | 20.2 ± 0.6 d |
| MPNF | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd |
| With apple by-products | |||||||||||||||
| AppMPLUHS245 | nd | nd | nd | nd | nd | 11.0 ± 0.7 a | nd | nd | nd | nd | nd | nd | 17.3 ± 0.1 d | nd | 17.3 ± 0.2 c |
| AppMPLUHS210 | nd | nd | nd | nd | nd | 12.6 ± 0.3 b | nd | nd | 12.3 ± 0.2 | 17.4 ± 0.3 d | nd | nd | 11.6 ± 0.2 a | nd | 18.6 ± 0.4 c |
| AppMPLUHS51 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 17.2 ± 0.4 d | nd | 14.5 ± 0.9 b |
| AppMPLUHS135 | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | nd | 18.9 ± 0.6 d | nd | 12.3 ± 0.5 a |
| AppMPLUHS29 | nd | nd | nd | nd | nd | nd | nd | nd | nd | 20.1 ± 0.4 e | nd | nd | 11.2 ± 0.1 a | nd | 15.2 ± 0.3 b |
| AppMPLUHS122 | nd | nd | 12.0 ± 0.4 a | nd | nd | 11.3 ± 0.2a | nd | nd | 11.6 ± 0.6 b | nd | nd | nd | nd | nd | 20.9 ± 0.6 d |
| AppMPLUHS71 | nd | nd | nd | nd | nd | 12.4 ± 0.5b | nd | nd | 10.4 ± 0.4 a | nd | nd | nd | nd | nd | 17.6 ± 0.4 c |
| AppMPLUHS244 | nd | nd | 12.3 ± 0.5 a b | nd | nd | nd | nd | nd | 11.2 ± 0.3 b | nd | nd | nd | nd | nd | 20.4 ± 0.3 d |
| AppMPLUHS183 | nd | nd | 13.4 ± 0.6 b | nd | nd | nd | nd | nd | 12.9 ± 0.7 c | nd | nd | nd | nd | nd | 17.9 ± 0.8 c |
| AppMPLUHS206 | nd | nd | 11.6 ± 0.2 a | nd | nd | nd | nd | nd | 11.2 ± 0.3 b | nd | nd | nd | nd | nd | 20.2 ± 0.4 d |
| AppMPNF | nd | nd | nd | nd | nd | nd | nd | nd | nd | 14.3 ± 0.6 | nd | nd | 10.8 ± 0.3 | nd | 16.6 ± 0.3 c |
| Growth ( + ) or Growth Absence (-) of Pathogenic and Opportunistic Bacteria | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogenic and Opportunistic Bacterial Strains | ||||||||||||||||
| Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | Number of the Inhibited Pathogens |
| Without apple by-products Experimental design: 0.5 mL tested sample + 0.1 mL pathogen | ||||||||||||||||
| MPLUHS245 | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 13 |
| MPLUHS210 | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 13 |
| MPLUHS51 | - | - | - | - | + | - | + | + | - | - | + | - | - | - | - | 11 |
| MPLUHS135 | + | + | + | + | + | - | + | + | - | - | + | + | - | - | - | 6 |
| MPLUHS29 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 12 |
| MPLUHS122 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 12 |
| MPLUHS71 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 12 |
| MPLUHS244 | + | - | - | - | - | - | + | + | - | - | + | - | - | - | - | 11 |
| MPLUHS183 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 12 |
| MPLUHS206 | + | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 12 |
| MPNF | + | + | + | + | - | - | + | + | + | + | + | + | + | + | + | 0 |
| Pathogen control | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - |
| LAB control | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - |
| With apple by-products Experimental design: 0.5 mL tested sample + 0.1 mL pathogen | ||||||||||||||||
| AppMPLUHS245 | - | + | - | - | + | + | - | + | - | - | + | - | - | - | - | 10 |
| AppMPLUHS210 | - | - | - | - | - | + | + | + | - | - | - | - | - | - | + | 11 |
| AppMPLUHS51 | + | + | - | - | + | + | + | + | - | - | - | - | - | - | - | 9 |
| AppMPLUHS135 | - | + | - | - | + | + | + | + | - | - | + | - | - | - | - | 9 |
| AppMPLUHS29 | - | - | - | - | - | + | - | + | - | - | + | - | - | - | - | 12 |
| AppMPLUHS122 | - | - | - | - | - | + | + | + | - | - | - | + | - | - | - | 11 |
| AppMPLUHS71 | - | - | - | - | - | - | + | + | - | - | - | - | - | - | - | 13 |
| AppMPLUHS244 | - | - | - | - | + | - | + | + | - | - | + | - | - | + | - | 10 |
| AppMPLUHS183 | + | - | - | - | + | + | + | + | - | - | + | - | - | - | - | 9 |
| AppMPLUHS206 | + | - | - | - | + | + | + | + | - | - | - | - | - | - | - | 10 |
| AppMPNF | + | - | - | - | + | + | - | + | - | - | + | - | - | + | - | 9 |
| Pathogen control | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - |
| LAB control | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | - |
| Beverages Samples | Overall Acceptability | Emotions Induced by the Beverages (from 0 to 1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Neutral | Happy | Sad | Angry | Surprised | Scared | Disgusted | Contempt | Valence | ||
| Without apple by-products | ||||||||||
| MPLUHS245 | 3.9 ± 0.12 a | 0.42 ± 0.01 d | 0.06 ± 0.001 a | 0.24 ± 0.01 c | 0.08 ± 0.002 a | 0.03 ± 0.001 a | 0.0009 ± 0.00002 a | 0.0009 ± 0.00002 a | 0.01 ± 0.0002 a | 0.19 ± 0.004 c |
| MPLUHS210 | 4.4 ± 0.13 b | 0.38 ± 0.01 c | 0.04 ± 0.001 a | 0.2 ± 0.004 b | 0.09 ± 0.002 a | 0.03 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.01 ± 0.0002 a | 0.19 ± 0.004 c |
| MPLUHS51 | 4.3 ± 0.12 b | 0.28 ± 0.01 a | 0.09 ± 0.002 a | 0.18 ± 0.004 a | 0.06 ± 0.001 a | 0.04 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.02 ± 0.0004 a | 0.18 ± 0.004 c |
| MPLUHS135 | 4.9 ± 0.12 c | 0.35 ± 0.01 c | 0.12 ± 0.002 b | 0.16 ± 0.003 a | 0.09 ± 0.002 a | 0.03 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.01 ± 0.0002 a | 0.22 ± 0.004 d |
| MPLUHS29 | 5.3 ± 0.13 c | 0.23 ± 0.004 a | 0.14 ± 0.003 b | 0.16 ± 0.003 a | 0.13 ± 0.003 b | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.03 ± 0.0006 b | 0.13 ± 0.003 b |
| MPLUHS122 | 4.5 ± 0.11 b | 0.39 ± 0.01 c | 0.03 ± 0.001 a | 0.15 ± 0.003 a | 0.1 ± 0.002 a | 0.04 ± 0.001 a | 0.01 ± 0.0002 b | 0.001 ± 0.00002 a | 0.02 ± 0.0004 a | 0.11 ± 0.002 a |
| MPLUHS71 | 4.8 ± 0.08 c | 0.43 ± 0.01 d | 0.16 ± 0.0032 c | 0.08 ± 0.002 a | 0.03 ± 0.001 a | 0.02 ± 0.0004 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.01 ± 0.0002 a | 0.13 ± 0.003 b |
| MPLUHS244 | 3.9 ± 0,07 a | 0.34 ± 0.01 b | 0.18 ± 0.004 c | 0.11 ± 0.002 a | 0.07 ± 0.001 a | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.01 ± 0.0002 a | 0.09 ± 0.002 a |
| MPLUHS183 | 5.1 ± 0.1 c | 0.31 ± 0.01 b | 0.16 ± 0.003 c | 0.2 ± 0.004 b | 0.05 ± 0.001 a | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.002 ± 0.00004 a | 0.02 ± 0.0004 a | 0.29 ± 0.01 e |
| MPLUHS206 | 3.8 ± 0,08 a | 0.34 ± 0.01 b | 0.17 ± 0.0034 c | 0.17 ± 0.003 a | 0.12 ± 0.002 b | 0.06 ± 0.0012 b | 0.002 ± 0.00004 a | 0.001 ± 0.00002 a | 0.03 ± 0.001 b | 0.18 ± 0.004 c |
| MPNF | 3.5 ± 0.07 a | 0.21 ± 0.004 a | 0.19 ± 0.004 c | 0.19 ± 0.004 a | 0.09 ± 0.002 a | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.09 ± 0.002 c | 0.22 ± 0.004 d |
| With apple by-products | ||||||||||
| AppMPLUHS245 | 5.3 ± 0.08 c | 0.45 ± 0.01 d | 0.08 ± 0.002 a | 0.16 ± 0.003 a | 0.1 ± 0.002 a | 0.02 ± 0.0004 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.11 ± 0.002 c | 0.18 ± 0.004 c |
| AppMPLUHS210 | 5.8 ± 0.09 d | 0.32 ± 0.01 b | 0.01 ± 0.0002 a | 0.43 ± 0.009 | 0.05 ± 0.001 a | 0.03 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.13 ± 0.003 d | 0.27 ± 0.01 e |
| AppMPLUHS51 | 5.9 ± 0.08 d | 0.29 ± 0.01 a | 0.02 ± 0.0004 a | 0.22 ± 0.004 b | 0.16 ± 0.003 c | 0.04 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.09 ± 0.002 c | 0.25 ± 0.005 e |
| AppMPLUHS135 | 5.5 ± 0.08 c | 0.3 ± 0.01 b | 0.02 ± 0.0004 a | 0.29 ± 0.006 c | 0.14 ± 0.003 b | 0.02 ± 0.0004 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.08 ± 0.0016 c | 0.25 ± 0.005 e |
| AppMPLUHS29 | 5.9 ± 0.06 d | 0.24 ± 0.01 a | 0.07 ± 0.0014 a | 0.28 ± 0.006 c | 0.21 ± 0.004 d | 0.04 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.12 ± 0.002 d | 0.38 ± 0.01f |
| AppMPLUHS122 | 6.1 ± 0.09 d | 0.26 ± 0.01 a | 0.05 ± 0.001 a | 0.24 ± 0.01 b | 0.17 ± 0.003 c | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.11 ± 0.002 c | 0.18 ± 0.004 c |
| AppMPLUHS71 | 6.4 ± 0.09 d | 0.36 ± 0.01 b | 0.06 ± 0.001 a | 0.16 ± 0.003 a | 0.09 ± 0.002 a | 0.02 ± 0.0004 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.09 ± 0.002 c | 0.16 ± 0.003 b |
| AppMPLUHS244 | 6.8 ± 0.23 e | 0.33 ± 0.01 b | 0.07 ± 0.001 a | 0.15 ± 0.003 a | 0.1 ± 0.002 a | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.05 ± 0.001 b | 0.15 ± 0.003 b |
| AppMPLUHS183 | 7.1 ± 0.25 e | 0.38 ± 0.02 c | 0.04 ± 0.001 a | 0.13 ± 0.003 a | 0.1 ± 0.002 a | 0.08 ± 0.002 b | 0.005 ± 0.0001 a | 0.002 ± 0.00004 a | 0.03 ± 0.001 b | 0.16 ± 0.003 b |
| AppMPLUHS206 | 6.5 ± 0.23 d e | 0.36 ± 0.02 c | 0.08 ± 0.002 a | 0.16 ± 0.003 a | 0.09 ± 0.002 a | 0.01 ± 0.0002 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.01 ± 0.0002 a | 0.14 ± 0.003 b |
| AppMPNF | 5.2 ± 0.18 c | 0.37 ± 0.02 c | 0.13 ± 0.003 b | 0.18 ± 0.004 a | 0.06 ± 0.001 a | 0.03 ± 0.001 a | 0.001 ± 0.00002 a | 0.001 ± 0.00002 a | 0.09 ± 0.002 c | 0.08 ± 0.002 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zokaityte, E.; Cernauskas, D.; Klupsaite, D.; Lele, V.; Starkute, V.; Zavistanaviciute, P.; Ruzauskas, M.; Gruzauskas, R.; Juodeikiene, G.; Rocha, J.M.; et al. Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides. Microorganisms 2020, 8, 1182. https://doi.org/10.3390/microorganisms8081182
Zokaityte E, Cernauskas D, Klupsaite D, Lele V, Starkute V, Zavistanaviciute P, Ruzauskas M, Gruzauskas R, Juodeikiene G, Rocha JM, et al. Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides. Microorganisms. 2020; 8(8):1182. https://doi.org/10.3390/microorganisms8081182
Chicago/Turabian StyleZokaityte, Egle, Darius Cernauskas, Dovile Klupsaite, Vita Lele, Vytaute Starkute, Paulina Zavistanaviciute, Modestas Ruzauskas, Romas Gruzauskas, Grazina Juodeikiene, João Miguel Rocha, and et al. 2020. "Bioconversion of Milk Permeate with Selected Lactic Acid Bacteria Strains and Apple By-Products into Beverages with Antimicrobial Properties and Enriched with Galactooligosaccharides" Microorganisms 8, no. 8: 1182. https://doi.org/10.3390/microorganisms8081182