Genomic and Transcriptome Analyses of a Thermophilic Bacterium Geobacillus stearothermophilus B5 Isolated from Compost Reveal Its Enzymatic Basis for Lignocellulose Degradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening, Isolation and Identification of the Cellulolytic Strain
2.2. Optimal Culture Conditions and Carbon Source Utilization by Strain B5
2.3. Extracellular Protein Extraction and Enzyme Activity Assays
2.4. Genome Sequencing, Assembly and Annotation
2.5. RNA Extraction and Transcriptome Sequencing
2.6. Statistical Analysis
3. Results and Discussion
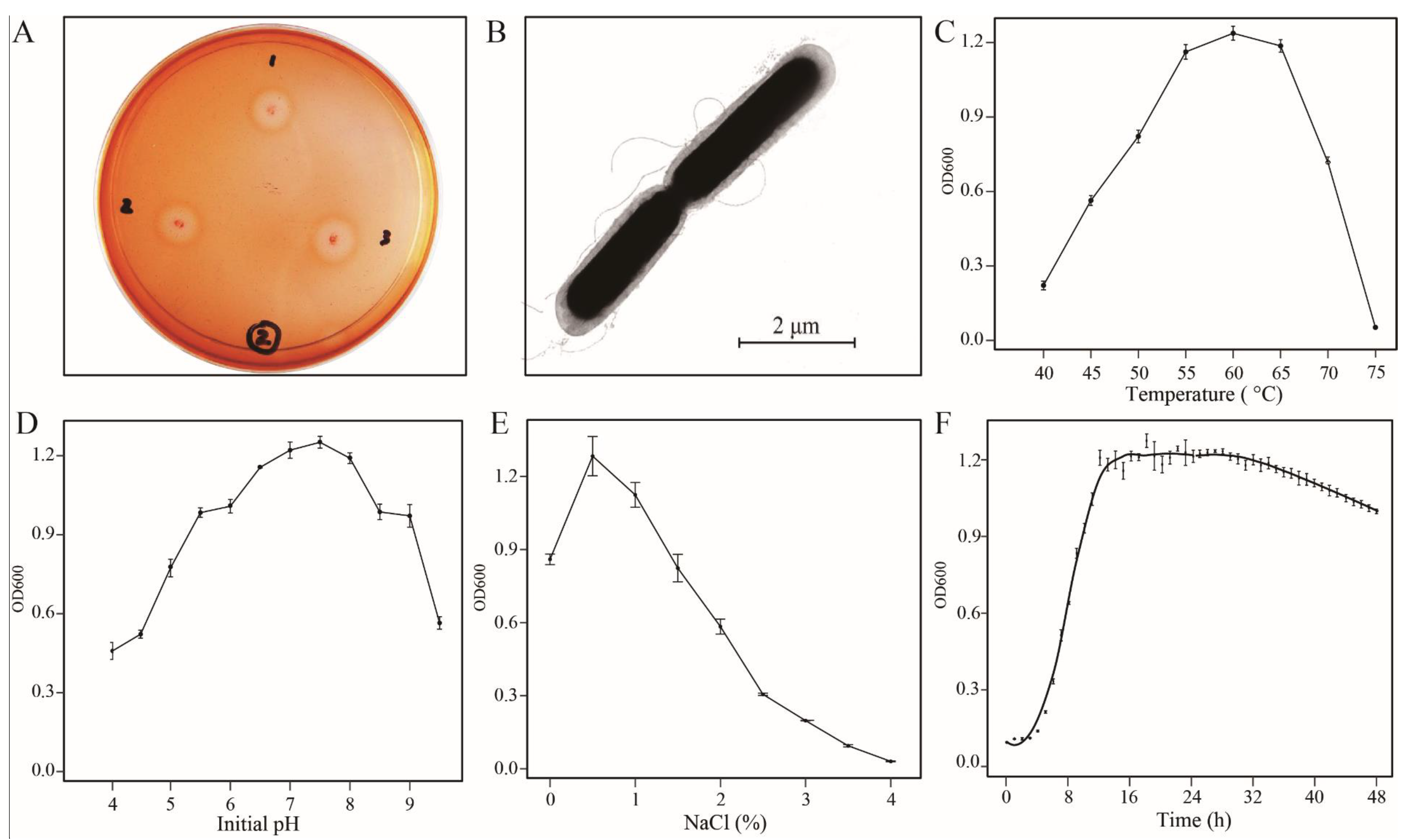
3.1. Characteristics of Strain B5
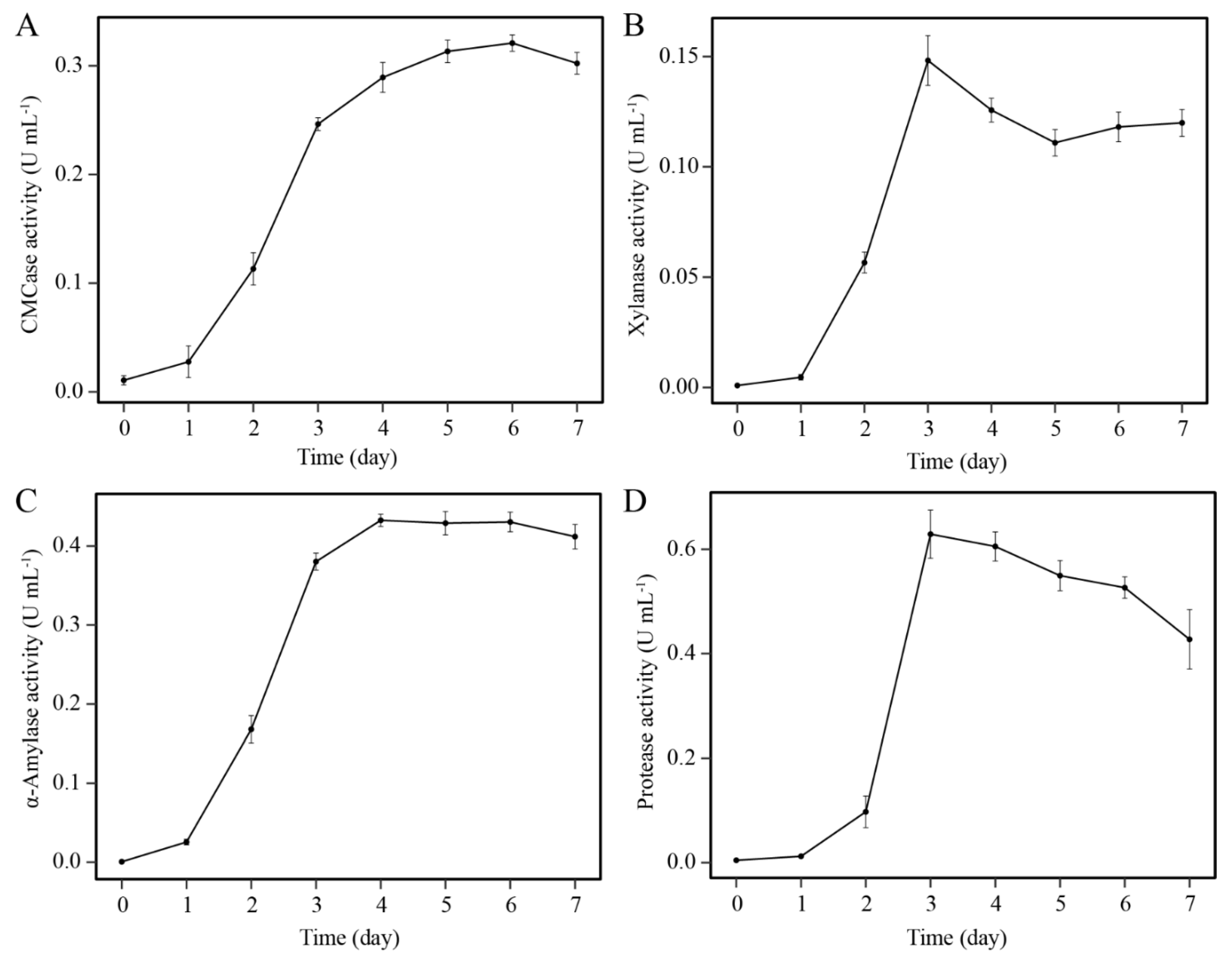
3.2. Determination of Enzyme Activities
3.3. Genomic Analysis of G. stearothermophilus B5 and Comparison of COG Categories
3.4. CAZyme Family Analysis of the Genome of Strain B5
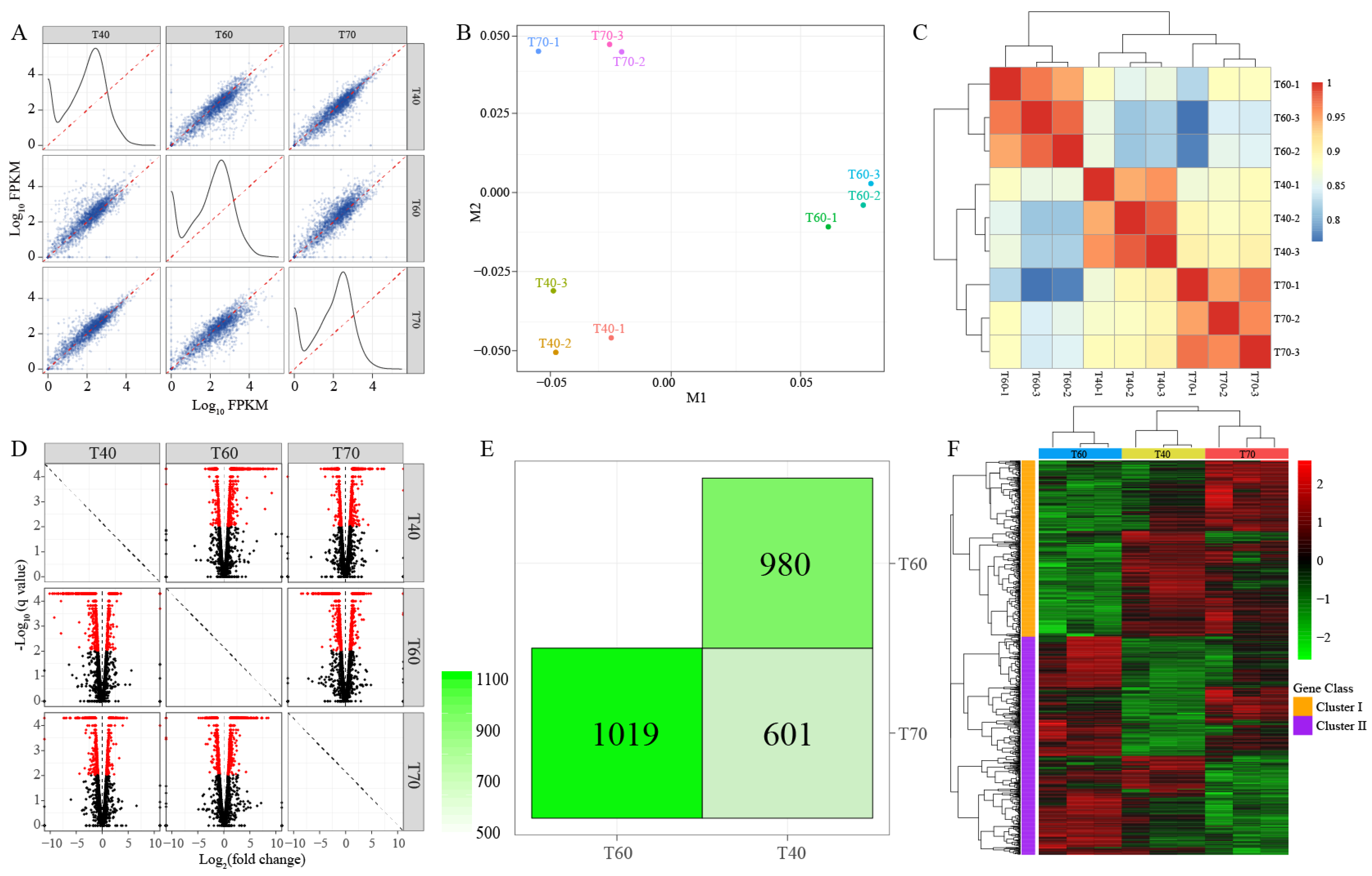
3.5. Global Analysis of Transcriptome and DEGs
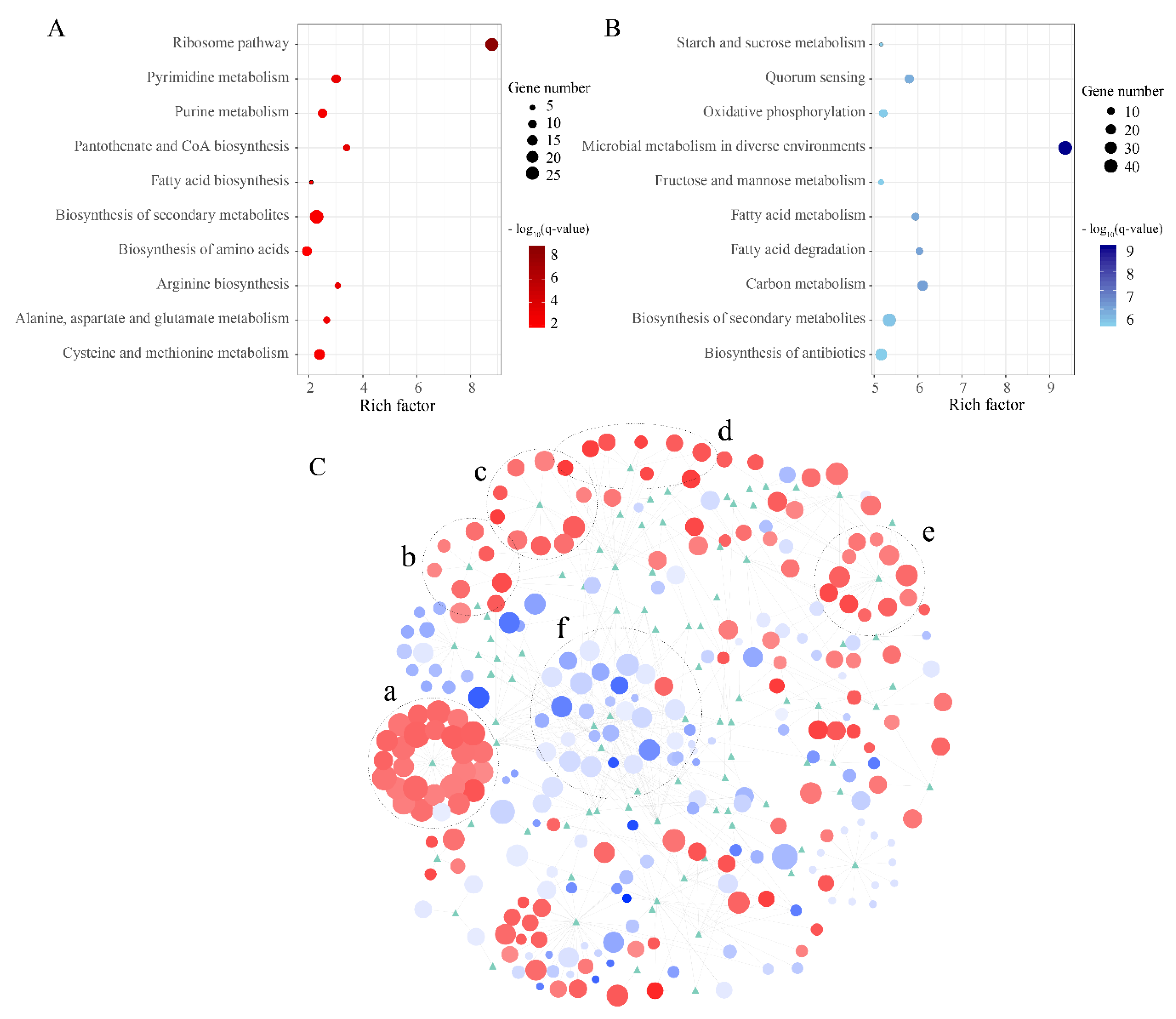
3.6. Metabolism Characteristics of Strain B5 under Mesophilic Conditions
3.7. Heat Shock Proteins (HSPs) and Enrichment Analyses of Strain B5 under Extreme Thermophilic Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, K.-C.; Yoo, S.-S.; Oh, Y.-A.; Kim, S.-J. Isolation and characteristics of Trichoderma harzianum FJI producing cellulases and xylanase. J. Microbiol. Biotechnol. 2003, 13, 1–8. [Google Scholar]
- Linton, S.M.; Greenaway, P. Presence and properties of cellulase and hemicellulase enzymes of the gecarcinid land crabs Gecarcoidea natalis and Discoplax hirtipes. J. Exp. Biol. 2004, 207, 4095–4104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deswal, D.; Khasa, Y.P.; Kuhad, R.C. Optimization of cellulase production by a brown rot fungus Fomitopsis sp. RCK2010 under solid state fermentation. Bioresour. Technol. 2011, 102, 6065–6072. [Google Scholar] [CrossRef] [PubMed]
- Vargas-García, M.; Suárez-Estrella, F.; López, M.; Moreno, J. Microbial population dynamics and enzyme activities in composting processes with different starting materials. Waste Manag. 2010, 30, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Mondini, C.; Fornasier, F.; Sinicco, T. Enzymatic activity as a parameter for the characterization of the composting process. Soil Biol. Biochem. 2004, 36, 1587–1594. [Google Scholar] [CrossRef]
- Gerardi, M.H.; Zimmerman, M.C. Wastewater Ppathogens; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Beyea, J.; Cannon, C.; Diddy, S. Composting of Yard Trimmings and Municipal Solid Waste; Environmental Protection Agency: Washington, DC, USA, 1994.
- Blumer-Schuette, S.E.; Brown, S.D.; Sander, K.B.; Bayer, E.A.; Kataeva, I.; Zurawski, J.V.; Conway, J.M.; Adams, M.W.; Kelly, R.M. Thermophilic lignocellulose deconstruction. Fems Microbiol. Rev. 2014, 38, 393–448. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.H.; Lisowska, B.K.; Leak, D.J. The genus Geobacillus and their biotechnological potential. In Advances in Aapplied Mmicrobiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 92, pp. 1–48. [Google Scholar]
- Bhalla, A.; Bischoff, K.M.; Uppugundla, N.; Balan, V.; Sani, R.K. Novel thermostable endo-xylanase cloned and expressed from bacterium Geobacillus sp. WSUCF1. Bioresour. Technol. 2014, 165, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Bartosiak-Jentys, J.; Hussein, A.H.; Lewis, C.J.; Leak, D.J. Modular system for assessment of glycosyl hydrolase secretion in Geobacillus thermoglucosidasius. Microbiology 2013, 159, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- López-Mondéjar, R.; Zühlke, D.; Becher, D.; Riedel, K.; Baldrian, P. Cellulose and hemicellulose decomposition by forest soil bacteria proceeds by the action of structurally variable enzymatic systems. Sci. Rep. 2016, 6, 25279. [Google Scholar] [CrossRef]
- Teather, R.M.; Wood, P.J. Use of Congo red-polysaccharide interactions in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl. Environ. Microbiol. 1982, 43, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Holt, J.G.; Krieg, N.; Sneath, P.H.; Staley, J.; Williams, S. Bergey’s Mmanual of Ddeterminative Bbacteriology, 9th ed.; Lippincott Williams & Wikins: Baltimore, MD, USA, 1994. [Google Scholar]
- Abuga, I.; Sulaiman, S.F.; Abdul Wahab, R.; Ooi, K.L.; Abdull Rasad, M.S.B. In vitro antibacterial effect of the leaf extract of Murraya koenigii on cell membrane destruction against pathogenic bacteria and phenolic compounds identification. Eur. J. Integr. Med. 2020, 33, 101010. [Google Scholar] [CrossRef]
- Canakci, S.; Inan, K.; Kacagan, M.; Belduz, A.O. Evaluation of arabinofuranosidase and xylanase activities of Geobacillus spp. isolated from some hot springs in Turkey. J. Microbiol. Biotechnol. Adv. 2007, 17, 1262. [Google Scholar]
- Dheeran, P.; Kumar, S.; Jaiswal, Y.K.; Adhikari, D.K.J.A.m.; Characterization of hyperthermostable α-amylase from Geobacillus sp. IIPTN. Appl. Microbiol. Biotechnol. 2010, 86, 1857–1866. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Li, J. Secretome diversity and quantitative analysis of cellulolytic Aspergillus fumigatus Z5 in the presence of different carbon sources. Biotechnol. Biofuels 2013, 6, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thebti, W.; Riahi, Y.; Belhadj, O. Purification and Characterization of a New Thermostable, Haloalkaline, Solvent Stable, and Detergent Compatible Serine Protease from Geobacillus toebii Strain LBT 77. Biomed. Res. Int. 2016, 2016, 9178962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. Bioinformatics 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Zeigler, D.R. Application of a recN sequence similarity analysis to the identification of species within the bacterial genus Geobacillus. Int. J. Syst. Evol. Microbiol. 2005, 55, 1171–1179. [Google Scholar] [CrossRef]
- Miller, F.C. Composting as a process based on the control of ecologically selective factors. In Soil Microbial Ecology. Applications in Agricultural and Environmental Management; Metting, F.B., Ed.; Marcel Dekker: Ottawa, ON, Canada, 1993; pp. 515–544. [Google Scholar]
- Raut, M.P.; Prince William, S.P.M.; Bhattacharyya, J.K.; Chakrabarti, T.; Devotta, S. Microbial dynamics and enzyme activities during rapid composting of municipal solid waste—A compost maturity analysis perspective. Bioresour. Technol. 2008, 99, 6512–6519. [Google Scholar] [CrossRef]
- Wang, X.-J.; Bai, J.-G.; Liang, Y.-X. Optimization of multienzyme production by two mixed strains in solid-state fermentation. Appl. Microbiol. Biotechnol. 2006, 73, 533–540. [Google Scholar] [CrossRef]
- Tai, S.-K.; Lin, H.-P.P.; Kuo, J.; Liu, J.-K. Isolation and characterization of a cellulolytic Geobacillus thermoleovorans T4 strain from sugar refinery wastewater. Extremophiles 2004, 8, 345–349. [Google Scholar] [CrossRef]
- Rastogi, G.; Bhalla, A.; Adhikari, A.; Bischoff, K.M.; Hughes, S.R.; Christopher, L.P.; Sani, R.K. Characterization of thermostable cellulases produced by Bacillus and Geobacillus strains. Bioresour. Technol. 2010, 101, 8798–8806. [Google Scholar] [CrossRef] [PubMed]
- Lynd, L.R.; Weimer, P.J.; Van Zyl, W.H.; Pretorius, I.S. Microbial cellulose utilization: Fundamentals and biotechnology. Microbiol. Mmolecular Bbiol. Rrev. 2002, 66, 506–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Lu, Q.; Wei, Y.; Cui, H.; Zhang, X.; Wang, X.; Shan, S.; Wei, Z.J.B.t. Effect of actinobacteria agent inoculation methods on cellulose degradation during composting based on redundancy analysis. Bioresour. Technol. 2016, 219, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.; Mamo, G.; Karlsson, E.N. Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microb. Cell Factories 2007, 6, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Fattah, Y.R.; El-Helow, E.R.; Ghanem, K.M.; Lotfy, W.A. Application of factorial designs for optimization of avicelase production by a thermophilic Geobacillus isolate. Res. J. Microbiol. 2007, 2, 13–23. [Google Scholar]
- Zhang, N.; Yang, D.; Wang, D.; Miao, Y.; Shao, J.; Zhou, X.; Xu, Z.; Li, Q.; Feng, H.; Li, S. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genom. 2015, 16, 685. [Google Scholar] [CrossRef] [Green Version]
- Madej, M.G.; Sun, L.; Yan, N.; Kaback, H.R. Functional architecture of MFS D-glucose transporters. Proc. Natl. Acad. Sci. USA 2014, 111, E719–E727. [Google Scholar] [CrossRef] [Green Version]
- Canal-Llaubères, R.M. 4-Enzymes and wine quality. In Managing Wine Quality; Reynolds, A.G., Ed.; Woodhead Publishing: Sawston, UK, 2010; pp. 93–132. [Google Scholar]
- Janeček, Š.; Gabriško, M. Remarkable evolutionary relatedness among the enzymes and proteins from the α-amylase family. Cell. Mol. Life Sci. 2016, 73, 2707–2725. [Google Scholar] [CrossRef]
- Tamaru, Y.; López-Contreras, A.M. Lignocellulosic biomass utilization toward biorefinery using meshophilic Clostridial species. In Cellulose-Biomass Conversion; InTech: London, UK, 2013; pp. 132–144. [Google Scholar]
- Field, R.; Pergolizzi, G.; Kuhaudomlarp, S.S.; Kalita, E. Glycan Phosphorylases in Multi-Enzyme Synthetic Processes. Protein Pept. Lett. 2017, 24, 696–709. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, H.; Okazaki, F.; Kondo, A.; Yoshida, K.-I. Genome mining and motif modifications of glycoside hydrolase family 1 members encoded by Geobacillus kaustophilus HTA426 provide thermostable 6-phospho-β-glycosidase and β-fucosidase. Appl. Microbiol. Biotechnol. 2013, 97, 2929–2938. [Google Scholar] [CrossRef]
- Plaza-Vinuesa, L.; Hernandez-Hernandez, O.; Moreno, F.J.; de las Rivas, B.; Muñoz, R. Unravelling the diversity of glycoside hydrolase family 13 α-amylases from Lactobacillus plantarum WCFS1. Microb. Cell Factories 2019, 18, 183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Siika-aho, M.; Tenkanen, M.; Viikari, L. The role of acetyl xylan esterase in the solubilization of xylan and enzymatic hydrolysis of wheat straw and giant reed. Biotechnol. Biofuels 2011, 4, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; Wiegel, J. Purification and characterization of two thermostable acetyl xylan esterases from Thermoanaerobacterium sp. strain JW/SL-YS485. Appl. Environ. Microbiol. 1995, 61, 729–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biely, P. Microbial carbohydrate esterases deacetylating plant polysaccharides. Biotechnol. Adv. 2012, 30, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, R.H.; Fraaije, M.W.; Mattevi, A.; van Berkel, W.J. Structure, function and redesign of vanillyl-alcohol oxidase. In Proceedings of The International Congress Series, London, UK, 12–15 May 2002; pp. 13–24. [Google Scholar]
- Magidovich, H.; Eichler, J. Glycosyltransferases and oligosaccharyltransferases in Archaea: Putative components of the N-glycosylation pathway in the third domain of life. FEMS Microbiol. Lett. 2009, 300, 122–130. [Google Scholar] [CrossRef] [Green Version]
- Coutinho, P.M.; Deleury, E.; Davies, G.J.; Henrissat, B. An Evolving Hierarchical Family Classification for Glycosyltransferases. J. Mol. Biol. 2003, 328, 307–317. [Google Scholar] [CrossRef]
- Geremia, S.; Campagnolo, M.; Schinzel, R.; Johnson, L.N. Enzymatic Catalysis in Crystals of Escherichia coli Maltodextrin Phosphorylase. J. Mol. Biol. 2002, 322, 413–423. [Google Scholar] [CrossRef]
- Ghosh, S.; Chan, C.-K.K. Analysis of RNA-Seq data using TopHat and Cufflinks. In Plant Bioinformatics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 339–361. [Google Scholar]
- Lam, H.; Oh, D.-C.; Cava, F.; Takacs, C.N.; Clardy, J.; de Pedro, M.A.; Waldor, M.K. D-amino acids govern stationary phase cell wall remodeling in bacteria. Science 2009, 325, 1552–1555. [Google Scholar] [CrossRef] [Green Version]
- Bentley, R.; Haslam, E. The shikimate pathway—A metabolic tree with many branche. Crit. Rev. Biochem. Mol. Biol. Evol. 1990, 25, 307–384. [Google Scholar] [CrossRef]
- Shen, T.; Liu, Q.; Xie, X.; Xu, Q.; Chen, N. Improved production of tryptophan in genetically engineered Escherichia coli with TktA and PpsA overexpression. Biomed Res. Int. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Vicent, I.; Navarro, A.; Mulet, J.M.; Sharma, S.; Serrano, R. Uptake of inorganic phosphate is a limiting factor for Saccharomyces cerevisiae during growth at low temperatures. FEMS Yeast Res. 2015, 15, fov008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.-J.; Seo, Y.-S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brauer, D.; Röming, R.J.F.l. The primary structure of protein S3 from the small ribosomal subunit of Escherichia coli. FEBS Lett. 1979, 106, 352–357. [Google Scholar] [CrossRef] [Green Version]
- Herold, M.; Nierhaus, K. Incorporation of six additional proteins to complete the assembly map of the 50 S subunit from Escherichia coli ribosomes. J. Biol. Chem. 1987, 262, 8826–8833. [Google Scholar] [PubMed]
- Ye, Y.; Zhu, Y.; Pan, L.; Li, L.; Wang, X.; Lin, Y. Gaining insight into the response logic of Saccharomyces cerevisiae to heat shock by combining expression profiles with metabolic pathways. Biochem. Biophys. Res. Commun. 2009, 385, 357–362. [Google Scholar] [CrossRef]
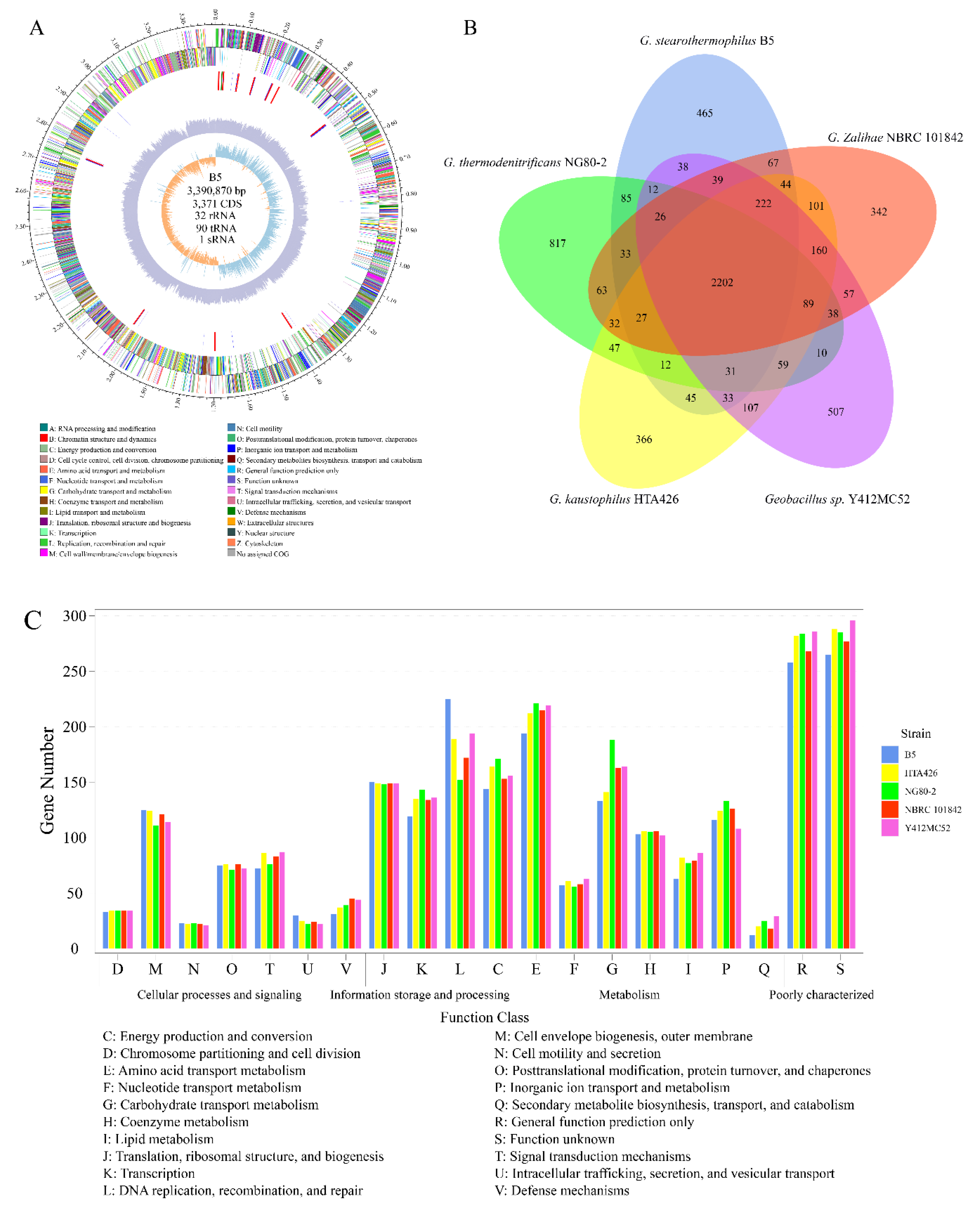
| Feature | B5 | HTA426 | NG80–2 | Y412MC52 | NBRC 101842 |
|---|---|---|---|---|---|
| Size (bp) | 3,390,870 | 3,592,666 | 3,608,012 | 3,673,940 | 3,539,687 |
| GC content (%) | 52.46 | 51.99 | 48.85 | 52.31 | 51.9 |
| Number of contigs | 1 | 2 | 2 | 2 | 164 |
| Protein-coding genes | 3371 | 3546 | 3554 | 3596 | 3515 |
| Mean gene length (bp) | 851 | 861 | 853 | 869 | 849 |
| Percent of coding region (%) | 84.60 | 84.98 | 84.02 | 85.06 | 84.31 |
| Number of tRNA | 90 | 87 | 88 | 87 | 82 |
| Number of rRNA | 32 | 27 | 30 | 25 | 12 |
| Gene ID | Gene | Description | Fold Change |
|---|---|---|---|
| gene0064 | Heat shock protein 33 (molecular chaperonin), a cytoplasmically localized protein | 2.3 | |
| gene0701 | clpB | Unfold insoluble protein aggregates, and co-factor of Hsp70/DnaK | 2.8 |
| gene1200 | clpP | Clp protease | 5.3 |
| gene2328 | dnaJ | Heat shock protein 40, co-factor of Hsp70 | 2.8 |
| gene2329 | dnaK | Folding and unfolding protein, providing thermotolerance on extreme environment | 2.7 |
| gene2330 | grpE | Encoding protein GrpE, HSP-70 cofactor | 4.5 |
| gene2447 | mreB | Rod shape-determining protein MreB | 3.1 |
| gene2482 | clpX | ATP-dependent Clp protease | 2.1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Miao, J.; Wang, X.; Li, T.; Zhu, H.; Liu, D.; Shen, Q. Genomic and Transcriptome Analyses of a Thermophilic Bacterium Geobacillus stearothermophilus B5 Isolated from Compost Reveal Its Enzymatic Basis for Lignocellulose Degradation. Microorganisms 2020, 8, 1357. https://doi.org/10.3390/microorganisms8091357
Wang M, Miao J, Wang X, Li T, Zhu H, Liu D, Shen Q. Genomic and Transcriptome Analyses of a Thermophilic Bacterium Geobacillus stearothermophilus B5 Isolated from Compost Reveal Its Enzymatic Basis for Lignocellulose Degradation. Microorganisms. 2020; 8(9):1357. https://doi.org/10.3390/microorganisms8091357
Chicago/Turabian StyleWang, Mengmeng, Jiaxi Miao, Xuanqing Wang, Tuo Li, Han Zhu, Dongyang Liu, and Qirong Shen. 2020. "Genomic and Transcriptome Analyses of a Thermophilic Bacterium Geobacillus stearothermophilus B5 Isolated from Compost Reveal Its Enzymatic Basis for Lignocellulose Degradation" Microorganisms 8, no. 9: 1357. https://doi.org/10.3390/microorganisms8091357
APA StyleWang, M., Miao, J., Wang, X., Li, T., Zhu, H., Liu, D., & Shen, Q. (2020). Genomic and Transcriptome Analyses of a Thermophilic Bacterium Geobacillus stearothermophilus B5 Isolated from Compost Reveal Its Enzymatic Basis for Lignocellulose Degradation. Microorganisms, 8(9), 1357. https://doi.org/10.3390/microorganisms8091357






