Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense
Abstract
1. Introduction
2. Materials and Methods
2.1. C. parvum and Cell Lines
2.2. Infection Models and Infection Measurements
2.3. Agilent Microarray Analysis
2.4. Quantitative Real-Time PCR and Western Blot
2.5. Statistical Analysis
3. Results
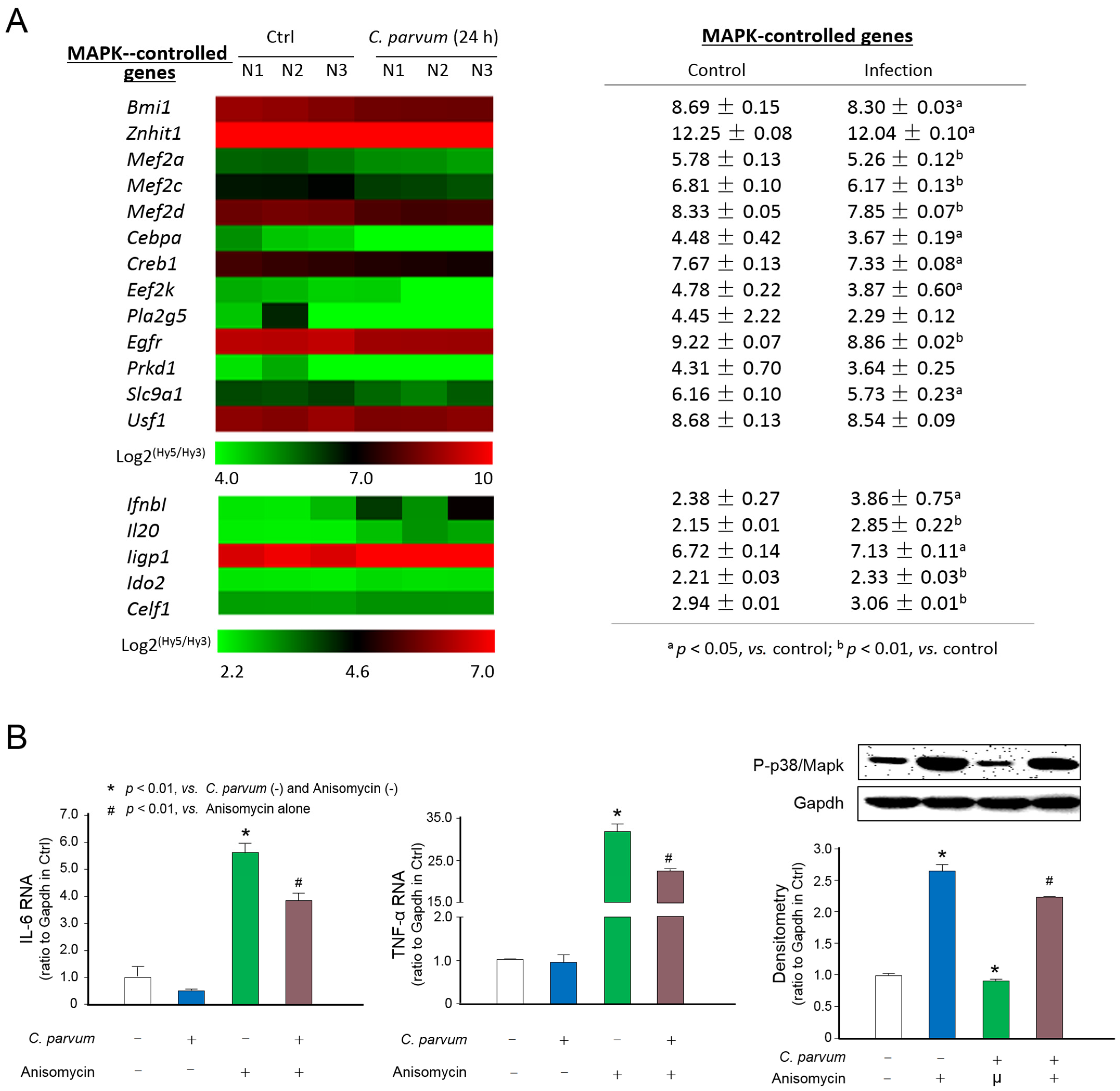
3.1. Suppression of MAPK Signaling in Intestinal Epithelium Following C. parvum Infection
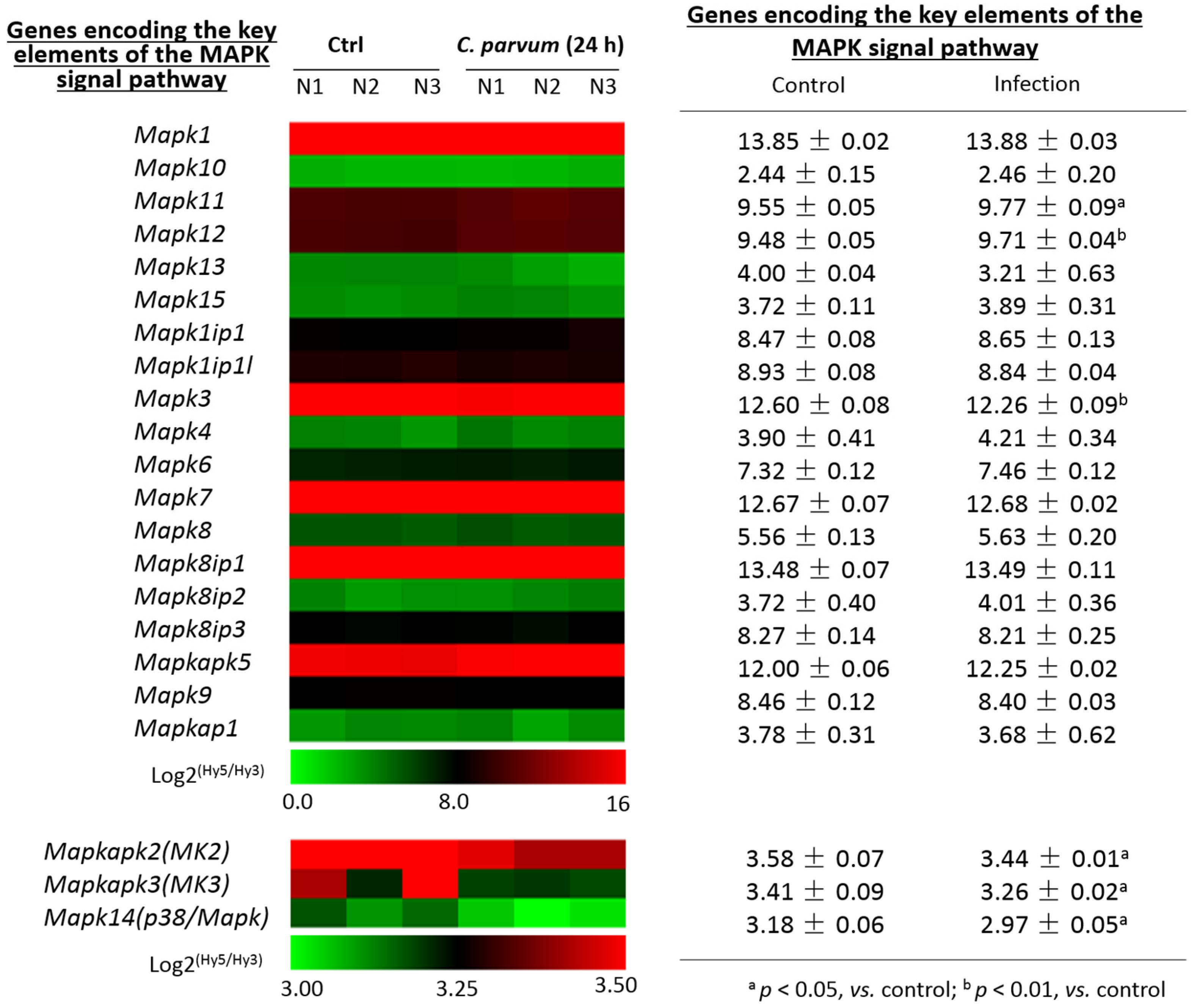
3.2. Expression Profile of Genes Encoding the Key Elements of the MAPK Signaling Pathway in Intestinal Epithelial Cells Following Infection
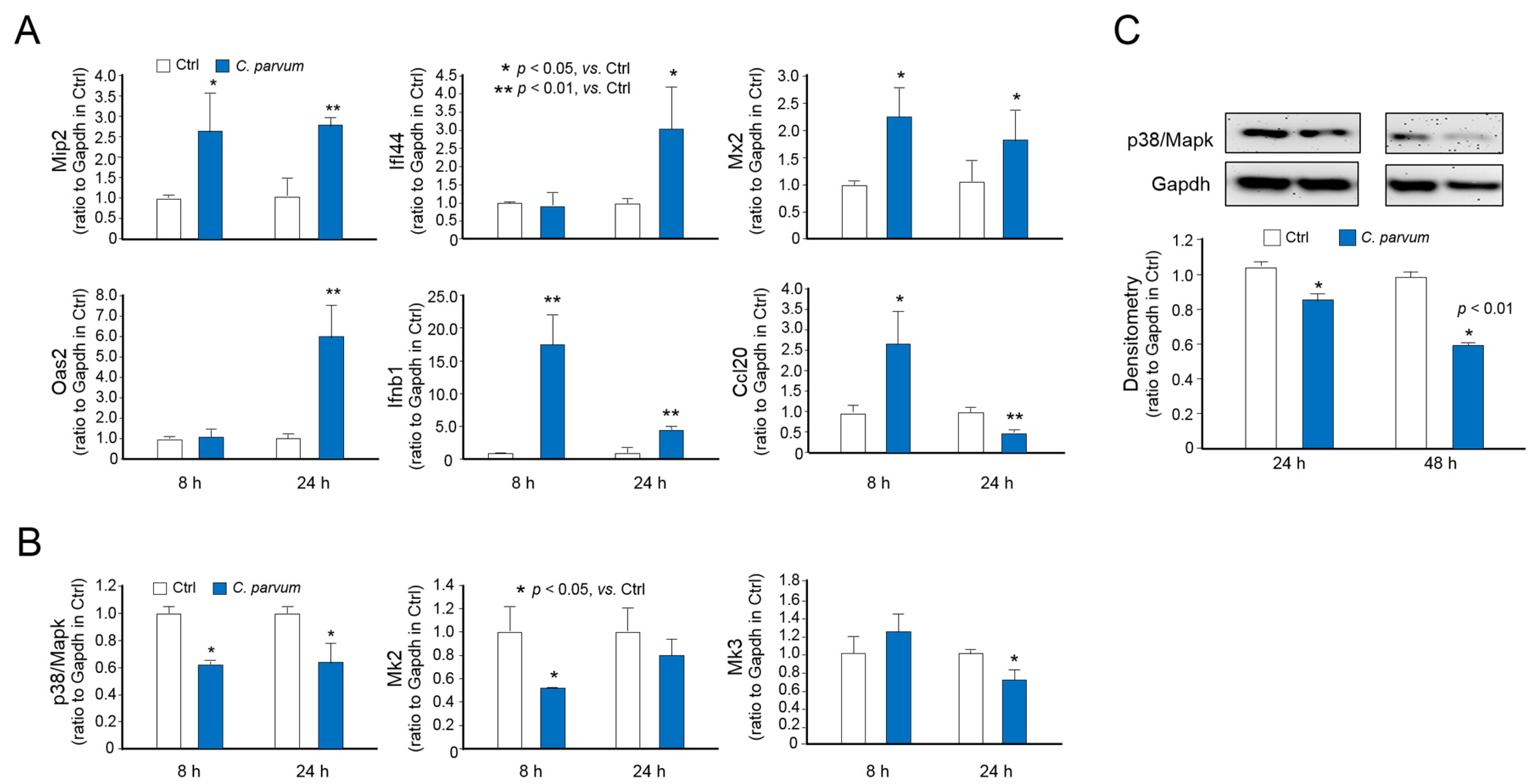
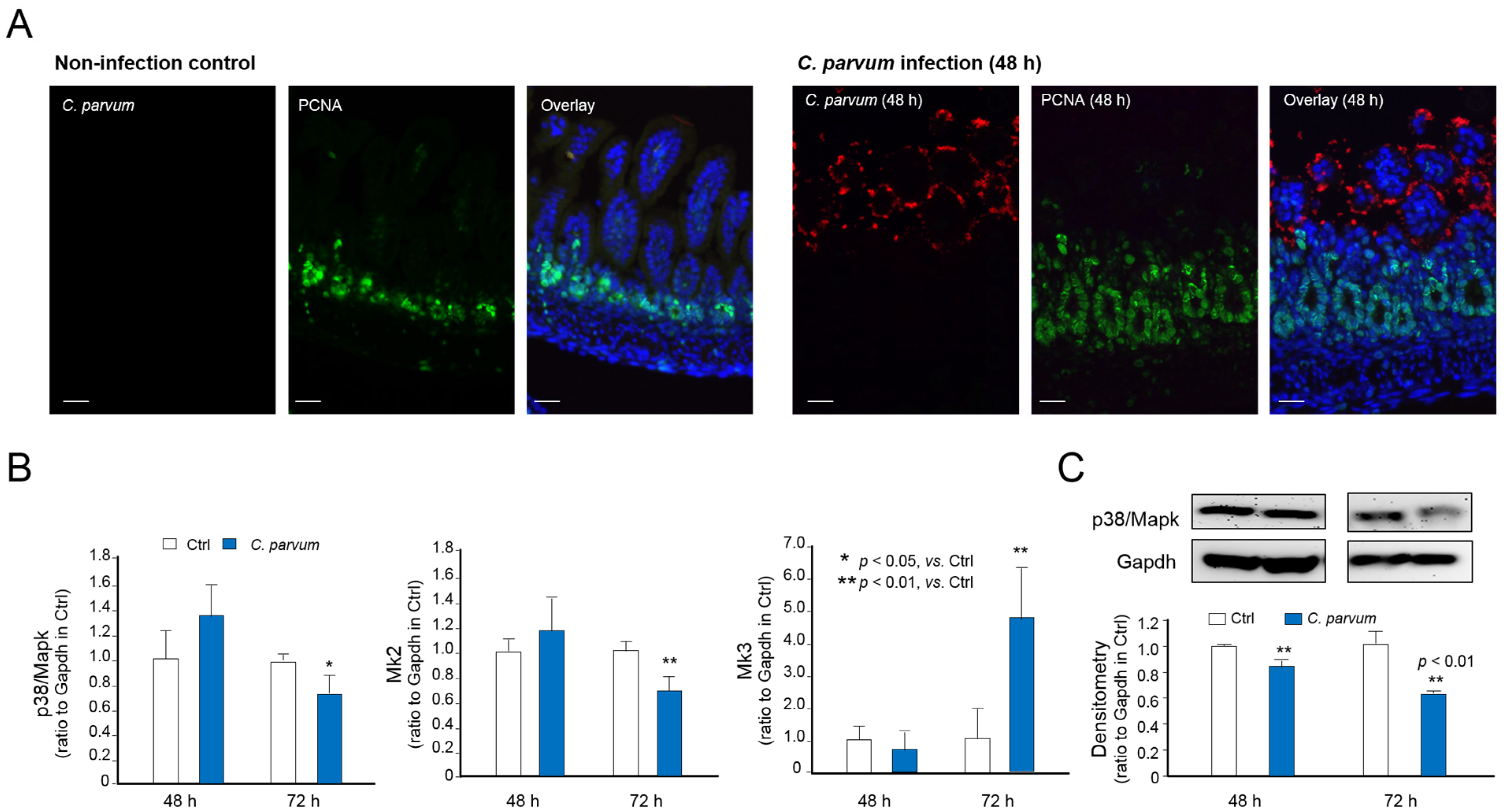
3.3. Downregulation of p38MAPK, MK2 and MK3 Genes in Infected Host Cells of Various Models of C. parvum Infection
3.4. Suppression of MAPK Signaling Impairs Intestinal Epithelial Innate Defense against C. parvum
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalmers, R.M.; Katzer, F. Looking for cryptosporidium: The application of advances in detection and diagnosis. Trends Parasitol. 2013, 5, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 13, 1667–1685. [Google Scholar] [CrossRef] [PubMed]
- Striepen, B. Parasitic infections: Time to tackle cryptosporidiosis. Nature 2013, 7475, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Checkley, W.; White, A.C., Jr.; Jaganath, D.; Arrowood, M.J.; Chalmers, R.M.; Chen, X.M.; Fayer, R.; Griffiths, J.K.; Guerrant, R.L.; Hedstrom, L.; et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015, 1, 85–94. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, gems): A prospective, case-control study. Lancet 2013, 9888, 209–222. [Google Scholar] [CrossRef]
- Putignani, L.; Menichella, D. Global distribution, public health and clinical impact of the protozoan pathogen cryptosporidium. Interdiscip. Perspect Infect. Dis. 2010, 2010, 753512. [Google Scholar] [CrossRef]
- Chen, X.M.; Keithly, J.S.; Paya, C.V.; LaRusso, N.F. Cryptosporidiosis. N. Engl. J. Med. 2002, 22, 1723–1731. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 3, 141–153. [Google Scholar] [CrossRef]
- McDonald, V.; Korbel, D.S.; Barakat, F.M.; Choudhry, N.; Petry, F. Innate immune responses against cryptosporidium parvum infection. Parasite Immunol. 2013, 2, 55–64. [Google Scholar] [CrossRef]
- Laurent, F.; Kagnoff, M.F.; Savidge, T.C.; Naciri, M.; Eckmann, L. Human intestinal epithelial cells respond to cryptosporidium parvum infection with increased prostaglandin h synthase 2 expression and prostaglandin e2 and f2alpha production. Infect. Immun. 1998, 4, 1787–1790. [Google Scholar] [CrossRef]
- Pollok, R.C.; Farthing, M.J.; Bajaj-Elliott, M.; Sanderson, I.R.; McDonald, V. Interferon gamma induces enterocyte resistance against infection by the intracellular pathogen cryptosporidium parvum. Gastroenterology 2001, 1, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Barakat, F.M.; McDonald, V.; Di Santo, J.P.; Korbel, D.S. Roles for nk cells and an nk cell-independent source of intestinal gamma interferon for innate immunity to cryptosporidium parvum infection. Infect. Immun. 2009, 11, 5044–5049. [Google Scholar] [CrossRef] [PubMed]
- Choudhry, N.; Petry, F.; van Rooijen, N.; McDonald, V. A protective role for interleukin 18 in interferon gamma-mediated innate immunity to cryptosporidium parvum that is independent of natural killer cells. J. Infect. Dis. 2012, 1, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bedi, B.; McNair, N.N.; Forster, I.; Mead, J.R. Il-18 cytokine levels modulate innate immune responses and cryptosporidiosis in mice. J. Eukaryot. Microbiol. 2015, 1, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Gong, A.Y.; Eischeid, A.N.; Chen, X.M. Mir-27b targets ksrp to coordinate tlr4-mediated epithelial defense against cryptosporidium parvum infection. PLoS Pathog. 2012, 5, e1002702. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; O’Hara, S.P.; Nelson, J.B.; Splinter, P.L.; Small, A.J.; Tietz, P.S.; Limper, A.H.; LaRusso, N.F. Multiple tlrs are expressed in human cholangiocytes and mediate host epithelial defense responses to cryptosporidium parvum via activation of nf-kappab. J. Immunol. 2005, 11, 7447–7456. [Google Scholar] [CrossRef]
- Chen, X.M.; Levine, S.A.; Splinter, P.L.; Tietz, P.S.; Ganong, A.L.; Jobin, C.; Gores, G.J.; Paya, C.V.; LaRusso, N.F. Cryptosporidium parvum activates nuclear factor kappab in biliary epithelia preventing epithelial cell apoptosis. Gastroenterology 2001, 7, 1774–1783. [Google Scholar] [CrossRef]
- Liu, J.; Deng, M.; Lancto, C.A.; Abrahamsen, M.S.; Rutherford, M.S.; Enomoto, S. Biphasic modulation of apoptotic pathways in cryptosporidium parvum-infected human intestinal epithelial cells. Infect. Immun. 2009, 2, 837–849. [Google Scholar] [CrossRef]
- Choudhry, N.; Korbel, D.S.; Edwards, L.A.; Bajaj-Elliott, M.; McDonald, V. Dysregulation of interferon-gamma-mediated signalling pathway in intestinal epithelial cells by cryptosporidium parvum infection. Cell Microbiol. 2009, 9, 1354–1364. [Google Scholar] [CrossRef]
- Guesdon, W.; Auray, G.; Pezier, T.; Bussiere, F.I.; Drouet, F.; Le Vern, Y.; Marquis, M.; Potiron, L.; Rabot, S.; Bruneau, A.; et al. Ccl20 displays antimicrobial activity against cryptosporidium parvum, but its expression is reduced during infection in the intestine of neonatal mice. J. Infect. Dis. 2015, 8, 1332–1340. [Google Scholar] [CrossRef]
- Li, X.C.; Jevnikar, A.M.; Grant, D.R. Expression of functional icam-1 and vcam-1 adhesion molecules by an immortalized epithelial cell clone derived from the small intestine. Cell Immunol. 1997, 1, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, T.A.; Bhushal, S.; Kalinke, U.; Wirth, D.; Hauser, H.; Koster, M.; Hornef, M.W. Identification of a predominantly interferon-lambda-induced transcriptional profile in murine intestinal epithelial cells. Front. Immunol. 2017, 8, 1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gong, A.Y.; Ma, S.; Chen, X. Delivery of parasite cdg7_flc_0990 rna transcript into intestinal epithelial cells during cryptosporidium parvum infection suppresses host cell gene transcription through epigenetic mechanisms. Cell. Microbiol. 2017, 11, 1462–5814. [Google Scholar]
- Li, M.; Gong, A.Y.; Zhang, X.T.; Wang, Y.; Mathy, N.W.; Martins, G.A.; Strauss-Soukup, J.K.; Chen, X.M. Induction of a long noncoding rna transcript, nr_045064, promotes defense gene transcription and facilitates intestinal epithelial cell responses against cryptosporidium infection. J. Immunol. 2018, 12, 3630–3640. [Google Scholar] [CrossRef]
- Lantier, L.; Lacroix-Lamande, S.; Potiron, L.; Metton, C.; Drouet, F.; Guesdon, W.; Gnahoui-David, A.; Le Vern, Y.; Deriaud, E.; Fenis, A.; et al. Intestinal cd103+ dendritic cells are key players in the innate immune control of cryptosporidium parvum infection in neonatal mice. PLoS Pathog. 2013, 12, e1003801. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, A.Y.; Ma, S.; Chen, X.; Li, Y.; Su, C.J.; Norall, D.; Chen, J.; Strauss-Soukup, J.K.; Chen, X.M. Delivery of parasite RNA transcripts into infected epithelial cells during cryptosporidium infection and its potential impact on host gene transcription. J. Infect. Dis. 2017, 4, 636–643. [Google Scholar] [CrossRef][Green Version]
- Cuadrado, A.; Corrado, N.; Perdiguero, E.; Lafarga, V.; Munoz-Canoves, P.; Nebreda, A.R. Essential role of p18hamlet/srcap-mediated histone h2a.Z chromatin incorporation in muscle differentiation. EMBO J. 2010, 12, 2014–2025. [Google Scholar] [CrossRef]
- Kim, J.; Hwangbo, J.; Wong, P.K. P38 mapk-mediated bmi-1 down-regulation and defective proliferation in atm-deficient neural stem cells can be restored by akt activation. PLoS ONE 2011, 1, e16615. [Google Scholar] [CrossRef]
- Malcolm, T.; Chen, J.; Chang, C.; Sadowski, I. Induction of chromosomally integrated hiv-1 ltr requires rbf-2 (usf/tfii-i) and ras/mapk signaling. Virus Genes 2007, 2, 215–223. [Google Scholar] [CrossRef]
- Hwang, Y.S.; Oh, S.W.; Park, S.H.; Lee, J.; Yoo, J.A.; Kwon, K.; Park, S.J.; Kim, J.; Yu, E.; Cho, J.Y.; et al. Melanogenic effects of maclurin are mediated through the activation of camp/pka/creb and p38 mapk/creb signaling pathways. Oxid. Med. Cell Longev. 2019, 2019, 9827519. [Google Scholar] [CrossRef]
- Hu, S.B.; Zou, Q.; Lv, X.; Zhou, R.L.; Niu, X.; Weng, C.; Chen, F.; Fan, Y.W.; Deng, Z.Y.; Li, J. 9t18:1 and 11t18:1 activate the mapk pathway to regulate the expression of pla2 and cause inflammation in huvecs. Food Funct. 2020, 1, 649–661. [Google Scholar] [CrossRef]
- Dreas, A.; Mikulski, M.; Milik, M.; Fabritius, C.H.; Brzozka, K.; Rzymski, T. Mitogen-activated protein kinase (mapk) interacting kinases 1 and 2 (mnk1 and mnk2) as targets for cancer therapy: Recent progress in the development of mnk inhibitors. Curr. Med. Chem. 2017, 28, 3025–3053. [Google Scholar] [CrossRef] [PubMed]
- Bardwell, A.J.; Frankson, E.; Bardwell, L. Selectivity of docking sites in mapk kinases. J. Biol. Chem. 2009, 19, 13165–13173. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Kojic, L.Z.; Zhang, L.; Prasad, S.S.; Douglas, R.; Wang, Y.; Cynader, M.S. Anisomycin activates p38 map kinase to induce ltd in mouse primary visual cortex. Brain Res. 2006, 1, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Zanello, G.; Berri, M.; Dupont, J.; Sizaret, P.Y.; D’Inca, R.; Salmon, H.; Meurens, F. Saccharomyces cerevisiae modulates immune gene expressions and inhibits etec-mediated erk1/2 and p38 signaling pathways in intestinal epithelial cells. PLoS ONE 2011, 4, e18573. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.; Chaudhuri, K. Vibrio cholerae o395 outer membrane vesicles modulate intestinal epithelial cells in a nod1 protein-dependent manner and induce dendritic cell-mediated th2/th17 cell responses. J. Biol. Chem. 2013, 6, 4299–4309. [Google Scholar] [CrossRef] [PubMed]
- Heo, I.; Dutta, D.; Schaefer, D.A.; Iakobachvili, N.; Artegiani, B.; Sachs, N.; Boonekamp, K.E.; Bowden, G.; Hendrickx, A.P.A.; Willems, R.J.L.; et al. Modelling cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018, 7, 814–823. [Google Scholar] [CrossRef]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. Sp600125, an anthrapyrazolone inhibitor of jun n-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 24, 13681–13686. [Google Scholar] [CrossRef]
- Xie, H.; Lei, N.; Gong, A.Y.; Chen, X.M.; Hu, G. Cryptosporidium parvum induces sirt1 expression in host epithelial cells through downregulating let-7i. Hum. Immunol. 2014, 8, 760–765. [Google Scholar] [CrossRef]
- Barber, G.N. Host defense, viruses and apoptosis. Cell Death Differ. 2001, 2, 113–126. [Google Scholar] [CrossRef]
- Liu, J.; Drescher, K.M.; Chen, X.M. Micrornas and epithelial immunity. Int. Rev. Immunol. 2009, 3–4, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 9, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Sandri, S.; Hatanaka, E.; Franco, A.G.; Pedrosa, A.M.; Monteiro, H.P.; Campa, A. Serum amyloid a induces ccl20 secretion in mononuclear cells through mapk (p38 and erk1/2) signaling pathways. Immunol. Lett. 2008, 1, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Dommisch, H.; Chung, W.O.; Jepsen, S.; Hacker, B.M.; Dale, B.A. Phospholipase c, p38/mapk, and nf-kappab-mediated induction of mip-3alpha/ccl20 by porphyromonas gingivalis. Innate Immun. 2010, 4, 226–234. [Google Scholar] [CrossRef]
- Halle, M.; Gomez, M.A.; Stuible, M.; Shimizu, H.; McMaster, W.R.; Olivier, M.; Tremblay, M.L. The leishmania surface protease gp63 cleaves multiple intracellular proteins and actively participates in p38 mitogen-activated protein kinase inactivation. J. Biol. Chem. 2009, 11, 6893–6908. [Google Scholar] [CrossRef]
- Turk, B.E. Manipulation of host signalling pathways by anthrax toxins. Biochem. J. 2007, 3, 405–417. [Google Scholar] [CrossRef]
- Trosky, J.E.; Liverman, A.D.; Orth, K. Yersinia outer proteins: Yops. Cell Microbiol. 2008, 3, 557–565. [Google Scholar] [CrossRef]
- Roy, C.R.; Mocarski, E.S. Pathogen subversion of cell-intrinsic innate immunity. Nat. Immunol. 2007, 11, 1179–1187. [Google Scholar] [CrossRef]
- Mukherjee, S.; Keitany, G.; Li, Y.; Wang, Y.; Ball, H.L.; Goldsmith, E.J.; Orth, K. Yersinia yopj acetylates and inhibits kinase activation by blocking phosphorylation. Science 2006, 5777, 1211–1214. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, W.; Li, J.; Gong, A.-Y.; Deng, S.; Li, M.; Wang, Y.; Mathy, N.W.; Feng, Y.; Xiao, L.; Chen, X.-M. Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense. Microorganisms 2021, 9, 151. https://doi.org/10.3390/microorganisms9010151
He W, Li J, Gong A-Y, Deng S, Li M, Wang Y, Mathy NW, Feng Y, Xiao L, Chen X-M. Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense. Microorganisms. 2021; 9(1):151. https://doi.org/10.3390/microorganisms9010151
Chicago/Turabian StyleHe, Wei, Juan Li, Ai-Yu Gong, Silu Deng, Min Li, Yang Wang, Nicholas W. Mathy, Yaoyu Feng, Lihua Xiao, and Xian-Ming Chen. 2021. "Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense" Microorganisms 9, no. 1: 151. https://doi.org/10.3390/microorganisms9010151
APA StyleHe, W., Li, J., Gong, A.-Y., Deng, S., Li, M., Wang, Y., Mathy, N. W., Feng, Y., Xiao, L., & Chen, X.-M. (2021). Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense. Microorganisms, 9(1), 151. https://doi.org/10.3390/microorganisms9010151




