Chlorine Disinfection of Legionella spp., L. pneumophila, and Acanthamoeba under Warm Water Premise Plumbing Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Chlorine Dosing and Residual Experiments
2.2. Simulated Glass Water Heaters (SGWHs)
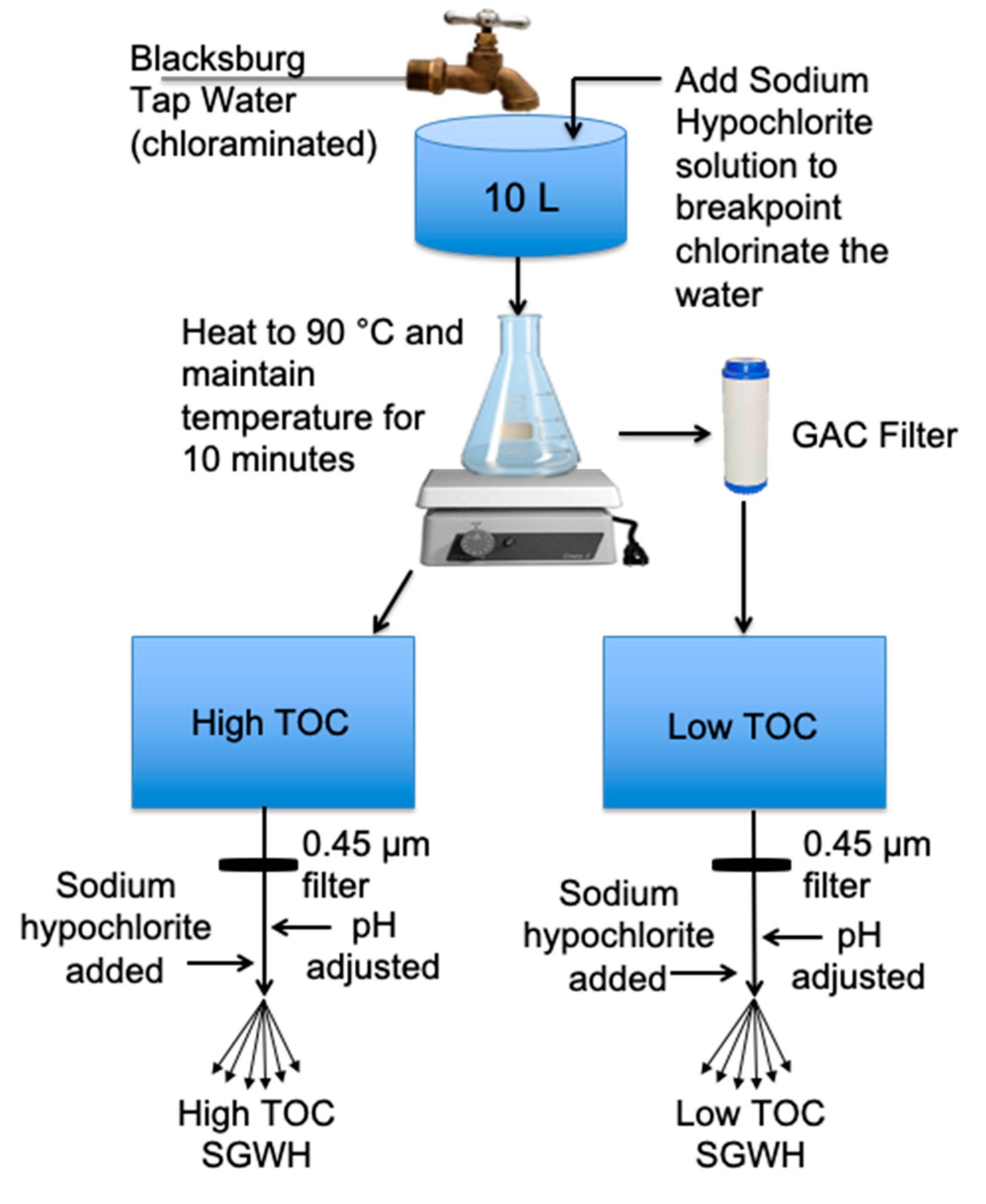
2.3. Water Preparation Method, Sequential Disinfection Addition, and Sampling Schedule
2.4. Sampling Methods
2.5. Statistical Analyses
3. Results
3.1. Instantaneous Residual Demand and Decay Without Premise Plumbing Materials at Room Temperature
3.2. Residual Decay Without Premise Plumbing Materials at 37 °C
3.3. Effects of Premise Plumbing Materials and Conditions at Warm Temperatures
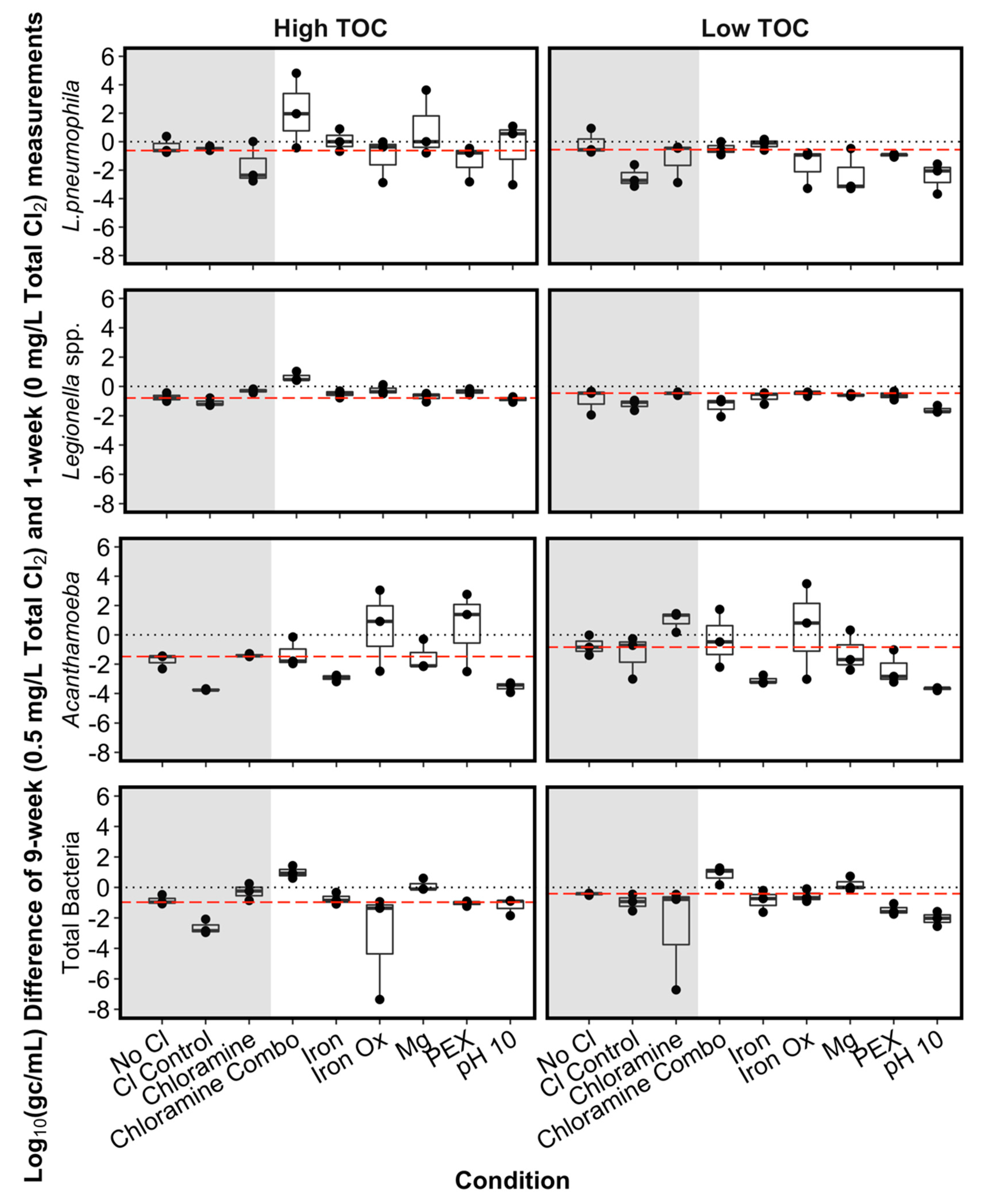
3.3.1. Accounting for Background Variation in the No Chlorine Control Condition
3.3.2. Chloramine versus Chlorine in the SGWHs
3.3.3. Effect of pH in the SGWHs Without Plumbing Materials
3.3.4. Plumbing Material Effects on Target Microorganisms
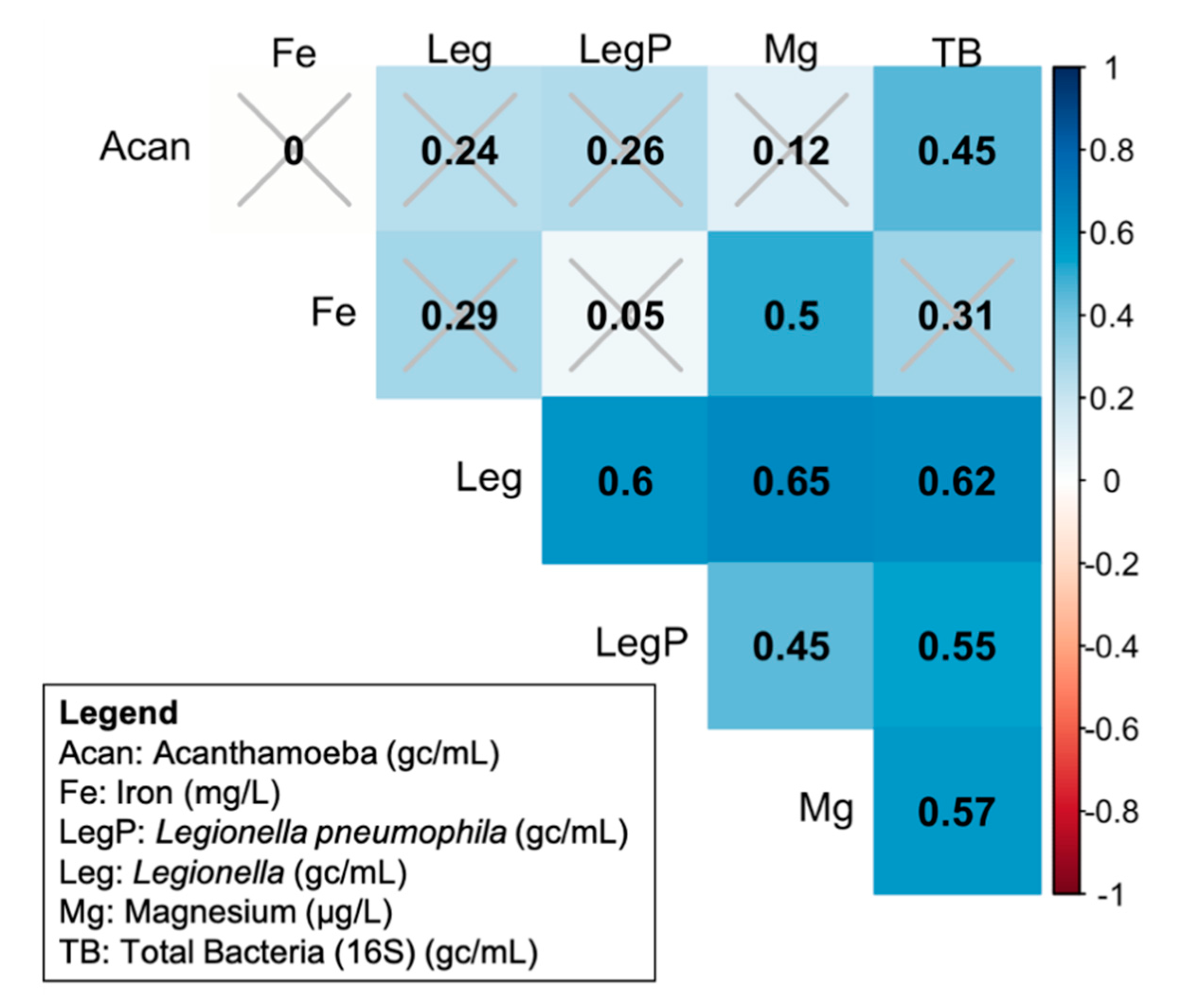
3.4. Correlation of Target Microbes with Each Other and with Chemical Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Garrison, L.E.; Kunz, J.M.; Cooley, L.A.; Moore, M.R.; Lucas, C.; Schrag, S.; Sarisky, J.; Whitney, C.G. Vital Signs: Deficiencies in Environmental Control Identified in Outbreaks of Legionnaires’ Disease—North America, 2000–2014. Morb. Mortal. Wkly. Rep. 2016, 16, 3049–3058. [Google Scholar]
- OSHA. Legionnaires’ Disease: Facts and Frequently Asked Questions. Available online: https://www.osha.gov/dts/osta/otm/legionnaires/faq.html (accessed on 14 January 2020).
- CDC. Legionella Fast Facts. Available online: https://www.cdc.gov/legionella/fastfacts.html (accessed on 21 September 2020).
- Collier, S.A.; Stockman, L.J.; Hicks, L.A.; Garrison, L.E.; Zhou, F.J.; Beach, M.J. Direct healthcare costs of selected diseases primarily or partially transmitted by water. Epidemiol. Infect. 2012, 140, 2003–2013. [Google Scholar] [CrossRef]
- Vaccaro, L.; Izquierdo, F.; Magnet, A.; Hurtado, C.; Salinas, M.A.; Gomes, T.S.; Angulo, S.; Salso, S.; Pelaez, J.; Tejeda, M.I.; et al. First Case of Legionnaire’s Disease Caused by Legionella anisa in Spain and the Limitations on the Diagnosis of Legionella non-pneumophila Infections. PLoS ONE 2016, 11, e0159726. [Google Scholar] [CrossRef]
- Fields, B.S.; Sanden, G.N.; Barbaree, J.M.; Morrill, W.E.; Wadowsky, R.M.; White, E.H.; Feeley, J.C. Intracellular multiplication ofLegionella pneumophila in amoebae isolated from hospital hot water tanks. Curr. Microbiol. 1989, 18, 131–137. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Management of Legionella in Water Systems; The National Academies Press: Washington, DC, USA, 2019; p. 304. [Google Scholar] [CrossRef]
- Hsu, B.-M. Surveillance and Evaluation of the Infection Risk of Free-Living Amoebae—Acanthamoeba in Aquatic Environments. Int. J. Environ. Sci. Dev. 2016, 7, 445–448. [Google Scholar] [CrossRef]
- Hsu, B.-M.; Huang, C.-C.; Chen, J.-S.; Chen, N.-H.; Huang, J.-T. Comparison of potentially pathogenic free-living amoeba hosts by Legionella spp. in substrate-associated biofilms and floating biofilms from spring environments. Water Res. 2011, 45, 5171–5183. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, W.J.; Garner, E.; Ji, P.; Zhu, N.; Parks, J.; Schwake, D.O.; Pruden, A.; Edwards, M.A. Distribution System Operational Deficiencies Coincide with Reported Legionnaires’ Disease Clusters in Flint, Michigan. Environ. Sci. Technol. 2017, 51, 11986–11995. [Google Scholar] [CrossRef]
- Zahran, S.; McElmurry, S.P.; Kilgore, P.E.; Mushinski, D.; Press, J.; Love, N.G.; Sadler, R.C.; Swanson, M.S. Assessment of the Legionnaires’ disease outbreak in Flint, Michigan. Proc. Natl. Acad. Sci. USA 2018, 115, E1730–E1739. [Google Scholar] [CrossRef]
- Schwake, D.O.; Garner, E.; Strom, O.R.; Pruden, A.; Edwards, M.A. Legionella DNA Markers in Tap Water Coincident with a Spike in Legionnaires’ Disease in Flint, MI. Environ. Sci. Technol. Lett. 2016. [Google Scholar] [CrossRef]
- EPA. Draft-Technologies for Legionella Control: Scientific Literature Reviewed; EPA Office of Water: Washington, DC, USA, 2016. [Google Scholar]
- Falkinham, J.O.; Pruden, A.; Edwards, M. Opportunistic Premise Plumbing Pathogens: Increasingly Important Pathogens in Drinking Water. Pathogens 2015, 4, 373. [Google Scholar] [CrossRef]
- Schoenen, D. Role of disinfection in suppressing the spread of pathogens with drinking water: Possibilities and limitations. Water Res. 2002, 36, 3874–3888. [Google Scholar] [CrossRef]
- Wahman, D.G.; Pressman, J.G. Distribution System Residuals—Is “Detectable” Still Acceptable for Chloramines? J. Awwa 2015, 107, 53–63. [Google Scholar] [CrossRef]
- WHO. Legionella and the Prevention of Legionellosis; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- Lehtola, M.J.; Lehtola, M.J.; Miettinen, I.T.; Lampola, T.; Hirvonen, A. Pipeline materials modify the effectiveness of disinfectants in drinking water distribution systems. Water Res. 2005, 39, 1962–1971. [Google Scholar] [CrossRef]
- Lehtola, M.J.; Miettinen, I.T.; Keinänen, M.M.; Kekki, T.K.; Laine, O.; Hirvonen, A.; Vartiainen, T.; Martikainen, P.J. Microbiology, chemistry and biofilm development in a pilot drinking water distribution system with copper and plastic pipes. Water Res. 2004, 38, 3769–3779. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.M. Chlorine fate and transport in drinking water distribution systems: Results from experimental and modeling studies. Front. Earth Sci. 2011, 5, 334–340. [Google Scholar] [CrossRef]
- Rhoads, W.J.; Pruden, A.; Edwards, M.K. Interactive Effects of Corrosion, Copper, and Chloramines on Legionella and Mycobacteria in Hot Water Plumbing. Environ. Sci. Technol. 2017, 51, 7065–7075. [Google Scholar] [CrossRef] [PubMed]
- Cohn, P.D.; Gleason, J.A.; Rudowski, E.; Tsai, S.M.; Genese, C.A.; Fagliano, J.A. Community outbreak of legionellosis and an environmental investigation into a community water system. Epidemiol. Infect. 2015, 143, 1322–1331. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, S.; Rodriguez-Martinez, S.; Sharaby, Y.; Pecellin, M.; Brettar, I. Spatial distribution of Legionella pneumophila MLVA-genotypes in a drinking water system. Water Res. 2015, 77, 119–132. [Google Scholar] [CrossRef]
- Sánchez-Busó, L.; Olmos, M.P.; Camaró, M.L.; Adrián, F.; Calafat, J.M.; González-Candelas, F. Phylogenetic analysis of environmental Legionella pneumophila isolates from an endemic area (Alcoy, Spain). Infect. Genet. Evol. 2015, 30, 45–54. [Google Scholar] [CrossRef]
- Nguyen, C.; Elfland, C.; Edwards, M. Impact of advanced water conservation features and new copper pipe on rapid chloramine decay and microbial regrowth. Water Res. 2012, 46, 611–621. [Google Scholar] [CrossRef]
- Zhang, Y.; Edwards, M. Accelerated chloramine decay and microbial growth by nitrification in premise plumbing. Am. Water Work. Assoc. J. 2009, 101, 51. [Google Scholar] [CrossRef]
- LeChevallier, M.W.; Lowry, C.D.; Lee, R.G.; Gibbon, D.L. Examining the relationship between iron corrosion and the disinfection of biofilm bacteria. J. Am. Water Work. Assoc. 1993, 85, 111–123. [Google Scholar] [CrossRef]
- Wang, H.; Masters, S.; Hong, Y.; Stallings, J.; Falkinham, J.O.; Edwards, M.A.; Pruden, A. Effect of Disinfectant, Water Age, and Pipe Material on Occurrence and Persistence of Legionella, mycobacteria, Pseudomonas aeruginosa, and Two Amoebas. Environ. Sci. Technol. 2012, 46, 11566–11574. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Griffin, A.; Edwards, M. Nitrification in premise plumbing: Role of phosphate, pH and pipe corrosion. Environ. Sci. Technol. 2008, 42, 4280–4284. [Google Scholar] [CrossRef] [PubMed]
- Långmark, J.; Storey, M.V.; Ashbolt, N.J.; Stenström, T.-A. The effects of UV disinfection on distribution pipe biofilm growth and pathogen incidence within the greater Stockholm area, Sweden. Water Res. 2007, 41, 3327–3336. [Google Scholar] [CrossRef] [PubMed]
- Weinrich, L.A.; Weinrich, L.A.; Jjemba, P.K.; Giraldo, E.; LeChevallier, M.W. Implications of organic carbon in the deterioration of water quality in reclaimed water distribution systems. Water Res. 2010, 44, 5367–5375. [Google Scholar] [CrossRef] [PubMed]
- Thomas, V.; Bouchez, T.; Nicolas, V.; Robert, S.; Loret, J.F.; Lévi, Y. Amoebae in domestic water systems: Resistance to disinfection treatments and implication in Legionella persistence. J. Appl. Microbiol. 2004, 97, 950–963. [Google Scholar] [CrossRef]
- Mustapha, P.; Epalle, T.; Allegra, S.; Girardot, F. Monitoring of Legionella pneumophila viability after chlorine dioxide treatment using flow cytometry. Res. Microbiol. 2015, 166, 215–219. [Google Scholar] [CrossRef]
- Singer, P.C. Control of disinfection by-products in drinking water. J. Environ. Eng. 1994, 120, 727–744. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. DBP formation during chlorination and chloramination: Effect of reaction time, pH, dosage, and temperature. J. Am. Water Work. Assoc. 2008, 100, 82–95. [Google Scholar] [CrossRef]
- Dupuy, M.; Mazoua, S.; Berne, F.; Bodet, C.; Garrec, N.; Herbelin, P.; Menard-Szczebara, F.; Oberti, S.; Rodier, M.-H.; Soreau, S.; et al. Efficiency of water disinfectants against Legionella pneumophila and Acanthamoeba. Water Res. 2011, 45, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Fisher, I.; Kastl, G.; Sathasivan, A. A suitable model of combined effects of temperature and initial condition on chlorine bulk decay in water distribution systems. Water Res. 2012, 46, 3293–3303. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, L.; Viegas, R.; Covas, D.I.; Menaia, J. Modelling chlorine residual decay as influenced by temperature. Water Environ. J. 2015, 29, 331–337. [Google Scholar] [CrossRef]
- Coniglio, M.A.; Melada, S.; Yassin, M.H. Monochloramine for controlling Legionella in biofilms: How much we know? J. Nat. Sci. 2015, 1, 44. [Google Scholar]
- Pioneer. Sodium Hypochlorite Handbook. Available online: http://www.forceflow.com/hypochlorite/PioneerHypo.pdf (accessed on 27 June 2017).
- Olin Corporation. Sodium Hypochlorite Manual. Available online: https://olinchloralkali.com/wp-content/uploads/2016/11/Olin_NaClO_Handbook.pdf (accessed on 21 June 2016).
- Muraca, P.; Stout, J.E.; Yu, V.L. Comparative assessment of chlorine, heat, ozone, and UV light for killing Legionella pneumophila within a model plumbing system. Appl. Environ. Microbiol. 1987, 53, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Garner, E.; Zhu, N.; Strom, L.; Edwards, M.; Pruden, A. A human exposome framework for guiding risk management and holistic assessment of recycled water quality. Environ. Sci. Water Res. Technol. 2016. [Google Scholar] [CrossRef]
- Bond, T.; Goslan, E.H.; Parsons, S.A.; Jefferson, B. Treatment of disinfection by-product precursors. Environ. Technol. 2011, 32, 1–25. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, L.; Zeng, G.; Deng, H.; Li, G. Impact of total organic carbon and chlorine to ammonia ratio on nitrification in a bench-scale drinking water distribution system. Front. Environ. Sci. Eng. China 2010, 4, 430–437. [Google Scholar] [CrossRef]
- Liu, W.; Wu, H.; Wang, Z.; Ong, S.L.; Hu, J.Y.; Ng, W.J. Investigation of assimilable organic carbon (AOC) and bacterial regrowth in drinking water distribution system. Water Res. 2002, 36, 891–898. [Google Scholar] [CrossRef]
- Liu, X.; Wang, J.; Liu, T.; Kong, W.; He, X.; Jin, Y.; Zhang, B. Effects of assimilable organic carbon and free chlorine on bacterial growth in drinking water. PLoS ONE 2015, 10, e0128825. [Google Scholar] [CrossRef]
- van der Kooij, D.; Veenendaal, H.R.; Scheffer, W.J.H. Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res. 2005, 39, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.K. Organic Carbon Generation Mechanisms in Main and Premise Distribution Systems. Masters’ Thesis, Virginia Tech, Blacksburg, VA, USA, 2012. [Google Scholar]
- Connell, M.; Stenson, A.; Weinrich, L.; LeChevallier, M.; Boyd, S.L.; Ghosal, R.R.; Dey, R.; Whelton, A.J. PEX and PP Water Pipes: Assimilable Carbon, Chemicals, and Odors. J. Am. Water Work. Assoc. 2016, 108, E192–E204. [Google Scholar] [CrossRef]
- Williams, K.; Pruden, A.; Falkinham, J.O.; Edwards, M. Relationship between Organic Carbon and Opportunistic Pathogens in Simulated Glass Water Heaters. Pathogens 2015, 4, 355–372. [Google Scholar] [CrossRef]
- Masten, S.J.; Davies, S.H.; Mcelmurry, S.P. Flint Water Crisis: What Happened and Why? J. Am. Water Work. Assoc. 2016, 108, 22–34. [Google Scholar] [CrossRef] [PubMed]
- van der Kooij, D.; Veenendaal, H.R.; Italiaander, R.; van der Mark, E.J.; Dignum, M. Primary-Colonizing Betaproteobacteriales Play a Key Role in Growth of Legionella Pneumophila in Biofilms on Surfaces Exposed to Drinking Water Treated By Slow Sand Filtration. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Dai, D.; Proctor, C.R.; Williams, K.; Edwards, M.A.; Pruden, A. Mediation of effects of biofiltration on bacterial regrowth, Legionella pneumophila, and the microbial community structure under hot water plumbing conditions. Environ. Sci. Water Res. Technol. 2018, 4, 183–194. [Google Scholar] [CrossRef]
- Van der Kooij, D.; van der Wielen, P.W.; Rosso, D.; Shaw, A.; Borchardt, D.; Ibisch, R.; Apgar, D.; Witherspoon, J.; Di Toro, D.M.; Paquin, P.R. Microbial Growth in Drinking Water Supplies; Iwa Publishing: London, UK, 2013. [Google Scholar]
- Wullings, B.A.; Bakker, G.; van der Kooij, D. Concentration and Diversity of Uncultured Legionella spp. in Two Unchlorinated Drinking Water Supplies with Different Concentrations of Natural Organic Matter. Appl. Environ. Microbiol. 2011, 77, 634–641. [Google Scholar] [CrossRef]
- Proctor, C.R.; Dai, D.; Edwards, M.A.; Pruden, A. Interactive effects of temperature, organic carbon, and pipe material on microbiota composition and Legionella pneumophila in hot water plumbing systems. Microbiome 2017, 5, 130. [Google Scholar] [CrossRef]
- Chang, S.L. Modern concept of disinfection. J. Sanit. Eng. Div. 1971, 97, 689–707. [Google Scholar]
- Ward, N.R.; Wolfe, R.; Olson, B.H. Effect of pH, application technique, and chlorine-to-nitrogen ratio on disinfectant activity of inorganic chloramines with pure culture bacteria. Appl. Environ. Microbiol. 1984, 48, 508–514. [Google Scholar] [CrossRef]
- Vatansever, C.; Irfan, T. Survival of Biofilm-Associated Legionella pneumophila Exposed to Various Stressors. Water Environ. Res. 2015, 87, 227. [Google Scholar] [CrossRef] [PubMed]
- Wadowsky, R.M.; Wolford, R.; McNamara, A.; Yee, R.B. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 1985, 49, 1197–1205. [Google Scholar] [CrossRef] [PubMed]
- States, S.J.; Conley, L.F.; Towner, S.G.; Wolford, R.S.; Stephenson, T.E.; McNamara, A.M.; Wadowsky, R.M.; Yee, R.B. An alkaline approach to treating cooling towers for control of Legionella pneumophila. Appl. Environ. Microbiol. 1987, 53, 1775–1779. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, W.J.; Pruden, A.; Edwards, M.A. Convective mixing in distal pipes exacerbates Legionella pneumophila growth in hot water plumbing. Pathogens 2016, 5, 29. [Google Scholar] [CrossRef]
- Wang, H.; Edwards, M.; Falkinham, J.O., III; Pruden, A. Molecular Survey of the Occurrence of Legionella spp., Mycobacterium spp., Pseudomonas aeruginosa, and Amoeba Hosts in Two Chloraminated Drinking Water Distribution Systems. Appl. Environ. Microbiol. 2012, 78, 6285–6294. [Google Scholar] [CrossRef]
- Masters, S.; Wang, H.; Pruden, A.; Edwards, M.A. Redox gradients in distribution systems influence water quality, corrosion, and microbial ecology. Water Res. 2015, 68, 140–149. [Google Scholar] [CrossRef]
- Feeley, J.; Gorman, G.; Weaver, R.; Mackel, D.C.; Smith, H. Primary isolation media for Legionnaires disease bacterium. J. Clin. Microbiol. 1978, 8, 320–325. [Google Scholar]
- Warren, W.J.; Miller, R.D. Growth of Legionnaires disease bacterium (Legionella pneumophila) in chemically defined medium. J. Clin. Microbiol. 1979, 10, 50–55. [Google Scholar] [CrossRef]
- Rakić, A.; Štambuk-Giljanović, N. Physical and chemical parameter correlations with technical and technological characteristics of heating systems and the presence of Legionella spp. in the hot water supply. Environ. Monit. Assess. 2016, 188, 1–12. [Google Scholar] [CrossRef]
- Bargellini, A.; Marchesi, I.; Righi, E.; Ferrari, A.; Cencetti, S.; Borella, P.; Rovesti, S. Parameters predictive of Legionella contamination in hot water systems: Association with trace elements and heterotrophic plate counts. Water Res. 2011, 45, 2315–2321. [Google Scholar] [CrossRef]
- Pieper, K.J.; Tang, M.; Edwards, M.A. Flint Water Crisis Caused By Interrupted Corrosion Control: Investigating “Ground Zero” Home. Environ. Sci. Technol. 2017, 51, 2007–2014. [Google Scholar] [CrossRef]
- Ramseier, M.K.; Ramseier, M.K.; Peter, A.; Traber, J.; von Gunten, U. Formation of assimilable organic carbon during oxidation of natural waters with ozone, chlorine dioxide, chlorine, permanganate, and ferrate. Water Res. 2011, 45, 2002–2010. [Google Scholar] [CrossRef] [PubMed]
- van der Wielen, P.W.; van der Kooij, D. Nontuberculous mycobacteria, fungi, and opportunistic pathogens in unchlorinated drinking water in The Netherlands. Appl. Environ. Microbiol. 2013, 79, 825–834. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.E.; Yu, V.L.; Yee, Y.C.; Vaccarello, S.; Diven, W.; Lee, T.C. Legionella pneumophila in residential water supplies: Environmental surveillance with clinical assessment for Legionnaires’ disease. Epidemiol. Infect. 1992, 109, 49–57. [Google Scholar]
- Reeves, M.; Pine, L.; Hutner, S.; George, J.; Harrell, W. Metal requirements of Legionella pneumophila. J. Clin. Microbiol. 1981, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Proctor, C.R.; Edwards, M.A.; Pryor, M.; Santo Domingo, J.W.; Ryu, H.; Camper, A.K.; Olson, A.; Pruden, A. Microbial community response to chlorine conversion in a chloraminated drinking water distribution system. Environ. Sci. Technol. 2014, 48, 10624–10633. [Google Scholar] [CrossRef]
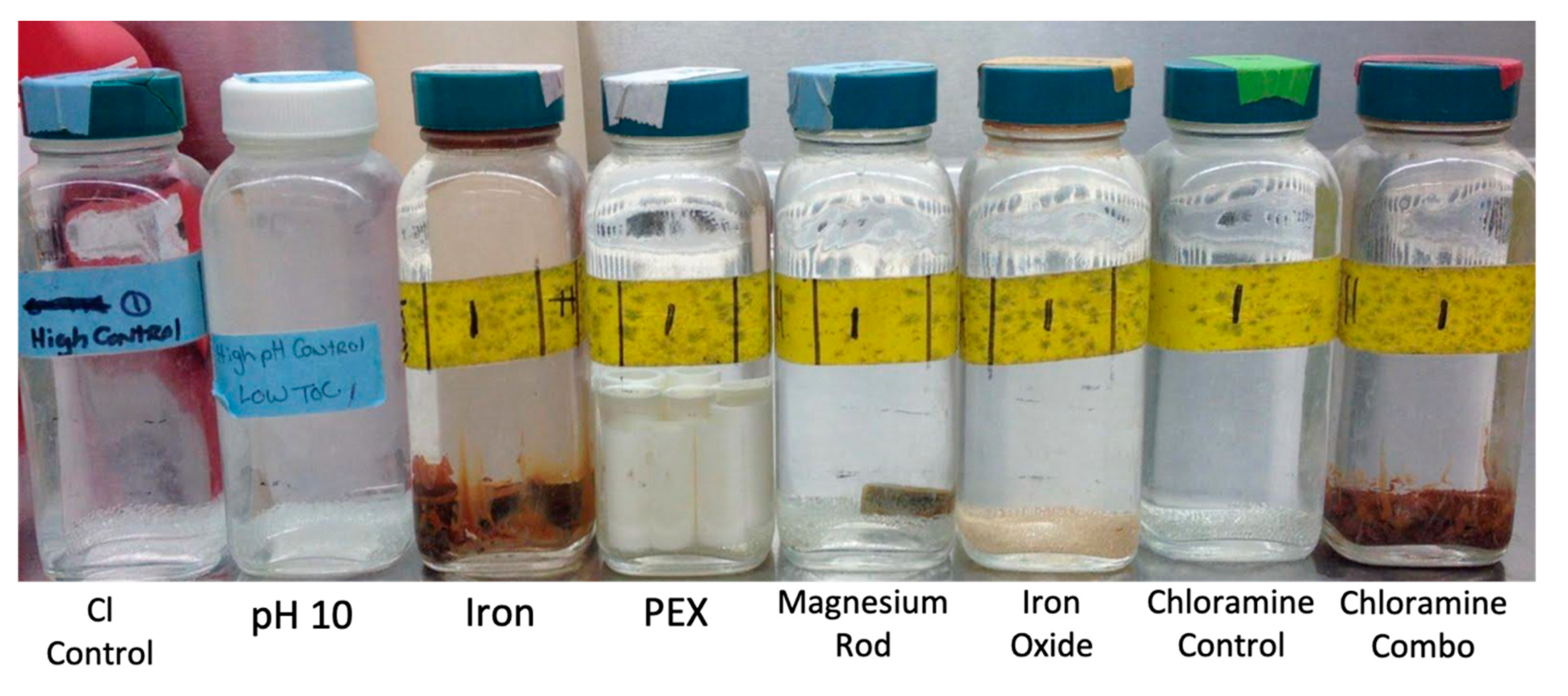

| SGWH Condition 1 | Premise Plumbing Material | Glass Beads? | Cl Added? | NH3 Added | pH of Influent 2 |
|---|---|---|---|---|---|
| No Cl | None | No | No | No | 7.5 ± 0.1 |
| Cl control | None | No | Yes | No | 7.5 ± 0.1 |
| ClNH2 | None | Yes | Yes | Yes | 7.5 ± 0.1 |
| ClNH2 combo | Iron and Magnesium anode rod | Yes | Yes | Yes | 7.5 ± 0.1 |
| Iron metal | Iron coupon | Yes | Yes | No | 7.5 ± 0.1 |
| Iron oxide | Fe(OH)3 (rust) | Yes | Yes | No | 7.5 ± 0.1 |
| Mg | Magnesium anode rod | Yes | Yes | No | 7.5 ± 0.1 |
| PEX | PEX coupons | Yes | Yes | No | 7.5 ± 0.1 |
| pH 10 | None | Yes | Yes | No | 10 ± 0.1 |
 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, R.L.; Harrison, K.; Proctor, C.R.; Martin, A.; Williams, K.; Pruden, A.; Edwards, M.A. Chlorine Disinfection of Legionella spp., L. pneumophila, and Acanthamoeba under Warm Water Premise Plumbing Conditions. Microorganisms 2020, 8, 1452. https://doi.org/10.3390/microorganisms8091452
Martin RL, Harrison K, Proctor CR, Martin A, Williams K, Pruden A, Edwards MA. Chlorine Disinfection of Legionella spp., L. pneumophila, and Acanthamoeba under Warm Water Premise Plumbing Conditions. Microorganisms. 2020; 8(9):1452. https://doi.org/10.3390/microorganisms8091452
Chicago/Turabian StyleMartin, Rebekah L., Kara Harrison, Caitlin R. Proctor, Amanda Martin, Krista Williams, Amy Pruden, and Marc A. Edwards. 2020. "Chlorine Disinfection of Legionella spp., L. pneumophila, and Acanthamoeba under Warm Water Premise Plumbing Conditions" Microorganisms 8, no. 9: 1452. https://doi.org/10.3390/microorganisms8091452
APA StyleMartin, R. L., Harrison, K., Proctor, C. R., Martin, A., Williams, K., Pruden, A., & Edwards, M. A. (2020). Chlorine Disinfection of Legionella spp., L. pneumophila, and Acanthamoeba under Warm Water Premise Plumbing Conditions. Microorganisms, 8(9), 1452. https://doi.org/10.3390/microorganisms8091452





