Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles
Abstract
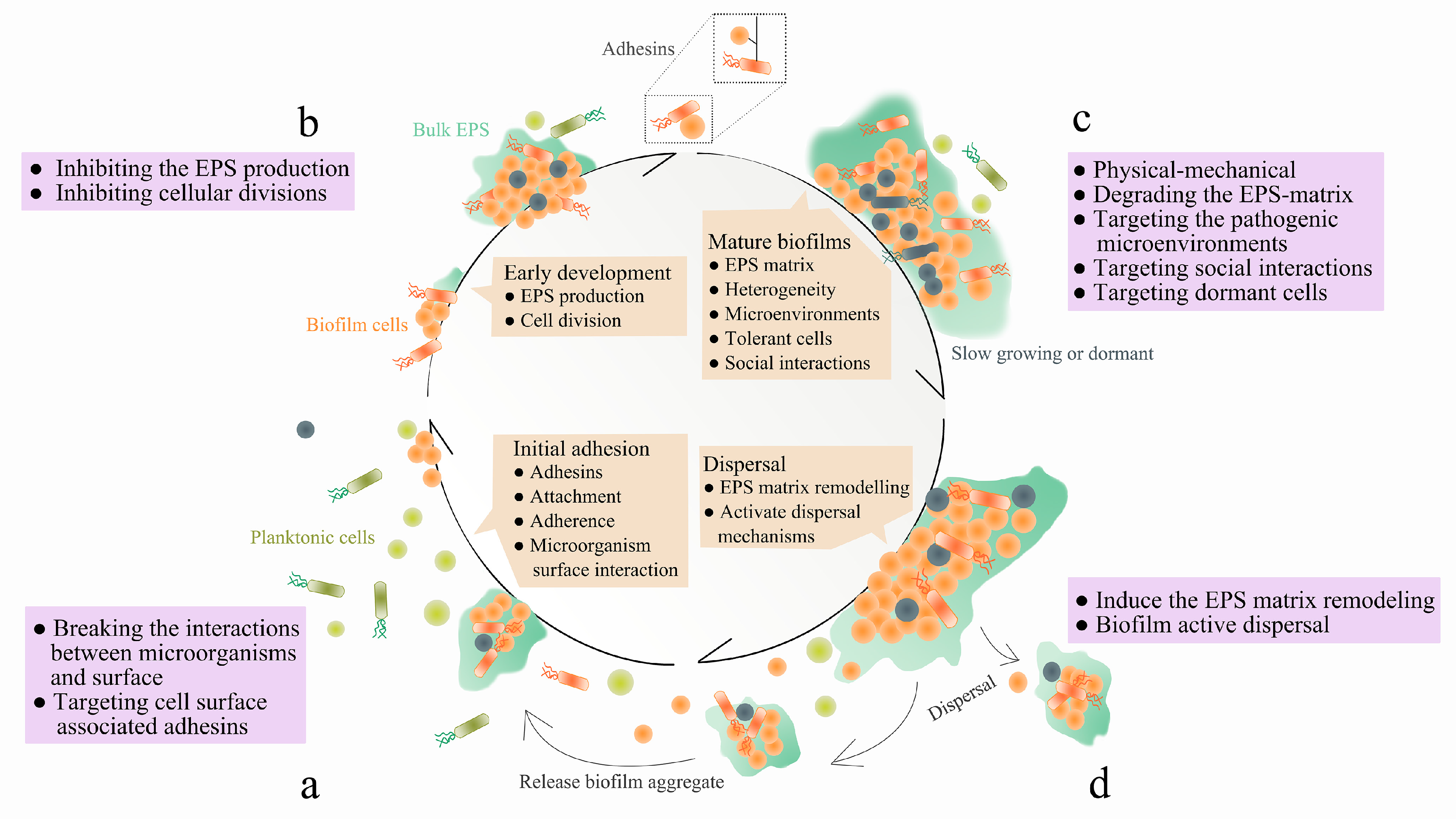
1. Introduction
2. Conventional Treatment Methods
3. EPS-Targeting Strategies
3.1. Targeting EPS Synthesis, Secretion and Adhesins
3.2. Targeting EPS Chemical Composition
3.2.1. Proteases
3.2.2. Deoxyribonuclease (DNase)
3.2.3. Glycoside Hydrolases
3.3. Targeting Specific Components in EPS and Nucleic-Acid-Binding Proteins
4. Dispersal Molecules
4.1. Dispersal Signals
4.2. Anti-Matrix Molecules
4.3. Sequestration Molecules
4.4. Metabolic Interference Molecules
5. Targeting Quorum Sensing
6. Targeting Dormant Cells in Biofilms
7. Hurdles to Development
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rumbaugh, K.P.; Diggle, S.P.; Watters, C.M.; Ross-Gillespie, A.; Griffin, A.S.; West, S.A. Quorum Sensing and the Social Evolution of Bacterial Virulence. Curr. Biol. 2009, 19, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Karatan, E.; Watnick, P. Signals, Regulatory Networks, and Materials That Build and Break Bacterial Biofilms. Microbiol. Mol. Biol. Rev. 2009, 73, 310. [Google Scholar] [CrossRef] [PubMed]
- Fleming, D.; Rumbaugh, K.P. Approaches to Dispersing Medical Biofilms. Microorganisms 2017, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Hoiby, N.; Bjarnsholt, T.; Moser, C.; Bassi, G.L.; Coenye, T.; Donelli, G.; Hall-Stoodley, L.; Hola, V.; Imbert, C.; Kirketerp-Moller, K.; et al. ESCMID guideline for the diagnosis and treatment of biofilm infections. Clin. Microbiol. Infect. 2015, 21, S1–S25. [Google Scholar] [CrossRef]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Howlin, R.P.; Brayford, M.J.; Webb, J.S.; Cooper, J.J.; Aiken, S.S.; Stoodley, P. Antibiotic-loaded synthetic calcium sulfate beads for prevention of bacterial colonization and biofilm formation in periprosthetic infections. Antimicrob. Agents Chemother. 2015, 59, 111–120. [Google Scholar] [CrossRef]
- Castaneda, P.; McLaren, A.; Tavaziva, G.; Overstreet, D. Biofilm Antimicrobial Susceptibility Increases with Antimicrobial Exposure Time. Clin. Orthop. Relat. Res. 2016, 474, 1659–1664. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, S.; Johnston, D.A.; Rmaile, A.; Gottenbos, B.; De Jager, M.; Aspiras, M.; Starke, E.M.; Ward, M.T.; Stoodley, P. Streptococcus mutans biofilm transient viscoelastic fluid behaviour during high-velocity microsprays. J. Mech. Behav. Biomed. Mater. 2016, 59, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Hobley, L.; Harkins, C.P.; Macphee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS Microbiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef]
- Gunn, J.S.; Bakaletz, L.O.; Wozniak, D.J. What’s on the Outside Matters: The Role of the Extracellular Polymeric Substance of Gram-negative Biofilms in Evading Host Immunity and as a Target for Therapeutic Intervention. J. Biol. Chem. 2016, 291, 12538–12546. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, Y.; Bai, G.; Zhou, X.; Wu, H. Cyclic di-AMP mediates biofilm formation. Mol. Microbiol. 2016, 99, 945–959. [Google Scholar] [CrossRef]
- Teschler, J.K.; Zamorano-Sánchez, D.; Utada, A.S.; Warner, C.J.A.; Wong, G.C.L.; Linington, R.G.; Yildiz, F. Living in the matrix: Assembly and control of Vibrio cholerae biofilms. Nat. Rev. Microbiol. 2015, 13, 255–268. [Google Scholar] [CrossRef]
- Mann, E.E.; Wozniak, D.J. Pseudomonas biofilm matrix composition and niche biology. FEMS Microbiol. Rev. 2012, 36, 893–916. [Google Scholar] [CrossRef]
- Ren, Z.; Cui, T.; Zeng, J.; Chen, L.; Zhang, W.; Xu, X.; Cheng, L.; Li, M.; Li, J.; Zhou, X.; et al. Molecule Targeting Glucosyltransferase Inhibits Streptococcus mutans Biofilm Formation and Virulence. Antimicrob. Agents Chemother. 2016, 60, 126–135. [Google Scholar] [CrossRef]
- Falsetta, M.L.; Klein, M.I.; Lemos, J.A.; Bueno-Silva, B.; Agidi, S.; Scott-Anne, K.K.; Koo, H. Novel Antibiofilm Chemotherapy Targets Exopolysaccharide Synthesis and Stress Tolerance in Streptococcus mutans To Modulate Virulence Expression in vivo. Antimicrob. Agents Chemother. 2012, 56, 6201–6211. [Google Scholar] [CrossRef]
- Fernicola, S.; Paiardini, A.; Giardina, G.; Rampioni, G.; Leoni, L.; Cutruzzolà, F.; Rinaldo, S. In Silico Discovery and in vitro Validation of Catechol-Containing Sulfonohydrazide Compounds as Potent Inhibitors of the Diguanylate Cyclase PleD. J. Bacteriol. 2016, 198, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Sambanthamoorthy, K.; Sloup, R.E.; Parashar, V.; Smith, J.M.; Kim, E.E.; Semmelhack, M.F.; Neiditch, M.B.; Waters, C.M. Identification of Small Molecules That Antagonize Diguanylate Cyclase Enzymes To Inhibit Biofilm Formation. Antimicrob. Agents Chemother. 2012, 56, 5202–5211. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Cusumano, C.K.; Kline, K.A.; Dodson, K.W.; Han, Z.; Janetka, J.W.; Henderson, J.P.; Caparon, M.G.; Hultgren, S.J. Combinatorial Small-Molecule Therapy Prevents Uropathogenic Escherichia coli Catheter-Associated Urinary Tract Infections in Mice. Antimicrob. Agents Chemother. 2012, 56, 4738–4745. [Google Scholar] [CrossRef] [PubMed]
- Totsika, M.; Kostakioti, M.; Hannan, T.; Upton, M.; Beatson, S.A.; Janetka, J.W.; Hultgren, S.J.; Schembri, M.A. A FimH inhibitor prevents acute bladder infection and treats chronic cystitis caused by multidrug-resistant uropathogenic Escherichia coli ST. J. Infect. Dis. 2013, 208, 921–928. [Google Scholar] [CrossRef]
- Spaulding, C.N.; Klein, R.D.; Ruer, S.; Kau, A.L.; Schreiber, H.L.; Cusumano, Z.T.; Dodson, K.W.; Pinkner, J.S.; Fremont, D.H.; Janetka, J.W.; et al. Selective depletion of uropathogenic E. coli from the gut by a FimH antagonist. Nature 2017, 546, 528–532. [Google Scholar] [CrossRef]
- Mydock-McGrane, L.; Cusumano, Z.; Han, Z.; Binkley, J.; Kostakioti, M.; Hannan, T.; Pinkner, J.S.; Klein, R.; Kalas, V.; Crowley, J.; et al. Antivirulence C-Mannosides as Antibiotic-Sparing, Oral Therapeutics for Urinary Tract Infections. J. Med. Chem. 2016, 59, 9390–9408. [Google Scholar] [CrossRef]
- Bouckaert, J.; Berglund, J.; Schembri, M.A.; De Genst, E.; Cools, L.; Wuhrer, M.; Hung, C.-S.; Pinkner, J.; Slättegård, R.; Zavialov, A.V.; et al. Receptor binding studies disclose a novel class of high-affinity inhibitors of the Escherichia coli FimH adhesin. Mol. Microbiol. 2005, 55, 441–455. [Google Scholar] [CrossRef]
- Han, Z.; Pinkner, J.S.; Ford, B.; Obermann, R.; Nolan, W.; Wildman, S.A.; Hobbs, D.; Ellenberger, T.; Cusumano, C.K.; Hultgren, S.J.; et al. Structure-Based Drug Design and Optimization of Mannoside Bacterial FimH Antagonists. J. Med. Chem. 2010, 53, 4779–4792. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Åberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Methods 2009, 5, 913–919. [Google Scholar] [CrossRef]
- Cozens, D.; Read, R.C. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef]
- Arita-Morioka, K.-I.; Yamanaka, K.; Mizunoe, Y.; Tanaka, Y.; Ogura, T.; Sugimoto, S. Inhibitory effects of Myricetin derivatives on curli-dependent biofilm formation in Escherichia coli. Sci. Rep. 2018, 8, 8452. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xie, Z.; Wei, H.; Zhang, S.; Song, Y.; Wang, M.; Zhang, Y. Antibacterial and Antibiofilm Activity of Temporin-GHc and Temporin-GHd Against Cariogenic Bacteria, Streptococcus mutans. Front. Microbiol. 2019, 10, 2854. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Cabezas-Olcoz, J.; Marchillo, K.; Mosher, D.F.; Andes, D.R. Targeting Fibronectin To Disrupt In Vivo Candida albicans Biofilms. Antimicrob. Agents Chemother. 2016, 60, 3152–3155. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Uppuluri, P.; Mamouei, Z.; Alqarihi, A.; Elhassan, H.; French, S.; Lockhart, S.R.; Chiller, T.; Edwards, J.E.; Ibrahim, A.S. The NDV-3A vaccine protects mice from multidrug resistant Candida auris infection. PLoS Pathog. 2019, 15, e1007460. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the Natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Lasa, I.; Penadés, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef]
- Jiao, Y.; Cody, G.D.; Harding, A.K.; Wilmes, P.; Schrenk, M.; Wheeler, K.E.; Banfield, J.F.; Thelen, M.P. Characterization of Extracellular Polymeric Substances from Acidophilic Microbial Biofilms. Appl. Environ. Microbiol. 2010, 76, 2916–2922. [Google Scholar] [CrossRef]
- Muthukrishnan, G.; Quinn, G.A.; Lamers, R.P.; Diaz, C.; Cole, A.L.; Chen, S.; Cole, A.M. Exoproteome of Staphylococcus aureus Reveals Putative Determinants of Nasal Carriage. J. Proteome Res. 2011, 10, 2064–2078. [Google Scholar] [CrossRef]
- Speziale, P.; Pietrocola, G.; Foster, T.J.; Geoghegan, J.A. Protein-based biofilm matrices in Staphylococci. Front. Cell. Infect. Microbiol. 2014, 4, 171. [Google Scholar] [CrossRef]
- Zhang, X.; Bishop, P.L. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 2003, 50, 63–69. [Google Scholar] [CrossRef]
- Kaplan, J.B. Biofilm dispersal: Mechanisms, clinical implications, and potential therapeutic uses. J. Dent. Res. 2010, 89, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Rao, T.S.; Shukla, S.K. Staphylococcus aureus biofilm removal by targeting biofilm-associated extracellular proteins. Indian J. Med Res. 2017, 146, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Marx, C.; Gardner, S.; Harman, R.M.; Van De Walle, G.R. The mesenchymal stromal cell secretome impairs methicillin-resistant Staphylococcus aureus biofilms via cysteine protease activity in the equine model. STEM CELLS Transl. Med. 2020, 9, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Loughran, A.J.; Atwood, D.N.; Anthony, A.C.; Harik, N.; Spencer, H.J.; Beenken, K.E.; Smeltzer, M.S. Impact of individual extracellular proteases on Staphylococcus aureus biofilm formation in diverse clinical isolates and their isogenic sarA mutants. Microbiologyopen 2014, 3, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Martí, M.; Trotonda, M.P.; Tormo-Más, M.Á.; Vergara-Irigaray, M.; Cheung, A.L.; Lasa, I.; Penadés, J.R. Extracellular proteases inhibit protein-dependent biofilm formation in Staphylococcus aureus. Microbes Infect. 2010, 12, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Gjermansen, M.; Nilsson, M.; Yang, L.; Tolker-Nielsen, T. Characterization of starvation-induced dispersion in Pseudomonas putida biofilms: Genetic elements and molecular mechanisms. Mol. Microbiol. 2010, 75, 815–826. [Google Scholar] [CrossRef]
- Chaignon, P.; Sadovskaya, I.; Ragunah, C.; Ramasubbu, N.; Kaplan, J.B.; Jabbouri, S. Susceptibility of staphylococcal biofilms to enzymatic treatments depends on their chemical composition. Appl. Microbiol. Biotechnol. 2007, 75, 125–132. [Google Scholar] [CrossRef]
- Cui, H.; Ma, C.; Lin, L. Co-loaded proteinase K/thyme oil liposomes for inactivation of Escherichia coli O157:H7 biofilms on cucumber. Food Funct. 2016, 7, 4030–4040. [Google Scholar] [CrossRef]
- Fredheim, E.G.A.; Klingenberg, C.; Rohde, H.; Frankenberger, S.; Gaustad, P.; Flaegstad, T.; Sollid, J.E.; Flægstad, T. Biofilm Formation by Staphylococcus haemolyticus. J. Clin. Microbiol. 2009, 47, 1172–1180. [Google Scholar] [CrossRef]
- Izano, E.A.; Shah, S.M.; Kaplan, J.B. Intercellular adhesion and biocide resistance in nontypeable Haemophilus influenzae biofilms. Microb. Pathog. 2009, 46, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Rao, T.S. Dispersal of Bap-mediated Staphylococcus aureus biofilm by proteinase K. J. Antibiot. 2013, 66, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Medina, A.A.; Kadouri, D.E. Biofilm formation of Bdellovibrio bacteriovorus host-independent derivatives. Res. Microbiol. 2009, 160, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, U.T.; Burrows, L.L. DNase I and proteinase K impair Listeria monocytogenes biofilm formation and induce dispersal of pre-existing biofilms. Int. J. Food Microbiol. 2014, 187, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.L.; Girerd, P.H.; Karjane, N.W.; Jefferson, K.K. Effect of biofilm phenotype on resistance of Gardnerella vaginalis to hydrogen peroxide and lactic acid. Am. J. Obstet. Gynecol. 2007, 197, 170.e1–170.e7. [Google Scholar] [CrossRef]
- Boles, B.R.; Horswill, A.R. agr-Mediated Dispersal of Staphylococcus aureus Biofilms. PLoS Pathog. 2008, 4, e1000052. [Google Scholar] [CrossRef]
- Lauderdale, K.J.; Boles, B.R.; Cheung, A.L.; Horswill, A.R. Interconnections between Sigma B, agr, and Proteolytic Activity in Staphylococcus aureus Biofilm Maturation. Infect. Immun. 2009, 77, 1623–1635. [Google Scholar] [CrossRef]
- Mootz, J.M.; Malone, C.L.; Shaw, L.N.; Horswill, A.R. Staphopains Modulate Staphylococcus aureus Biofilm Integrity. Infect. Immun. 2013, 81, 3227–3238. [Google Scholar] [CrossRef]
- Connolly, K.L.; Roberts, A.L.; Holder, R.C.; Reid, S.D. Dispersal of Group a Streptococcal Biofilms by the Cysteine Protease SpeB Leads to Increased Disease Severity in a Murine Model. PLoS ONE 2011, 6, e18984. [Google Scholar] [CrossRef]
- Nelson, D.C.; Garbe, J.; Collin, M. Cysteine proteinase SpeB from Streptococcus pyogenes—A potent modifier of immunologically important host and bacterial proteins. Biol. Chem. 2011, 392, 1077–1088. [Google Scholar] [CrossRef]
- Carothers, K.E.; Liang, Z.; Mayfield, J.; Donahue, D.L.; Lee, M.; Boggess, B.; Ploplis, V.A.; Castellino, F.J.; Lee, S.W. The Streptococcal Protease SpeB Antagonizes the Biofilms of the Human Pathogen Staphylococcus aureus USA300 through Cleavage of the Staphylococcal SdrC Protein. J. Bacteriol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.F.; Li, Y.H.; Bowden, G.H. Detachment of Streptococcus mutans biofilm cells by an endogenous enzymatic activity. Infect. Immun. 1996, 64, 1035–1038. [Google Scholar] [CrossRef] [PubMed]
- Banar, M.; Emaneini, M.; Satarzadeh, M.; Abdellahi, N.; Beigverdi, R.; Van Leeuwen, W.B.; Jabalameli, F. Evaluation of Mannosidase and Trypsin Enzymes Effects on Biofilm Production of Pseudomonas aeruginosa Isolated from Burn Wound Infections. PLoS ONE 2016, 11, e0164622. [Google Scholar] [CrossRef] [PubMed]
- Niazi, S.A.; Clark, D.; Do, T.; Gilbert, S.C.; Foschi, F.; Mannocci, F.; Beighton, D. The effectiveness of enzymic irrigation in removing a nutrient-stressed endodontic multispecies biofilm. Int. Endod. J. 2014, 47, 756–768. [Google Scholar] [CrossRef]
- McGavin, M.J.; Zahradka, C.; Rice, K.; Scott, J.E. Modification of the Staphylococcus aureus fibronectin binding phenotype by V8 protease. Infect. Immun. 1997, 65, 2621–2628. [Google Scholar] [CrossRef]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Jakubovics, N.; Shields, R.; Rajarajan, N.; Burgess, J. Life after death: The critical role of extracellular DNA in microbial biofilms. Lett. Appl. Microbiol. 2013, 57, 467–475. [Google Scholar] [CrossRef]
- Alhede, M.; Bjarnsholt, T.; Givskov, M.; Alhede, M. Pseudomonas aeruginosa biofilms: Mechanisms of immune evasion. Adv. Appl. Microbiol. 2014, 86, 1–40. [Google Scholar] [CrossRef]
- Okshevsky, M.; Meyer, R.L. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit. Rev. Microbiol. 2013, 41, 341–352. [Google Scholar] [CrossRef]
- Das, T.; Sehar, S.; Manefield, M. The roles of extracellular DNA in the structural integrity of extracellular polymeric substance and bacterial biofilm development. Environ. Microbiol. Rep. 2013, 5, 778–786. [Google Scholar] [CrossRef]
- Shak, S.; Capon, D.J.; Hellmiss, R.; Marsters, S.A.; Baker, C.L. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc. Natl. Acad. Sci. USA 1990, 87, 9188–9192. [Google Scholar] [CrossRef] [PubMed]
- Manzenreiter, R.; Kienberger, F.; Marcos, V.; Schilcher, K.; Krautgartner, W.D.; Obermayer, A.; Huml, M.; Stoiber, W.; Hector, A.; Griese, M.; et al. Ultrastructural characterization of cystic fibrosis sputum using atomic force and scanning electron microscopy. J. Cyst. Fibros. 2012, 11, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, J.; Yasuda, T.; Kunito, T.; Fujii, Y.; Takatsuka, H.; Moritani, T.; Takeshita, H. Two N-Linked Glycosylation Sites (Asn18 and Asn106) Are Both Required for Full Enzymatic Activity, Thermal Stability, and Resistance to Proteolysis in Mammalian Deoxyribonuclease I. Biosci. Biotechnol. Biochem. 2008, 72, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Okshevsky, M.; Regina, V.R.; Meyer, R.L. Extracellular DNA as a target for biofilm control. Curr. Opin. Biotechnol. 2015, 33, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Lu, S.-C.; Liao, T.-H. Cloning, sequencing and expression of a cDNA encoding bovine pancreatic deoxyribonuclease I in Escherichia coli: Purification and characterization of the recombinant enzyme. Gene 1998, 206, 181–184. [Google Scholar] [CrossRef]
- Demain, A.L.; Vaishnav, P. Production of recombinant proteins by microbes and higher organisms. Biotechnol. Adv. 2009, 27, 297–306. [Google Scholar] [CrossRef]
- Cho, E.-S.; Kim, J.-H.; Yoon, K.-H.; Kim, Y.H.; Nam, S.W. Overexpression and Characterization of Bovine Pancreatic Deoxyribonuclease I in Saccharomyces cerevisiae and Pichia pastoris. Microbiol. Biotechnol. Lett. 2012, 40, 348–355. [Google Scholar] [CrossRef]
- Hymes, S.; Randis, T.M.; Sun, T.Y.; Ratner, A.J. DNase Inhibits Gardnerella vaginalis Biofilms in vitro and in vivo. J. Infect. Dis. 2013, 207, 1491–1497. [Google Scholar] [CrossRef]
- Qin, Z.; Ou, Y.; Yang, L.; Zhu, Y.; Tolker-Nielsen, T.; Molin, S.; Qu, D. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology 2007, 153, 2083–2092. [Google Scholar] [CrossRef]
- Seper, A.; Fengler, V.H.; Roier, S.; Wolinski, H.; Kohlwein, S.D.; Bishop, A.L.; Camilli, A.; Reidl, J.; Schild, S. Extracellular nucleases and extracellular DNA play important roles in Vibrio cholerae biofilm formation. Mol. Microbiol. 2011, 82, 1015–1037. [Google Scholar] [CrossRef]
- Eckhart, L.; Fischer, H.; Barken, K.; Tolker-Nielsen, T.; Tschachler, E. DNase1L2 suppresses biofilm formation by Pseudomonas aeruginosa and Staphylococcus aureus. Br. J. Dermatol. 2007, 156, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Nistico, L.; Sambanthamoorthy, K.; Dice, B.; Nguyen, D.; Mershon, W.J.; Johnson, C.; Hu, F.Z.; Stoodley, P.; Ehrlich, G.D.; et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008, 8, 173. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; LoVetri, K.; Cardona, S.T.; Madhyastha, S.; Sadovskaya, I.; Jabbouri, S.; Izano, E.A. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J. Antibiot. 2011, 65, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Nijland, R.; Hall, M.; Burgess, J.G. Dispersal of Biofilms by Secreted, Matrix Degrading, Bacterial DNase. PLoS ONE 2010, 5, e15668. [Google Scholar] [CrossRef]
- Shakir, A.; ElBadawey, M.R.; Shields, R.; Jakubovics, N.; Burgess, J.G. Removal of Biofilms from Tracheoesophageal Speech Valves Using a Novel Marine Microbial Deoxyribonuclease. Otolaryngol. Head Neck Surg. 2012, 147, 509–514. [Google Scholar] [CrossRef]
- Shields, R.; Mokhtar, N.; Ford, M.; Hall, M.; Burgess, J.G.; ElBadawey, M.R.; Jakubovics, N. Efficacy of a Marine Bacterial Nuclease against Biofilm Forming Microorganisms Isolated from Chronic Rhinosinusitis. PLoS ONE 2013, 8, e55339. [Google Scholar] [CrossRef]
- Nemoto, K.; Hirota, K.; Murakami, K.; Taniguti, K.; Murata, H.; Viducic, D.; Miyake, Y. Effect of Varidase (streptodornase) on biofilm formed by Pseudomonas aeruginosa. Chemotherapy 2003, 49, 121–125. [Google Scholar] [CrossRef]
- Bales, P.M.; Renke, E.M.; May, S.L.; Shen, Y.; Nelson, D.C. Purification and Characterization of Biofilm-Associated EPS Exopolysaccharides from ESKAPE Organisms and Other Pathogens. PLoS ONE 2013, 8, e67950. [Google Scholar] [CrossRef]
- Wingender, J.; Strathmann, M.; Rode, A.; Leis, A.; Flemming, H.-C. [25] Isolation and biochemical characterization of extracellular polymeric substances from Pseudomonas aeruginosa. Methods Enzymol. 2001, 336, 302–314. [Google Scholar] [CrossRef]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial Extracellular Polysaccharides in Biofilm Formation and Function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef]
- Watters, C.; Fleming, D.; Bishop, D.; Rumbaugh, K. Host Responses to Biofilm. Prog. Mol. Biol. Transl. Sci. 2016, 142, 193–239. [Google Scholar] [CrossRef]
- Pestrak, M.J.; Baker, P.; Dellos-Nolan, S.; Hill, P.J.; Da Silva, D.P.; Silver, H.; Lacdao, I.; Raju, D.; Parsek, M.R.; Wozniak, D.J.; et al. Treatment with the Pseudomonas aeruginosa Glycoside Hydrolase PslG Combats Wound Infection by Improving Antibiotic Efficacy and Host Innate Immune Activity. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed]
- Pleszczyńska, M.; Wiater, A.; Janczarek, M.; Szczodrak, J. (1→3)-α-d-Glucan hydrolases in dental biofilm prevention and control: A review. Int. J. Biol. Macromol. 2015, 79, 761–778. [Google Scholar] [CrossRef]
- Fleming, D.; Chahin, L.; Rumbaugh, K.P. Glycoside Hydrolases Degrade Polymicrobial Bacterial Biofilms in Wounds. Antimicrob. Agents Chemother. 2016, 61. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B. Biofilm Matrix-Degrading Enzymes. In Microbial Biofilms; Humana Press: New York, NY, USA, 2014; Volume 1147, pp. 203–213. [Google Scholar] [CrossRef]
- Schmelcher, M.; Shen, Y.; Nelson, D.C.; Eugster, M.R.; Eichenseher, F.; Hanke, D.C.; Loessner, M.J.; Dong, S.; Pritchard, D.G.; Lee, J.C.; et al. Evolutionarily distinct bacteriophage endolysins featuring conserved peptidoglycan cleavage sites protect mice from MRSA infection. J. Antimicrob. Chemother. 2015, 70, 1453–1465. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Roach, D.R.; Chauhan, V.S.; Shen, Y.; Foster-Frey, J.; Powell, A.M.; Bauchan, G.R.; Lease, R.A.; Mohammadi, H.; Harty, W.J.; et al. Triple-acting Lytic Enzyme Treatment of Drug-Resistant and Intracellular Staphylococcus aureus. Sci. Rep. 2016, 6, 25063. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Hess, D.; Sturm, A. The N-glycans of jack bean alpha-mannosidase. Structure, topology and function. JBIC J. Biol. Inorg. Chem. 1999, 264, 168–175. [Google Scholar] [CrossRef] [PubMed]
- McCleary, B.V.; Matheson, N.K. Action patterns and substrate-binding requirements of β-d-mannanase with mannosaccharides and mannan-type polysaccharides. Carbohydr. Res. 1983, 119, 191–219. [Google Scholar] [CrossRef]
- Alkawash, M.A.; Soothill, J.S.; Schiller, N.L. Alginate lyase enhances antibiotic killing of mucoid Pseudomonas aeruginosa in biofilms. Apmis 2006, 114, 131–138. [Google Scholar] [CrossRef]
- Bayer, A.S.; Speert, D.P.; Park, S.; Tu, J.; Witt, M.; Nast, C.C.; Norman, D.C. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of Pseudomonas aeruginosa. Infect. Immun. 1991, 59, 302–308. [Google Scholar] [CrossRef]
- Hisano, T.; Nishimura, M.; Yonemoto, Y.; Abe, S.; Yamashita, T.; Sakaguchi, K.; Kimura, A.; Murata, K. Bacterial alginate lyase highly active on acetylated alginates. J. Ferment. Bioeng. 1993, 75, 332–335. [Google Scholar] [CrossRef]
- Lamppa, J.W.; Griswold, K.E. Alginate Lyase Exhibits Catalysis-Independent Biofilm Dispersion and Antibiotic Synergy. Antimicrob. Agents Chemother. 2013, 57, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Bradford, C.; Craigen, B.; Dashiff, A.; Kadouri, D.K. The Use of Commercially Available Alpha-Amylase Compounds to Inhibit and Remove Staphylococcus aureus Biofilms. Open Microbiol. J. 2011, 5, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, B.J.; Aarthy, S.; Pandian, S.K. Antibiofilm Activity of α-Amylase from Bacillus subtilis S8-18 Against Biofilm Forming Human Bacterial Pathogens. Appl. Biochem. Biotechnol. 2012, 167, 1778–1794. [Google Scholar] [CrossRef]
- Millenbaugh, N.J.; Watters, C.M.; Burton, T.; Kirui, D.K. Enzymatic degradation of in vitro Staphylococcus aureus biofilms supplemented with human plasma. Infect. Drug Resist. 2016, 9, 71–78. [Google Scholar] [CrossRef]
- Fazekas, E.; Kandra, L.; Gyémánt, G. Model for β-1, 6-N-acetylglucosamine oligomer hydrolysis catalysed by DispersinB, a biofilm degrading enzyme. Carbohydr. Res. 2012, 363, 7–13. [Google Scholar] [CrossRef]
- Gawande, P.V.; Leung, K.P.; Madhyastha, S. Antibiofilm and Antimicrobial Efficacy of DispersinB®-KSL-W Peptide-Based Wound Gel Against Chronic Wound Infection Associated Bacteria. Curr. Microbiol. 2014, 68, 635–641. [Google Scholar] [CrossRef]
- Itoh, Y.; Wang, X.; Hinnebusch, B.J.; Preston, J.F.; Romeo, T. Depolymerization of β-1, 6-N-Acetyl-d-Glucosamine Disrupts the Integrity of Diverse Bacterial Biofilms. J. Bacteriol. 2005, 187, 382–387. [Google Scholar] [CrossRef]
- Izano, E.A.; Sadovskaya, I.; Vinogradov, E.V.; Mulks, M.H.; Velliyagounder, K.; Ragunath, C.; Kher, W.B.; Ramasubbu, N.; Jabbouri, S.; Perry, M.B.; et al. Poly-N-acetylglucosamine mediates biofilm formation and antibiotic resistance in Actinobacillus pleuropneumoniae. Microb. Pathog. 2007, 43, 1–9. [Google Scholar] [CrossRef]
- Izano, E.; Wang, H.; Ragunath, C.; Ramasubbu, N.; Kaplan, J.; Bos, T.V.D.; Handoko, G.; Niehof, A.; Ryan, L.; Coburn, S.; et al. Detachment and Killing of Aggregatibacter actinomycetemcomitans Biofilms by Dispersin B and SDS. J. Dent. Res. 2007, 86, 618–622. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Ragunath, C.; Velliyagounder, K.; Fine, D.H.; Ramasubbu, N. Enzymatic Detachment of Staphylococcus epidermidis Biofilms. Antimicrob. Agents Chemother. 2004, 48, 2633–2636. [Google Scholar] [CrossRef] [PubMed]
- Waryah, C.; Wells, K.; Ulluwishewa, D.; Chen-Tan, N.; Gogoi-Tiwari, J.; Ravensdale, J.T.; Costantino, P.; Gökçen, A.; Vilcinskas, A.; Wiesner, J.; et al. in vitro Antimicrobial Efficacy of Tobramycin Against Staphylococcus aureus Biofilms in Combination With or Without DNase I and/or Dispersin B: A Preliminary Investigation. Microb. Drug Resist. 2017, 23, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Yakandawala, N.; Gawande, P.V.; LoVetri, K.; Cardona, S.T.; Romeo, T.; Nitz, M.; Madhyastha, S. Characterization of the Poly-β-1,6-N-Acetylglucosamine Polysaccharide Component of Burkholderia Biofilms. Appl. Environ. Microbiol. 2011, 77, 8303–8309. [Google Scholar] [CrossRef] [PubMed]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.K.; Horswill, A.R. Hyaluronan Modulation Impacts Staphylococcus aureus Biofilm Infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Pecharki, D.; Petersen, F.C.; Scheie, A.A. Role of hyaluronidase in Streptococcus intermedius biofilm. Microbiology 2008, 154, 932–938. [Google Scholar] [CrossRef]
- Baker, P.; Hill, P.J.; Snarr, B.D.; Alnabelseya, N.; Pestrak, M.J.; Lee, M.J.; Jennings, L.K.; Tam, J.; Melnyk, R.A.; Parsek, M.R.; et al. Exopolysaccharide biosynthetic glycoside hydrolases can be utilized to disrupt and prevent Pseudomonas aeruginosa biofilms. Sci. Adv. 2016, 2, e1501632. [Google Scholar] [CrossRef]
- Little, D.J.; Pfoh, R.; Le Mauff, F.; Bamford, N.C.; Notte, C.; Baker, P.; Guragain, M.; Robinson, H.; Pier, G.B.; Nitz, M.; et al. PgaB orthologues contain a glycoside hydrolase domain that cleaves deacetylated poly-β(1,6)-N-acetylglucosamine and can disrupt bacterial biofilms. PLoS Pathog. 2018, 14, e1006998. [Google Scholar] [CrossRef]
- Bamford, N.C.; Le Mauff, F.; Subramanian, A.S.; Yip, P.; Millán, C.; Zhang, Y.; Zacharias, C.; Forman, A.; Nitz, M.; Codée, J.D.C.; et al. Ega3 from the fungal pathogen Aspergillus fumigatus is an endo-α-1,4-galactosaminidase that disrupts microbial biofilms. J. Biol. Chem. 2019, 294, 13833–13849. [Google Scholar] [CrossRef]
- Le Mauff, F.; Bamford, N.C.; Alnabelseya, N.; Zhang, Y.; Baker, P.; Robinson, H.; Codée, J.D.C.; Howell, P.L.; Sheppard, D.C. Molecular mechanism of Aspergillus fumigatus biofilm disruption by fungal and bacterial glycoside hydrolases. J. Biol. Chem. 2019, 294, 10760–10772. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Wozniak, D.J.; Stoodley, P.; Hall-Stoodley, L. Prevention and treatment of Staphylococcus aureus biofilms. Expert Rev. Anti-Infect. Ther. 2015, 13, 1499–1516. [Google Scholar] [CrossRef]
- DiGiandomenico, A.; Warrener, P.; Hamilton, M.; Guillard, S.; Ravn, P.; Minter, R.; Camara, M.M.; Venkatraman, V.; MacGill, R.S.; Lin, J.; et al. Identification of broadly protective human antibodies to Pseudomonas aeruginosa exopolysaccharide Psl by phenotypic screening. J. Exp. Med. 2012, 209, 1273–1287. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Pinkner, J.S.; Caparon, M.G.; Hultgren, S.J. EbpA vaccine antibodies block binding of Enterococcus faecalis to fibrinogen to prevent catheter-associated bladder infection in mice. Sci. Transl. Med. 2014, 6, 254ra127. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Hung, C.S.; Hancock, L.E.; Caparon, M.G.; Hultgren, S.J. Enterococcal Biofilm Formation and Virulence in an Optimized Murine Model of Foreign Body-Associated Urinary Tract Infections. Infect. Immun. 2010, 78, 4166–4175. [Google Scholar] [CrossRef] [PubMed]
- Guiton, P.S.; Hung, C.S.; Kline, K.A.; Roth, R.; Kau, A.L.; Hayes, E.; Heuser, J.; Dodson, K.W.; Caparon, M.G.; Hultgren, S.J. Contribution of Autolysin and Sortase A during Enterococcus faecalis DNA-Dependent Biofilm Development. Infect. Immun. 2009, 77, 3626–3638. [Google Scholar] [CrossRef]
- Goodman, S.D.; Obergfell, K.P.; Jurcisek, J.A.; Novotny, L.A.; Downey, J.S.; Ayala, E.A.; Tjokro, N.; Li, B.; Justice, S.S.; Bakaletz, L.O. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011, 4, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.A.; Jurcisek, J.A.; Goodman, S.D.; Bakaletz, L.O. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine 2016, 10, 33–44. [Google Scholar] [CrossRef]
- Rocco, C.J.; Davey, M.E.; Bakaletz, L.O.; Goodman, S.D. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Mol. Oral Microbiol. 2016, 32, 118–130. [Google Scholar] [CrossRef]
- Devaraj, A.; Justice, S.S.; Bakaletz, L.O.; Goodman, S.D. DNABII proteins play a central role in UPEC biofilm structure. Mol. Microbiol. 2015, 96, 1119–1135. [Google Scholar] [CrossRef]
- Estellés, A.; Woischnig, A.-K.; Liu, K.; Stephenson, R.; Lomongsod, E.; Nguyen, D.; Zhang, J.; Heidecker, M.; Yang, Y.; Simon, R.J.; et al. A High-Affinity Native Human Antibody Disrupts Biofilm from Staphylococcus aureus Bacteria and Potentiates Antibiotic Efficacy in a Mouse Implant Infection Model. Antimicrob. Agents Chemother. 2016, 60, 2292–2301. [Google Scholar] [CrossRef]
- Novotny, L.A.; Jurcisek, J.A.; Ward, M.O.; Jordan, Z.B.; Goodman, S.D.; Bakaletz, L.O. Antibodies against the majority subunit of type IV Pili disperse nontypeable Haemophilus influenzae biofilms in a LuxS-dependent manner and confer therapeutic resolution of experimental otitis media. Mol. Microbiol. 2015, 96, 276–292. [Google Scholar] [CrossRef]
- Novotny, L.A.; Goodman, S.D.; Bakaletz, L.O. Redirecting the immune response towards immunoprotective domains of a DNABII protein resolves experimental otitis media. Npj Vaccines 2019, 4, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rocco, C.J.; Bakaletz, L.O.; Goodman, S.D. Targeting the HUβ Protein Prevents Porphyromonas gingivalis from Entering into Preexisting Biofilms. J. Bacteriol. 2018, 200. [Google Scholar] [CrossRef] [PubMed]
- Brady, R.A.; O’May, G.A.; Leid, J.G.; Prior, M.L.; Costerton, J.W.; Shirtliff, M.E. Resolution of Staphylococcus aureus Biofilm Infection Using Vaccination and Antibiotic Treatment. Infect. Immun. 2011, 79, 1797–1803. [Google Scholar] [CrossRef]
- Brockson, M.E.; Novotny, L.A.; Mokrzan, E.M.; Malhotra, S.; Jurcisek, J.A.; Akbar, R.; Devaraj, A.; Goodman, S.D.; Bakaletz, L.O. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol. Microbiol. 2014, 93, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Freire, M.; Devaraj, A.; Young, A.; Navarro, J.; Downey, J.; Chen, C.; Bakaletz, L.; Zadeh, H.; Goodman, S.D. A bacterial-biofilm-induced oral osteolytic infection can be successfully treated by immuno-targeting an extracellular nucleoid-associated protein. Mol. Oral Microbiol. 2017, 32, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Novotny, L.A.; Amer, A.O.; Brockson, M.E.; Goodman, S.D.; Bakaletz, L.O. Structural Stability of Burkholderia cenocepacia Biofilms Is Reliant on eDNA Structure and Presence of a Bacterial Nucleic Acid Binding Protein. PLoS ONE 2013, 8, e67629. [Google Scholar] [CrossRef]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2011, 10, 39–50. [Google Scholar] [CrossRef]
- Christensen, L.D.; Van Gennip, M.; Rybtke, M.T.; Wu, H.; Chiang, W.-C.; Alhede, M.; Høiby, N.; Nielsen, T.E.; Givskov, M.; Tolker-Nielsen, T. Clearance of Pseudomonas aeruginosa Foreign-Body Biofilm Infections through Reduction of the Cyclic Di-GMP Level in the Bacteria. Infect. Immun. 2013, 81, 2705–2713. [Google Scholar] [CrossRef]
- Pu, L.; Yang, S.; Xia, A.; Jin, F. Optogenetics Manipulation Enables Prevention of Biofilm Formation of Engineered Pseudomonas aeruginosa on Surfaces. ACS Synth. Biol. 2018, 7, 200–208. [Google Scholar] [CrossRef]
- Mangalea, M.R.; Plumley, B.A.; Borlee, B.R. Nitrate Sensing and Metabolism Inhibit Biofilm Formation in the Opportunistic Pathogen Burkholderia pseudomallei by Reducing the Intracellular Concentration of c-di-GMP. Front. Microbiol. 2017, 8, 1353. [Google Scholar] [CrossRef]
- Barraud, N.; Kelso, M.; Rice, S.; Kjelleberg, S. Nitric Oxide: A Key Mediator of Biofilm Dispersal with Applications in Infectious Diseases. Curr. Pharm. Des. 2014, 21, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Schleheck, D.; Klebensberger, J.; Webb, J.S.; Hassett, D.J.; Rice, S.A.; Kjelleberg, S. Nitric Oxide Signaling in Pseudomonas aeruginosa Biofilms Mediates Phosphodiesterase Activity, Decreased Cyclic Di-GMP Levels, and Enhanced Dispersal. J. Bacteriol. 2009, 191, 7333–7342. [Google Scholar] [CrossRef] [PubMed]
- Sauer, K.; Cullen, M.C.; Rickard, A.H.; Zeef, L.A.H.; Davies, D.G.; Gilbert, P. Characterization of Nutrient-Induced Dispersion in Pseudomonas aeruginosa PAO1 Biofilm. J. Bacteriol. 2004, 186, 7312–7326. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.-G.; O’Toole, G.A. c-di-GMP and its Effects on Biofilm Formation and Dispersion: A Pseudomonas Aeruginosa Review. Microbiol. Spectr. 2015, 3, 301–317. [Google Scholar] [CrossRef]
- Roy, A.B.; Petrova, O.E.; Sauer, K. The Phosphodiesterase DipA (PA5017) Is Essential for Pseudomonas aeruginosa Biofilm Dispersion. J. Bacteriol. 2012, 194, 2904–2915. [Google Scholar] [CrossRef]
- Barraud, N.; Kardak, B.G.; Yepuri, N.R.; Howlin, R.P.; Webb, J.S.; Faust, S.N.; Kjelleberg, S.; Rice, S.A.; Kelso, M.J. Cephalosporin-3′-diazeniumdiolates: Targeted NO-Donor Prodrugs for Dispersing Bacterial Biofilms. Angew. Chem. Int. Ed. 2012, 51, 9057–9060. [Google Scholar] [CrossRef]
- De La Fuente-Núñez, C.; Reffuveille, F.; Fairfull-Smith, K.E.; Hancock, R.E.W. Effect of Nitroxides on Swarming Motility and Biofilm Formation, Multicellular Behaviors in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2013, 57, 4877–4881. [Google Scholar] [CrossRef]
- Reffuveille, F.; De La Fuente-Nunez, C.; Fairfull-Smith, K.E.; Hancock, R.E.W. Potentiation of ciprofloxacin action against Gram-negative bacterial biofilms by a nitroxide. Pathog. Dis. 2015, 73. [Google Scholar] [CrossRef]
- Boles, B.R.; Thoendel, M.; Singh, P.K. Rhamnolipids mediate detachment of Pseudomonas aeruginosa from biofilms. Mol. Microbiol. 2005, 57, 1210–1223. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Nusca, T.D.; Hochbaum, A.I. Rhamnolipids Mediate an Interspecies Biofilm Dispersal Signaling Pathway. ACS Chem. Biol. 2016, 11, 3068–3076. [Google Scholar] [CrossRef]
- De Rienzo, M.D.; Martin, P.J. Effect of Mono and Di-rhamnolipids on Biofilms Pre-formed by Bacillus subtilis BBK006. Curr. Microbiol. 2016, 73, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Quinn, G.A.; Maloy, A.P.; Banat, M.M.; Banat, I.M. A Comparison of Effects of Broad-Spectrum Antibiotics and Biosurfactants on Established Bacterial Biofilms. Curr. Microbiol. 2013, 67, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Periasamy, S.; Joo, H.-S.; Duong, A.C.; Bach, T.-H.L.; Tan, V.Y.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. How Staphylococcus aureus biofilms develop their characteristic structure. Proc. Natl. Acad. Sci. USA 2012, 109, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus aureus Biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef]
- Böttcher, T.; Kolodkin-Gal, I.; Kolter, R.; Losick, R.; Clardy, J. Synthesis and Activity of Biomimetic Biofilm Disruptors. J. Am. Chem. Soc. 2013, 135, 2927–2930. [Google Scholar] [CrossRef]
- Burrell, M.; Hanfrey, C.C.; Murray, E.J.; Stanley-Wall, N.R.; Michael, A.J. Evolution and Multiplicity of Arginine Decarboxylases in Polyamine Biosynthesis and Essential Role in Bacillus subtilis Biofilm Formation. J. Biol. Chem. 2010, 285, 39224–39238. [Google Scholar] [CrossRef]
- Karatan, E.; Duncan, T.R.; Watnick, P.I. NspS, a Predicted Polyamine Sensor, Mediates Activation of Vibrio cholerae Biofilm Formation by Norspermidine. J. Bacteriol. 2005, 187, 7434–7443. [Google Scholar] [CrossRef]
- Li, B.; Maezato, Y.; Kim, S.H.; Kurihara, S.; Liang, J.; Michael, A.J. Polyamine-independent growth and biofilm formation, and functional spermidine/spermine N -acetyltransferases in Staphylococcus aureus and Enterococcus faecalis. Mol. Microbiol. 2018, 111, 159–175. [Google Scholar] [CrossRef]
- Hochbaum, A.I.; Kolodkin-Gal, I.; Foulston, L.; Kolter, R.; Aizenberg, J.; Losick, R. Inhibitory Effects of D-Amino Acids on Staphylococcus aureus Biofilm Development. J. Bacteriol. 2011, 193, 5616–5622. [Google Scholar] [CrossRef]
- Kolodkin-Gal, I.; Romero, D.; Cao, S.; Clardy, J.; Kolter, R.; Losick, R.; Kolodkin-Gal, I.; Kolodkin-Gal, I. D-Amino Acids Trigger Biofilm Disassembly. Science 2010, 328, 627–629. [Google Scholar] [CrossRef]
- Romero, D.; Vlamakis, H.; Losick, R.; Kolter, R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011, 80, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Harmata, A.J.; Ma, Y.; Sanchez, C.J.; Zienkiewicz, K.J.; Elefteriou, F.; Wenke, J.C.; Guelcher, S.A. d-amino Acid Inhibits Biofilm but not New Bone Formation in an Ovine Model. Clin. Orthop. Relat. Res. 2015, 473, 3951–3961. [Google Scholar] [CrossRef]
- Leiman, S.A.; May, J.M.; Lebar, M.D.; Kahne, D.; Kolter, R.; Losick, R. D-Amino Acids Indirectly Inhibit Biofilm Formation in Bacillus subtilis by Interfering with Protein Synthesis. J. Bacteriol. 2013, 195, 5391–5395. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Akers, K.S.; Romano, D.R.; Woodbury, R.L.; Hardy, S.K.; Murray, C.K.; Wenke, J.C. d-Amino Acids Enhance the Activity of Antimicrobials against Biofilms of Clinical Wound Isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2014, 58, 4353–4361. [Google Scholar] [CrossRef]
- Sanchez, C.J.; Prieto, E.M.; Krueger, C.A.; Zienkiewicz, K.J.; Romano, D.R.; Ward, C.L.; Akers, K.S.; Guelcher, S.A.; Wenke, J.C. Effects of local delivery of d-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials 2013, 34, 7533–7543. [Google Scholar] [CrossRef]
- Yu, C.; Wu, J.; Contreras, A.E.; Li, Q. Control of nanofiltration membrane biofouling by Pseudomonas aeruginosa using d-tyrosine. J. Membr. Sci. 2012, 423, 487–494. [Google Scholar] [CrossRef]
- Lord, D.M.; Baran, A.U.; Wood, T.K.; Peti, W.; Page, R. BdcA, a Protein Important for Escherichia coli Biofilm Dispersal, Is a Short-Chain Dehydrogenase/Reductase that Binds Specifically to NADPH. PLoS ONE 2014, 9, e105751. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Yang, Z.; Pu, M.; Peti, W.; Wood, T.K. Engineering a novel c-di-GMP-binding protein for biofilm dispersal. Environ. Microbiol. 2010, 13, 631–642. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, G.; Wood, T.K. Escherichia coli BdcA controls biofilm dispersal in Pseudomonas aeruginosa and Rhizobium meliloti. BMC Res. Notes 2011, 4, 447. [Google Scholar] [CrossRef]
- Singh, P.K.; Parsek, M.R.; Greenberg, E.P.; Welsh, M.J. A component of innate immunity prevents bacterial biofilm development. Nature 2002, 417, 552–555. [Google Scholar] [CrossRef]
- Banin, E.; Vasil, M.L.; Greenberg, E.P. From The Cover: Iron and Pseudomonas aeruginosa biofilm formation. Proc. Natl. Acad. Sci. USA 2005, 102, 11076–11081. [Google Scholar] [CrossRef] [PubMed]
- Kolodkin-Gal, I.; Elsholz, A.K.; Muth, C.; Girguis, P.; Kolter, R.; Losick, R. Respiration control of multicellularity in Bacillus subtilis by a complex of the cytochrome chain with a membrane-embedded histidine kinase. Genes Dev. 2013, 27, 887–899. [Google Scholar] [CrossRef] [PubMed]
- Ramos, I.; Dietrich, L.E.; Price-Whelan, A.; Newman, D.K. Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol. 2010, 161, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Moreau-Marquis, S.; O’Toole, G.A.; Stanton, B.A. Tobramycin and FDA-Approved Iron Chelators Eliminate Pseudomonas aeruginosa Biofilms on Cystic Fibrosis Cells. Am. J. Respir. Cell Mol. Biol. 2009, 41, 305–313. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Browngardt, C.; Xiaohui, X.; Klepac-Ceraj, V.; Paster, B.; Burne, R.A. The effect of arginine on oral biofilm communities. Mol. Oral Microbiol. 2013, 29, 45–54. [Google Scholar] [CrossRef]
- Jakubovics, N.; Robinson, J.C.; Samarian, D.S.; Kolderman, E.; Yassin, S.A.; Bettampadi, D.; Bashton, M.; Rickard, A.H. Critical roles of arginine in growth and biofilm development by Streptococcus gordonii. Mol. Microbiol. 2015, 97, 281–300. [Google Scholar] [CrossRef]
- He, J.; Hwang, G.; Liu, Y.; Gao, L.; Kilpatrick-Liverman, L.; Santarpia, P.; Zhou, X.; Koo, H. l-Arginine Modifies the Exopolysaccharide Matrix and Thwarts Streptococcus mutans Outgrowth within Mixed-Species Oral Biofilms. J. Bacteriol. 2016, 198, 2651–2661. [Google Scholar] [CrossRef]
- Kolderman, E.; Bettampadi, D.; Samarian, D.; Dowd, S.E.; Foxman, B.; Jakubovics, N.; Rickard, A.H. L-Arginine Destabilizes Oral Multi-Species Biofilm Communities Developed in Human Saliva. PLoS ONE 2015, 10, e0121835. [Google Scholar] [CrossRef]
- Zara, G.; Zeidan, M.B.; Fancello, F.; Sanna, M.L.; Mannazzu, I.; Budroni, M.; Zara, S. The administration of L-cysteine and L-arginine inhibits biofilm formation in wild-type biofilm-forming yeast by modulating FLO11 gene expression. Appl. Microbiol. Biotechnol. 2019, 103, 7675–7685. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Li, B.-S.; Liu, W.-W.; Cai, Q.; Wang, H.-Y.; Liu, Y.-Q.; Liu, Y.-J.; Meng, W.-Y. Effects of D-arginine on Porphyromonas gingivalis biofilm. J. Oral Sci. 2020, 62, 57–61. [Google Scholar] [CrossRef]
- Gnanadhas, D.P.; Elango, M.; Datey, A.; Chakravortty, D. Chronic lung infection by Pseudomonas aeruginosa biofilm is cured by L-Methionine in combination with antibiotic therapy. Sci. Rep. 2015, 5, 16043. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.A.; Alcaraz, E.S.; Franco, M.A.; De Rossi, B.N.P. Iron is a signal for Stenotrophomonas maltophilia biofilm formation, oxidative stress response, OMPs expression, and virulence. Front. Microbiol. 2015, 6, 926. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.-H.; Shu, J.-C.; Huang, H.-Y.; Cheng, Y.-C. Involvement of Iron in Biofilm Formation by Staphylococcus aureus. PLoS ONE 2012, 7, e34388. [Google Scholar] [CrossRef] [PubMed]
- Oglesby-Sherrouse, A.G.; Djapgne, L.; Nguyen, A.T.; Vasil, A.I.; Vasil, M.L. The complex interplay of iron, biofilm formation, and mucoidy affecting antimicrobial resistance of Pseudomonas aeruginosa. Pathog. Dis. 2014, 70, 307–320. [Google Scholar] [CrossRef]
- Moreau-Marquis, S.; Bomberger, J.M.; Anderson, G.G.; Swiatecka-Urban, A.; Ye, S.; O’Toole, G.A.; A Stanton, B. The ΔF508-CFTR mutation results in increased biofilm formation by Pseudomonas aeruginosa by increasing iron availability. Am. J. Physiol. Cell. Mol. Physiol. 2008, 295, L25–L37. [Google Scholar] [CrossRef]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts Pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef]
- Schlag, S.; Nerz, C.; Birkenstock, T.A.; Altenberend, F.; Götz, F. Inhibition of Staphylococcal Biofilm Formation by Nitrite. J. Bacteriol. 2007, 189, 7911–7919. [Google Scholar] [CrossRef]
- Van Alst, N.E.; Picardo, K.F.; Iglewski, B.H.; Haidaris, C.G. Nitrate Sensing and Metabolism Modulate Motility, Biofilm Formation, and Virulence in Pseudomonas aeruginosa. Infect. Immun. 2007, 75, 3780–3790. [Google Scholar] [CrossRef]
- Dean, S.N.; Chung, M.-C.; Van Hoek, M.L. Burkholderia Diffusible Signal Factor Signals to Francisella novicida To Disperse Biofilm and Increase Siderophore Production. Appl. Environ. Microbiol. 2015, 81, 7057–7066. [Google Scholar] [CrossRef]
- Dow, J.; Crossman, L.; Findlay, K.; He, Y.-Q.; Feng, J.-X.; Tang, J.-L. Biofilm dispersal in Xanthomonas campestris is controlled by cell-cell signaling and is required for full virulence to plants. Proc. Natl. Acad. Sci. USA 2003, 100, 10995–11000. [Google Scholar] [CrossRef]
- Davies, D.G.; Marques, C.N.H. A Fatty Acid Messenger Is Responsible for Inducing Dispersion in Microbial Biofilms. J. Bacteriol. 2008, 191, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Badi, A.; Sepehr, S.; Babaie-Naiej, H. A combination of cis-2-decenoic acid and chlorhexidine removes dental plaque. Arch. Oral Biol. 2015, 60, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Rahmani-Badi, A.; Sepehr, S.; Mohammadi, P.; Soudi, M.R.; Babaie-Naiej, H.; Fallahi, H. A combination of cis-2-decenoic acid and antibiotics eradicates pre-established catheter-associated biofilms. J. Med. Microbiol. 2014, 63, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Sepehr, S.; Rahmani-Badi, A.; Babaie-Naiej, H.; Soudi, M.R. Unsaturated Fatty Acid, cis-2-Decenoic Acid, in Combination with Disinfectants or Antibiotics Removes Pre-Established Biofilms Formed by Food-Related Bacteria. PLoS ONE 2014, 9, e101677. [Google Scholar] [CrossRef] [PubMed]
- Brindle, E.R.; Miller, D.; Stewart, P.S. Hydrodynamic deformation and removal of Staphylococcus epidermidis biofilms treated with urea, chlorhexidine, iron chloride, or DispersinB. Biotechnol. Bioeng. 2011, 108, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Stewart, P.S. Biofilm removal caused by chemical treatments. Water Res. 2000, 34, 4229–4233. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Han, G.; Frases, S.; Cordero, R.J.; Casadevall, A.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. The use of chitosan to damage Cryptococcus neoformans biofilms. Biomaterials 2010, 31, 669–679. [Google Scholar] [CrossRef]
- Martinez, L.R.; Mihu, M.R.; Tar, M.; Cordero, R.J.B.; Han, G.; Friedman, A.J.; Friedman, J.M.; Nosanchuk, J.D. Demonstration of Antibiofilm and Antifungal Efficacy of Chitosan against Candidal Biofilms, Using an in vivo Central Venous Catheter Model. J. Infect. Dis. 2010, 201, 1436–1440. [Google Scholar] [CrossRef]
- Mu, H.; Guo, F.; Niu, H.; Liu, Q.; Wang, S.; Duan, J. Chitosan Improves Anti-Biofilm Efficacy of Gentamicin through Facilitating Antibiotic Penetration. Int. J. Mol. Sci. 2014, 15, 22296–22308. [Google Scholar] [CrossRef]
- Orgaz, B.; Lobete, M.M.; Puga, C.H.; Jose, C.S. Effectiveness of Chitosan against Mature Biofilms Formed by Food Related Bacteria. Int. J. Mol. Sci. 2011, 12, 817–828. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, H.; Zhang, W.; Cui, G.; Zhu, J.; Duan, J. Chitosan Coupling Makes Microbial Biofilms Susceptible to Antibiotics. Sci. Rep. 2013, 3, 3364. [Google Scholar] [CrossRef] [PubMed]
- Banin, E.; Brady, K.M.; Greenberg, E.P. Chelator-Induced Dispersal and Killing of Pseudomonas aeruginosa Cells in a Biofilm. Appl. Environ. Microbiol. 2006, 72, 2064–2069. [Google Scholar] [CrossRef] [PubMed]
- De Almeida, J.; Hoogenkamp, M.; Felippe, W.T.; Crielaard, W.; Van Der Waal, S.V. Effectiveness of EDTA and Modified Salt Solution to Detach and Kill Cells from Enterococcus faecalis Biofilm. J. Endod. 2016, 42, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Jothiprakasam, V.; Sambantham, M.; Chinnathambi, S.; Vijayaboopathi, S. Candida tropicalis biofilm inhibition by ZnO nanoparticles and EDTA. Arch. Oral Biol. 2017, 73, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, E.; Vighetto, C.; Di Martino, P.; Véronique, L.G.; Seyer, D. Synergistic antibiofilm efficacy of various commercial antiseptics, enzymes and EDTA: A study of Pseudomonas aeruginosa and Staphylococcus aureus biofilms. Int. J. Antimicrob. Agents 2016, 48, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lin, Y.; Lu, Q.; Li, F.; Yu, J.-L.; Wang, Z.; He, Y.; Song, C. in vitro and in vivo activity of EDTA and antibacterial agents against the biofilm of mucoid Pseudomonas aeruginosa. Infection. 2016, 45, 23–31. [Google Scholar] [CrossRef]
- Maisetta, G.; Grassi, L.; Di Luca, M.; Bombardelli, S.; Medici, C.; Brancatisano, F.L.; Esin, S.; Batoni, G. Anti-biofilm properties of the antimicrobial peptide temporin 1Tb and its ability, in combination with EDTA, to eradicate Staphylococcus epidermidis biofilms on silicone catheters. Biofouling 2016, 32, 787–800. [Google Scholar] [CrossRef]
- Alves, F.R.F.; Silva, M.G.; Rôças, I.N.; Siqueira, J.F., Jr. Biofilm biomass disruption by natural substances with potential for endodontic use. Braz. Oral Res. 2013, 27, 20–25. [Google Scholar] [CrossRef]
- Ammons, M.C.B.; Copie, V. Mini-review: Lactoferrin: A bioinspired, anti-biofilm therapeutic. Biofouling 2013, 29, 443–455. [Google Scholar] [CrossRef]
- Srivastava, D.; Waters, C.M. A Tangled Web: Regulatory Connections between Quorum Sensing and Cyclic Di-GMP. J. Bacteriol. 2012, 194, 4485–4493. [Google Scholar] [CrossRef]
- Sauer, K.; Camper, A.K.; Ehrlich, G.D.; Costerton, J.W.; Davies, D.G. Pseudomonas aeruginosa Displays Multiple Phenotypes during Development as a Biofilm. J. Bacteriol. 2002, 184, 1140–1154. [Google Scholar] [CrossRef] [PubMed]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.K.; Huang, J.Y.; Wreden, C.; Sweeney, E.G.; Goers, J.; Remington, S.J.; Guillemin, K. Chemorepulsion from the Quorum Signal Autoinducer-2 Promotes Helicobacter pylori Biofilm Dispersal. mBio 2015, 6, e00379-15. [Google Scholar] [CrossRef] [PubMed]
- Mukherji, R.; Prabhune, A.A. Novel Glycolipids Synthesized Using Plant Essential Oils and Their Application in Quorum Sensing Inhibition and as Antibiofilm Agents. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.-H.; Xu, J.-L.; Li, X.-Z.; Zhang, L.-H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum-sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA 2000, 97, 3526–3531. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, P.; Rai, V.R. Inhibition of QS-regulated virulence factors in Pseudomonas aeruginosa PAO1 and Pectobacterium carotovorum by AHL-lactonase of endophytic bacterium Bacillus cereus VT96. Biocatal. Agric. Biotechnol. 2016, 7, 154–163. [Google Scholar] [CrossRef]
- Saipriya, K.; Swathi, C.; Ratnakar, K.; Sritharan, V.; Kamaraju, S.; Ch, S. Quorum-sensing system in Acinetobacter baumannii: A potential target for new drug development. J. Appl. Microbiol. 2019, 128, 15–27. [Google Scholar] [CrossRef]
- Brackman, G.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Quorum Sensing Inhibitors Increase the Susceptibility of Bacterial Biofilms to Antibiotics in vitro and in vivo. Antimicrob. Agents Chemother. 2011, 55, 2655–2661. [Google Scholar] [CrossRef]
- Lauderdale, K.J.; Malone, C.L.; Boles, B.R.; Morcuende, J.; Horswill, A.R. Biofilm dispersal of community-associated methicillin-resistant Staphylococcus aureus on orthopedic implant material. J. Orthop. Res. 2009, 28, 55–61. [Google Scholar] [CrossRef]
- Anguita-Alonso, P.; Giacometti, A.; Cirioni, O.; Ghiselli, R.; Orlando, F.; Saba, V.; Scalise, G.; Sevo, M.; Tuzova, M.; Patel, R.; et al. RNAIII-Inhibiting-Peptide-Loaded Polymethylmethacrylate Prevents In Vivo Staphylococcus aureus Biofilm Formation. Antimicrob. Agents Chemother. 2006, 51, 2594–2596. [Google Scholar] [CrossRef]
- Balaban, N.; Cirioni, O.; Gov, Y.; Ghiselli, R.; Saba, V.; Scalise, G.; Dell’Acqua, G.; Giacometti, A.; Mocchegiani, F.; Viticchi, C.; et al. Use of the quorum-sensing inhibitor RNAIII-inhibiting peptide to prevent biofilm formation in vivo by drug-resistant Staphylococcus epidermidis. J. Infect. Dis. 2003, 187, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Balaban, N.; Goldkorn, T.; Nhan, R.T.; Dang, L.B.; Scott, S.; Ridgley, R.M.; Rasooly, A.; Wright, S.C.; Larrick, J.W.; Rasooly, R.; et al. Autoinducer of Virulence As a Target for Vaccine and Therapy Against Staphylococcus aureus. Science 1998, 280, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Dell’Acqua, G.; Gov, Y.; Kamysz, W.; Lukasiak, J.; Mocchegiani, F.; Orlando, F.; D’Amato, G.; et al. Prophylactic Efficacy of Topical Temporin A and RNAIII-Inhibiting Peptide in a Subcutaneous Rat Pouch Model of Graft Infection Attributable to Staphylococci With Intermediate Resistance to Glycopeptides. Circulation 2003, 108, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Giacometti, A.; Ghiselli, R.; Acqua, G.D.; Orlando, F.; Mocchegiani, F.; Silvestri, C.; Licci, A.; Saba, V.; Scalise, G.; et al. RNAIII-Inhibiting Peptide Significantly Reduces Bacterial Load and Enhances the Effect of Antibiotics in the Treatment of Central Venous Catheter–Associated Staphylococcus aureus Infections. J. Infect. Dis. 2006, 193, 180–186. [Google Scholar] [CrossRef]
- Simonetti, O.; Cirioni, O.; Ghiselli, R.; Goteri, G.; Scalise, A.; Orlando, F.; Silvestri, C.; Riva, A.; Saba, V.; Madanahally, K.D.; et al. RNAIII-Inhibiting Peptide Enhances Healing of Wounds Infected with Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2008, 52, 2205–2211. [Google Scholar] [CrossRef]
- Starkey, M.; Lépine, F.; Maura, D.; Bandyopadhaya, A.; Lesic, B.; He, J.; Kitao, T.; Righi, V.; Milot, S.; Tzika, A.; et al. Identification of Anti-virulence Compounds That Disrupt Quorum-Sensing Regulated Acute and Persistent Pathogenicity. PLoS Pathog. 2014, 10, e1004321. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef]
- Başaran, T.I.; Berber, D.; Gökalsın, B.; Tramice, A.; Tommonaro, G.; Abbamondi, G.R.; Hasköylü, M.E.; Öner, E.T.; Iodice, C.; Sesal, C. Extremophilic Natrinema versiforme Against Pseudomonas aeruginosa Quorum Sensing and Biofilm. Front. Microbiol. 2020, 11, 79. [Google Scholar] [CrossRef]
- Peppoloni, S.; Pericolini, E.; Colombari, B.; Pinetti, D.; Cermelli, C.; Fini, F.; Prati, F.; Caselli, E.; Blasi, E. The β-Lactamase Inhibitor Boronic Acid Derivative SM23 as a New Anti-Pseudomonas aeruginosa Biofilm. Front. Microbiol. 2020, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Overhage, J.; Campisano, A.; Bains, M.; Torfs, E.C.; Rehm, B.H.; Hancock, R.E. Human host defense peptide LL-37 prevents bacterial biofilm formation. Infect. Immun. 2008, 76, 4176–4182. [Google Scholar] [CrossRef] [PubMed]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta (BBA) Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Pletzer, D.; Coleman, S.R.; Hancock, R.E.W. Anti-biofilm peptides as a new weapon in antimicrobial warfare. Curr. Opin. Microbiol. 2016, 33, 35–40. [Google Scholar] [CrossRef]
- De La Fuente-Núñez, C.; Reffuveille, F.; Mansour, S.C.; Reckseidler-Zenteno, S.L.; Hernández, D.; Brackman, G.; Coenye, T.; Hancock, R.E.W. D-enantiomeric peptides that eradicate wild-type and multidrug-resistant biofilms and protect against lethal Pseudomonas aeruginosa infections. Chem. Biol. 2015, 22, 196–205. [Google Scholar] [CrossRef]
- Jones, E.A.; McGillivary, G.; Bakaletz, L.O. Extracellular DNA within a nontypeable Haemophilus influenzae induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J. Innate Immun. 2012, 5, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kamesh, A.C.; Xiao, Y.; Sun, V.; Hayes, M.; Daniell, H.; Koo, H. Topical delivery of low-cost protein drug candidates made in chloroplasts for biofilm disruption and uptake by oral epithelial cells. Biomaterials 2016, 105, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, R.; Tafin, U.F.; Corvec, S.; Oliva, A.; Betrisey, B.; Borens, O.; Trampuz, A. High Activity of Fosfomycin and Rifampin against Methicillin-Resistant Staphylococcus aureus Biofilm in vitro and in an Experimental Foreign-Body Infection Model. Antimicrob. Agents Chemother. 2014, 58, 2547–2553. [Google Scholar] [CrossRef]
- Chowdhury, N.; Wood, T.L.; Martínez-Vázquez, M.; García-Contreras, R.; Wood, T.K. DNA-crosslinker cisplatin eradicates bacterial persister cells. Biotechnol. Bioeng. 2016, 113, 1984–1992. [Google Scholar] [CrossRef]
- Kwan, B.W.; Chowdhury, N.; Wood, T.K. Combatting bacterial infections by killing persister cells with mitomycin C. Environ. Microbiol. 2015, 17, 4406–4414. [Google Scholar] [CrossRef]
- De La Fuente-Núñez, C.; Reffuveille, F.; Haney, E.F.; Straus, S.; Hancock, R.E.W. Broad-Spectrum Anti-biofilm Peptide That Targets a Cellular Stress Response. PLoS Pathog. 2014, 10, e1004152. [Google Scholar] [CrossRef]
- Wang, Z.; De La Fuente-Núñez, C.; Shen, Y.; Haapasalo, M.; Hancock, R.E.W. Treatment of Oral Multispecies Biofilms by an Anti-Biofilm Peptide. PLoS ONE 2015, 10, e0132512. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.; Golla, R.M.; Lau, K.; Lushnikova, T.; Wang, G. Anti-Staphylococcal Biofilm Effects of Human Cathelicidin Peptides. ACS Med. Chem. Lett. 2015, 7, 117–121. [Google Scholar] [CrossRef]
- Haisma, E.M.; De Breij, A.; Chan, H.; Van Dissel, J.T.; Drijfhout, J.W.; Hiemstra, P.S.; El Ghalbzouri, A.; Nibbering, P.H. LL-37-Derived Peptides Eradicate Multidrug-Resistant Staphylococcus aureus from Thermally Wounded Human Skin Equivalents. Antimicrob. Agents Chemother. 2014, 58, 4411–4419. [Google Scholar] [CrossRef] [PubMed]
- Haisma, E.M.; Göblyös, A.; Ravensbergen, B.; Adriaans, A.E.; Cordfunke, R.A.; Schrumpf, J.; Limpens, R.W.A.L.; Schimmel, K.J.M.; Hartigh, J.D.; Hiemstra, P.S.; et al. Antimicrobial Peptide P60.4Ac-Containing Creams and Gel for Eradication of Methicillin-Resistant Staphylococcus aureus from Cultured Skin and Airway Epithelial Surfaces. Antimicrob. Agents Chemother. 2016, 60, 4063–4072. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.V.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, M.; Tomasinsig, L.; Arzese, A.; D’Este, F.; Oro, D.; Skerlavaj, B. Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections. Peptides 2015, 71, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, S.L.S.; De La Fuente-Núñez, C.; Baquir, B.; Faria-Junior, C.; Franco, O.L.; Hancock, R.E.W. Antibiofilm Peptides Increase the Susceptibility of Carbapenemase-Producing Klebsiella pneumoniae Clinical Isolates to β-Lactam Antibiotics. Antimicrob. Agents Chemother. 2015, 59, 3906–3912. [Google Scholar] [CrossRef] [PubMed]
- Ahire, J.J.; Kashikar, M.S.; Lakshmi, S.G.; Madempudi, R. Identification and characterization of antimicrobial peptide produced by indigenously isolated Bacillus paralicheniformis UBBLi30 strain. 3 Biotech 2020, 10, 112–113. [Google Scholar] [CrossRef]
- Kokilakanit, P.; Koontongkaew, S.; Roytrakul, S.; Utispan, K. A novel non-cytotoxic synthetic peptide, Pug-1, exhibited an antibiofilm effect on Streptococcus mutans adhesion. Lett. Appl. Microbiol. 2020, 70, 151–158. [Google Scholar] [CrossRef]
- Von Borowski, R.G.; Barros, M.P.; Da Silva, D.B.; Lopes, N.P.; Zimmer, K.R.; Staats, C.C.; De Oliveira, C.B.; Giudice, E.; Gillet, R.; Macedo, A.J.; et al. Red pepper peptide coatings control Staphylococcus epidermidis adhesion and biofilm formation. Int. J. Pharm. 2020, 574, 118872. [Google Scholar] [CrossRef]
- Xie, Z.; Wei, H.; Meng, J.; Cheng, T.; Song, Y.; Wang, M.; Zhang, Y. The Analogs of Temporin-GHa Exhibit a Broader Spectrum of Antimicrobial Activity and a Stronger Antibiofilm Potential against Staphylococcus aureus. Molecules 2019, 24, 4173. [Google Scholar] [CrossRef]
- Martínez, M.; Polizzotto, A.; Flores, N.; Semorile, L.; Maffia, P. Antibacterial, anti-biofilm and in vivo activities of the antimicrobial peptides P5 and P6.2. Microb. Pathog. 2020, 139, 103886. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Xie, Z.; Zhang, S.; Wei, H.; Song, Y.; Zhang, Y.; Wang, M. Brevinin-GR23 from frog Hylarana guentheri with antimicrobial and antibiofilm activities against Staphylococcus aureus. Biosci. Biotechnol. Biochem. 2019, 84, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Chua, S.L.; Liu, Y.; Yam, J.K.H.; Chen, Y.; Vejborg, R.M.; Tan, B.G.C.; Kjelleberg, S.; Tolker-Nielsen, T.; Givskov, M.; Yang, L. Dispersed cells represent a distinct stage in the transition from bacterial biofilm to planktonic lifestyles. Nat. Commun. 2014, 5, 4462. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Tan, K.S. The Danger Signal Extracellular ATP Is an Inducer of Fusobacterium nucleatum Biofilm Dispersal. Front. Microbiol. 2016, 6, 155. [Google Scholar] [CrossRef]
| Biomolecules That Target EPS Synthesis and Secretion | ||
|---|---|---|
| Name | Summary | References |
| 2-(4-methoxyphenyl)-N-(3-{[2-(4-methoxyphenyl)ethyl]imino}-1,4-dihydro-2-quinoxalinylidene)ethanamine | A kind of quinoxaline derivative which inhibits extracellular polymeric substance (EPS) synthesis and biofilm formation in Streptococcus mutans by selectively antagonizing Gtfs instead of killing the bacteria directly. | [19] |
| tt-farnesol | Targets the expression of key genes during biofilm formation. Those key genes are associated with exopolysaccharide matrix synthesis (gtfB) and exogenous stress modulation (e.g., sloA) that are essential for cariogenic biofilm assembly. It has been proved to be effective against S. mutans in vitro and in vivo. | [20] |
| myricetin | Targeting the expression of key genes during biofilm formation in vitro and in vivo. Key genes are associated with exopolysaccharide matrix synthesis (gtfB) and exogenous stress modulation (e.g., sloA) that are essential for cariogenic biofilm assembly. It has been proved to be effective against S. mutans and Escherichia coli. | [20,31] |
| Ring-fused 2-pyridones | A member of curlicides, such as FN075 and BibC6, sharing a common chemical lineage with other ring-fused 2-pyridones termed pilicides. Retain pilicide activities and inhibit both curli-dependent and type 1-dependent biofilms. | [29] |
| Temporin-GHc, Temporin-GHd | Impede the initial adhesion of biofilm and downregulate the expression of glucosyltransferases biosynthesis genes, having been proved to be effective against S. mutans. | [32] |
| Biomolecules that Target Adhesins | ||
| Mannosides | A small molecule inhibitor of type 1 fimbriae adhesin FimH that effectively inhibit the invasion of E. coli. | [23,25] |
| ZFH-04269 | 4′-[α-d-Mannopyranosyloxy]-N,3′-dimethylbiphenyl-3-carboxamide, a small molecular weight compound which inhibits the type 1 fimbriae adhesin FimH and significantly reduces E. coli colonization. | [24] |
| C-mannosides | Replacing O-mannosides with C-mannosides to improve the half-life and bioavailability of mannosides, which was due to the metabolic instability of O-glycoside linkage. C-mannosides have been proved to effectively reduce the E. coli burden. Alkyl-substituted mannose residues also have 100-fold higher affinities to the E. coli adhesin FimH than mannose. | [26,27] |
| Arylmannoside | Arylmannoside displays low nanomolar binding affinity to FimH, which is likely due to its hydrophobic interactions with the isoleucine and two tyrosine resodies within the binding pocket. | [28] |
| FUD | An inhibitory protein that targets Candida-fibronectin interactions by blocking the surface adhesion of Candida to halt biofilm formation. | [33] |
| NDV-3A | A vaccine based on the N-terminus of Als3 protein formulated with alum and has been proved to be effective against biofilm formed by Candida auris. | [34] |
| Name | Summary | References |
|---|---|---|
| Esp | A kind of serine protease secreted by a subset of Staphylococcus epidermidis. Purified S. epidermidis serine protease (Esp) can inhibit biofilm formation and destroy pre-existing Staphylococcus aureus biofilms. | [42] |
| Cysteine Proteases | Cysteine proteases secreted by equine mesenchymal stromal cells (MSCs) has been shown to destabilize methicillin-resistant Staphylococcus aureus (MRSA) biofilms, thereby increasing the efficacy of antibiotics that were previously tolerated by the biofilms (penicillin/streptomycin), and the equine MSCs secretome can inhibits biofilm formation of various bacteria, such as Pseudomonas aeruginosa, S. aureus, and S. epidermidis. | [44] |
| Aureolysin (Aur) | A staphylococcal metalloprotease that has been shown to disrupt S. aureus biofilms by degrading Bap and clumping factor b. | [45,46] |
| LapG | A protease produced by Pseudomonas putida and has been shown to trigger biofilm dispersal event through modification of the outer membrane-associated and exopolysaccharide-binding protein LapA. | [47] |
| Proteinase K | A highly reactive and stable serine protease with a broad range of cleavage activity that targets peptide bonds which are adjacent to the carboxylic group of aliphatic and aromatic amino acids. It is active against the biofilms produced by a wide range of bacteria strains, including S. aureus, Listeria monocytogenes, Staphylococcus lugdunensis, Staphylococcus heamolyticus, Gardnerella vaginalis, and E. coli, Heamophilus influenza, and Bdellovibrio bacteriovorus. | [48,49,50,51,52,53,54,55] |
| Spl | A group of six Staphylococcal serine proteases that are involved in S. aureus biofilm dispersal, possibly through the cleavage of a cell wall-associated protein EbpS. | [56,57] |
| ScpA, SspB | Belongs to Staphylococcal cysteine proteases and have been shown to disperse S. aureus biofilms through degrading unknown target(s). | [45,58] |
| SpeB | A Streptococcus pyogenes cysteine protease which has recently been shown to be involved in in vivo dispersal of S. pyogenes biofilms through the hydrolysis of surface proteins M and F1, which are hypothesized to be involved in microcolony formation. | [59,60,61] |
| SPRE | An endogenous Streptococcal protease which results in S. mutans monolayer biofilm detachment from colonized surface through releasing the surface protein antigen P1. | [62] |
| Trypsin | A member of pancreatic serine protease that cleaves peptides at the carboxyl side of lysine or arginine, and actives against biofilms produced by multiple bacterial species, including P. aeruginosa, Streptococcus mitis, Actinomyces radicidentis, S. epidermidis, and G. vaginalis. | [48,55,63,64] |
| SspA | A staphylococcal serine protease that degrades fibronectin binding proteins and Bap in S. aureus biofilms. | [46,65] |
| Name | Summary | References |
|---|---|---|
| DNase I | It has been demonstrated that pancreatic deoxyribonuclease (DNase) can deconstruct the established biofilms of a wide range of microbes, including P. aeruginosa, Vibrio cholerae, E. coli, S. pyogenes, Klebsiella pneumoniae, Acinetobacter baumannii, Aggregatibacter actinomycetemcomitans, Shewanella oneidensis, S. heamolyticus, Bordetella pertussis, Bordetella bronchiseptica, Campylobacter jejuni, H. influenza, B. bacteriovorus, S. aureus, Enterococcus faecalis, L. monocytogenes, Candida albicans, and Aspergillus | [74] |
| λ Exonuclease | A kind of viral DNase that can disrupt established V. cholerae biofilms. | [80] |
| DNase1L2 | A human DNase found in keratinocytes that has been demonstrated to degrade the established biofilms of P. aeruginosa and S. aureus. | [81] |
| Dornase alpha | A highly purified form of recombinant human DNase I (rhDNase I), which has been demonstrated to be effective against the established biofilms of S. aureus, G. vaginalis and Streptococcus pneumoniae. | [72,78,82,83] |
| NucB | A bacterial DNase produced by the marine bacterium, Bacillus licheniformis, which has been shown to be able to degrade the established biofilms of multiple bacterial species, including B. licheniformis, S. aureus, S. epidermidis, Staphylococcus salivarius, Staphylococcus constellatus, S. Staphylococcus lugdunesis, Staphylococcus anginosus, E. coli, Streptococcus intermedius, Micrococcus luteus, and Bacillus subtilis. | [84,85,86] |
| Streptodornase | A streptococcal DNase that can disrupt the established biofilms of P. aeruginosa. | [87] |
| Name | Summary | References |
|---|---|---|
| Cellulase | A glycoside hydrolase produced by multiple microbes that hydrolyzes the β(1,4) glycosidic linkage, and has been demonstrated to induce the dispersal of biofilms formed by S. aureus and P. aeruginosa. | [94] |
| α- mannosidase | An acid hydrolase that is thought to be involved in the turnover of N-linked glycoproteins and has been demonstrated to disrupt P. aeruginosa biofilms. However, it has cytotoxic effect on A-431 human epidermoid carcinoma cell lines. | [63,98] |
| β- mannosidase | Hydrolyzes the terminal mannose residues, which are β(1,4) linked to oligosaccharides or glycopeptides, can disrupt P. aeruginosa biofilms. However, it has cytotoxic effect on A-431 human epidermoid carcinoma cell lines. | [63,99] |
| Alginate lyase | A glycoside hydrolase that degrades the exopolysaccharide alginate, which is common in mucoid P. aeruginosa biofilms, causing bacterial cell dispersal and increasing antibiotics’ efficacy and phagocytosis. | [100,101,102,103] |
| α-amylase | A glycoside hydrolase derived from multiple sources that hydrolyzes α(1,4) glycosidic linkages, mediating the dispersal of mature biofilms of multiple bacterial strains, including V. cholerae, S. aureus and P. aeruginosa. | [94,104,105,106] |
| Dispersin B | A glycoside hydrolase produced by A. actinomycetemcomitans, and has been shown to degrade the polysaccharide poly(1,6)-N-acetyl-d-glucosamine (PNAG) through hydrolyzing β(1,6) glycosidic linkages. This enzyme can effectively act against the biofilms formed by multiple bacteria, including S. aureus, A. actinomycetemcomitans, S. epidermidis, A. baumannii, K. pneumoniae, E. coli, Burkholderia spp., Actinobacillus Pleuropeumoniae, Yersinia pestis and Pseudomonas fluorescens. | [107,108,109,110,111,112,113,114] |
| Hyaluronidase | An enzyme that cleaves hyaluronic acid (HA), a component which has been found to be incorporated into the biofilms formed by multiple pathogens, including S. aureus, and S. intermedius. When utilized against HA-containing biofilms, biofilms dispersal has been observed. | [115,116] |
| PelAh, PslGh | Glycoside hydrolases that can disperse mature biofilms formed by P. aeruginosa through hydrolyzing the Pel or Psl polysaccharide, respectively. | [117] |
| PgaB | Disrupts PNAG-dependent biofilms formed by B. pertussis, Staphylococcus carnosus, S. epidermidis, and E. coli, through hydrolyzing PNAG, a major biofilm component of many pathogenic bacteria. | [118] |
| Ega3 | An endo-acting α-1,4-galactosaminidase that has been demonstrated to disrupt biofilms formed by GAG-dependent Aspergillus fumigatus and Pel polysaccharide-dependent P. aeruginosa. | [119] |
| Sph3 | A retaining endo-α-1,4-N-acetylgalactosaminidase which can hydrolyze galactosaminogalactan (GAG), a cationic polymer of α-1,4-linked galactose and partially deacetylated N-acetylgalactosamine (GalNAc) and has been demonstrated to disrupt biofilms formed by A. fumigatus. | [120] |
| Targeting Specific Components in EPS | ||
|---|---|---|
| Name | Summary | References |
| Cam-003 | A monoclonal antibody that can bind three distinct epitopes on Psl, and have been demonstrated to block the attachment of P. aeruginosa to cultured epithelial cells, to inhibit the adherence or formation of denser biofilms. | [122] |
| EbpAFull, EbpANTD | Vaccine-elicited antibodies based on EbpA which mediates serum antibody response, blocks the interaction between EbpA and host, and inhibits the formation of biofilm. | [123] |
| Quadrivalent vaccine | It is a vaccine that targets four biofilm upregulated immunogens: SA0037, SA0486, SA0688, and glucosaminidase. The combination of quadrivalent vaccine with vancomycin can significantly reduce S. aureus numbers. | [134] |
| Targeting Nucleic-Acid-Binding Proteins | ||
| Antisera | Antisera, which is derived against DNABII proteins, has been demonstrated to be effective against biofilms formed by oral bacteria, E. coli and P. aeruginosa. | [127,128,129] |
| TRL1068 | A native human monoclonal antibody which has low-picomolar affinity to DNABII homologs from important Gram-positive and Gram-negative bacterial pathogens, and it has been demonstrated to be effective in disrupting biofilms of MRSA. | [130] |
| Anti-IHFEc | Hyperimmune antiserum is derived against purified E. coli integration host factor (IHF), and has been demonstrated to be effective on biofilms formed by nontypeable Haemophilus influenzae (NTHi) and Burkholderia cenocepacia. | [126,135,136,137] |
| Dispersal Signals | ||
|---|---|---|
| Name | Summary | References |
| YhjH | E. coli phosphodiesterase that can be induced in vivo, led to the reduction of c-di-GMP and dispersal of biofilms on silicone implants in a mouse foreign body infection model. | [139] |
| PA2133 | A functional protein gene containing an EAL domain to degrade c-di-GMP, and can inhibit biofilms formation of P. aeruginosa, resulting in much sparser and thinner biofilms. | [140] |
| Nitrate | Nitrate shows the effect of reducing intracellular levels of the second messenger c-di-GMP and inhibiting biofilm formation of P. aeruginosa, S. aureus and Burkholderia pseudomallei. | [141,188,189] |
| NO | An endogenously produced dispersal signal which can be generated and recognized by both prokaryotes and eukaryotes and are highly conserved. It has been shown to be involved in the dispersal of biofilms formed by P. aeruginosa, E. coli, Fusobacterium nucleatum, Serratia marcescens, V. cholerae, B. licheniformis, Shewanella woodyi, Neisseria gonorrhoeae, Pseudoalteromonas, Vibrio fischeri, S. aureus, B. subtilis, Legionella pneumophila, Nitrosomonas europaea, P. putida, C. albicans, Candida tropicalis, and Ulva linza. | [142] |
| Glutamate | The second molecule known to induce the release of cells from P. aeruginosa biofilms, and does so in nutrient-induced dispersion process, and may share the same mechanism with NO-induced biofilm dispersions. | [144,145,146] |
| C3Ds | A NO-donor prodrug that selectively release NO from the prodrug through contacting with biofilm β-lactamases, and allows targeted enhancement of bacterial killing by conventional antimicrobials at sites of biofilm infections, while also minimizing NO- mediated toxicity. It has been proved to effectively disperse P. aeruginosa biofilms in vitro. | [147] |
| Nitroxides | Sterically hindered NO analogues, which exert biological responses via NO-mimetic properties, and has been proved to induce biofilm dispersal in P. aeruginosa and E. coli, including carboxy-TEMPO, CTMIO and DCTEIO. | [148,149] |
| Cis-2-decenoic acid (CDA) | A kind of fatty acid cross-kingdom signaling molecule, also known as a diffusible signal factor (DSF), which was originally found to be produced by P. aeruginosa. This particular DSF has been shown to trigger the dispersal of biofilms formed by P. aeruginosa, E. coli, K. pneumoniae, Proteus. mirabilis, S. pyogenes, B. subtilis, S. aureus, C. albicans, Salmonella enterica, and S. mutans. It should be noted that other DSFs, such as Burkholderia diffusible signal factor (BDSF) [190] and Xanthomonas diffusible signal factor (XDSF), have been isolated and exhibited similar inductions of dispersal events [191]. | [192,193,194,195] |
| Anti-Matrix Molecules | ||
| Rhamnolipids | A microbial-produced surfactant that, at normal levels, is important for the maintenance of mature biofilms, particularly for fluid channel maintenance and cellular migration. At elevated levels, however, these rhamnolipids have been shown to trigger the dispersal of P. aeruginosa, E. coli, S. aureus, B. subtilis, M. luteus, and Yarrowia lipolytica biofilms. | [150,151,152,153] |
| PSM | Surfactant-like peptides that promote biofilm disassembly in their monomeric form, by reducing the surface tension, but form amyloid-like fibers when they undergo orderly aggregations. | [154,155] |
| Polyamines | Polyamines such as spermidine and norspermidine have been proved to effectively inhibit the biofilm formation of B. subtilis and S. aureus. However, in some cases both spermidine and norspermidine serve as signaling molecules that induce biofilm formation. | [156,157,158,159] |
| D-amino acids | D-isoforms of certain amino acids, including D-Leu, D-Met, D-Trp, D-Tyr, and D-Phe, have been shown to cause the disassembly of biofilms through multiple hypothesized mechanisms, including (1) inhibition of genes involved in EPS production; (2) incorporation of D-amino acids into the bacterial cell wall, resulting in the loss of cell-surface fibers which are vital to biofilm formation. D-amino acids have been demonstrated to exhibit efficacy against S. aureus, P. aeruginosa, and B. subtilis biofilms. | [161,163,164,165] |
| Urea | An amide that is theorized to break down biofilms by disrupting the hydrogen bonds that are vital for EPS mechanical stability, and has exhibited dispersal ability against S. epidermidis, P. aeruginosa and K. pneumoniae biofilms. | [196,197] |
| Chitosan | A polycationic macromolecule derived from the polysaccharide chitin, and has been shown to penetrate and possibly disrupt biofilms formed by Cryptococcus neoformans, L. monocytogenes, P. fluorescens, Bacillus cereus, S. enterica, C. albicans, and P. aeruginosa. It is important to note that it has not been proved that chitosan has any direct effect on the biofilm matrix, and it is possible that the molecule achieves biofilm disruption by penetrating the matrix and acting on the microbes themselves. | [198,199,200,201,202] |
| Sequestration Molecules | ||
| BdcA | A protein that reduces unbound c-di-GMP concentrations by binding to, but not degrading, the molecules, hindering the activation of biofilm-related cellular processes, and has been shown to disperse biofilms formed by E. coli, P. aeruginosa, P. fluorescens, and Rhizobium meliloti. | [168,169,170] |
| EDTA | Ethylenediaminetetraacetic acid (EDTA) is a metal-ion chelator that can sequester EPS-matrix-stabilizing ions, triggering biofilms dispersal of P. aeruginosa, H. influenzae, S. epidermidis, C. tropicalis, and Enterococcus faecalis. | [203,204,205,206,207,208] |
| Lactoferrin | An iron-binding protein from the innate immune system which triggers active dispersal through chelating irons, an essential bacterial nutrient and global regulator of a variety of processes, including biofilm development and growth. It has been shown to be effective against P. aeruginosa, E. coli, S. aureus, E. faecalis and S. epidermidis biofilms. | [209,210] |
| Metabolic Interference Molecules | ||
| Deferoxamine, Deferasirox | FDA-approved iron chelators that have been proved to interfere with bacterial iron metabolism, preventing the formation of P. aeruginosa biofilms and reducing established biofilm biomass. | [175] |
| L-Arg | Exogenous amino acids can affect both biofilm metabolism and development, and it has been proved that L-Arg can effectively disrupt biofilm of Streptococcus gordonii and S. mutans. | [176,178] |
| D-Arg | D-Arg can inhibit and dissociate EPS production from biofilm and can alter the Porphyromonas gingivalis biofilm structure in relatively high concentrations. | [181] |
| L-Met | L-Met can up-regulate DNase gene expression and target eDNA components in biofilms. It has been proved to be effective on biofilm formed by P. aeruginosa. | [182] |
| Ga | A transition metal that is chemically similar to Fe, thus it can substitute for Fe in many biologic systems and inhibit Fe-dependent processes. It was shown that Ga can inhibit P. aeruginosa growth and biofilm formation and kill planktonic and biofilm bacteria. | [187] |
| Name | Summary | References |
|---|---|---|
| AI-2 | QS autoinducer, functions as a chemorepellent in Helicobacter pylori by regulating the proportion and spatial organization of biofilm cells and has been proved to effectively reduce the proportion of adherent cells and induce biofilm dispersal. | [214] |
| AIP-I | The autoinducing peptide type I activates a regulatory cascade called agr system, resulting in the increased expression of invasive factors, including toxins, hemolysins, proteases, and other tissue-degrading enzymes. It has been proved to effectively disrupt biofilm formed by MRSA. | [220] |
| RIP | RNAIII-inhibiting peptide, a heptapeptide that has been shown to inhibit the biofilms formation of both methicillin-resistant and vancomycin-resistant S. aureus and S. epidermidis. | [221,222,223,224,225,226] |
| M64 | A small molecule that target the MvfR-regulated QS virulence pathway, which can effectively silence the MvfR communication system, thus blocks P. aeruginosa virulence both in vitro and in vivo. | [227] |
| Cinnamic acid | Acts as a competitive inhibitor for the natural ligands towards the ligand binding domain of the transcriptional activators of the quorum sensing circuit in P. aeruginosa, LasR and RhlR. It has been proved to effectively inhibit both the production of the QS-dependent virulence factors and biofilm formation in P. aeruginosa. | [228] |
| Trans 4-(2-carboxy-vinyl) benzoic acid | Cell-free extracts of Natrinema versiforme which show QSI properties against P. aeruginosa and is efficient for biofilm inhibition. | [229] |
| SM23 | A boronic acid derivative of β-lactamase inhibitor, acting as a powerful inhibitor of P. aeruginosa biofilm. | [230] |
| Name | Summary | References |
|---|---|---|
| Anti-Biofilm Peptides | ||
| 1018 | A synthetic, modified form of the cationic antimicrobial peptide bactenecin, which can effectively disrupt the established biofilms of P. aeruginosa, E. coli, A. baumannii, K. pneumoniae, S. aureus, Salmonella typhimurium, and B. cenocepacia. | [240,241] |
| 17BIPHE2, GF-17 | A 17-amino-acid derivative of human cathelicidin LL-37 which has exhibited efficacy in disrupting S. aureus biofilms. | [242] |
| P60.4Ac | A synthetic peptide derived from human cathelicidin LL-37, which consists of 24 amino acids and has been shown to effectively degrade biofilms formed by S. aureus. | [243,244] |
| BMAP-28 | Cathelicidin-derived peptides that can effectively degrade biofilms formed by S. aureus, P. aeruginosa, and Stenotrophomonas maltophilia. | [245,246] |
| DJK-5, DJK-6 | Synthetic protease-resistant peptide, D-enantiomeric, partly works through degrading the (p)ppGpp bacterial stringent response signal. It has been demonstrated to effectively disrupt biofilm formed by P. aeruginosa, A. baumannii, S. enterica and K. pneumoniae. | [234,247] |
| UBBLi30 | A kind of bacitracin produced by Bacillus paralicheniformis UBBLi30 that can significantly inhibit biofilms formed by M. luteus and MRSA. | [248] |
| Pug-1 | Pomegranate-derived peptides exhibit both antibacterial activity and anti-adherence activity against S. mutans and can inhibit the expression of virulence-associated genes and enzymes. | [249] |
| CSP | Antibiofilm peptides from Capsicum baccatum (red pepper) that can effectively restrict the biofilm formation by S. epidermidis, without any antibiotic activity. | [250] |
| GHaK, GHa4K | Temporin-GHa (GHa) derived peptides that can effectively inhibit the initial adhesion and the formation of S. aureus biofilms and eradicate the mature biofilms. | [251] |
| P5, P6.2 | Two synthetic designed AMPs which have the ability to interact with bacterial or eukaryotes membranes. P5 displayed antibiofilm activity on both P. aeruginosa and S. aureus, while P6.2 only on S. aureus. | [252] |
| B-GR23 | A brevinin-2 like antimicrobial peptide with antimicrobial activity against S. aureus, can reduce the production of EPS on the planktonic growth of S. aureus and inhibit nearly all planktonic bacteria to start the initial attachment of biofilm. | [253] |
| DNA Cross-Linking Drugs | ||
| mitomycin C (MMC) | FDA-approved anti-cancer drug, the first broad-spectrum compound capable of eliminating persister cells, passively transported and bioreductively activated leading to spontaneous cross-linking of DNA, and also worked as potent bactericide for a broad range of bacterial persisters, including commensal E. coli K-12, pathogenic species of E. coli, S. aureus and P. aeruginosa. | [239] |
| cisplatin | FDA-approved anti-cancer drug, cis-diamminodichloroplatinum(II), can eradicates persister cells of E. coli K-12, enterohemorrhagic E. coli, S. aureus and P. aeruginosa, more effective at killing P. aeruginosa persister cells than MMC, also highly effective against clinical isolates of S. aureus and P. aeruginosa. | [238] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Geng, M.; Bai, L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms 2020, 8, 1222. https://doi.org/10.3390/microorganisms8081222
Jiang Y, Geng M, Bai L. Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms. 2020; 8(8):1222. https://doi.org/10.3390/microorganisms8081222
Chicago/Turabian StyleJiang, Yu, Mengxin Geng, and Liping Bai. 2020. "Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles" Microorganisms 8, no. 8: 1222. https://doi.org/10.3390/microorganisms8081222
APA StyleJiang, Y., Geng, M., & Bai, L. (2020). Targeting Biofilms Therapy: Current Research Strategies and Development Hurdles. Microorganisms, 8(8), 1222. https://doi.org/10.3390/microorganisms8081222





