Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Randomized Clinical Trial
2.2.1. Trial Design
2.2.2. Participants
2.2.3. Interventions and Outcomes
2.2.4. Sample Size
2.2.5. Randomization and Blinding
2.2.6. Statistical Methods
3. Results
3.1. Periodontal Parameters
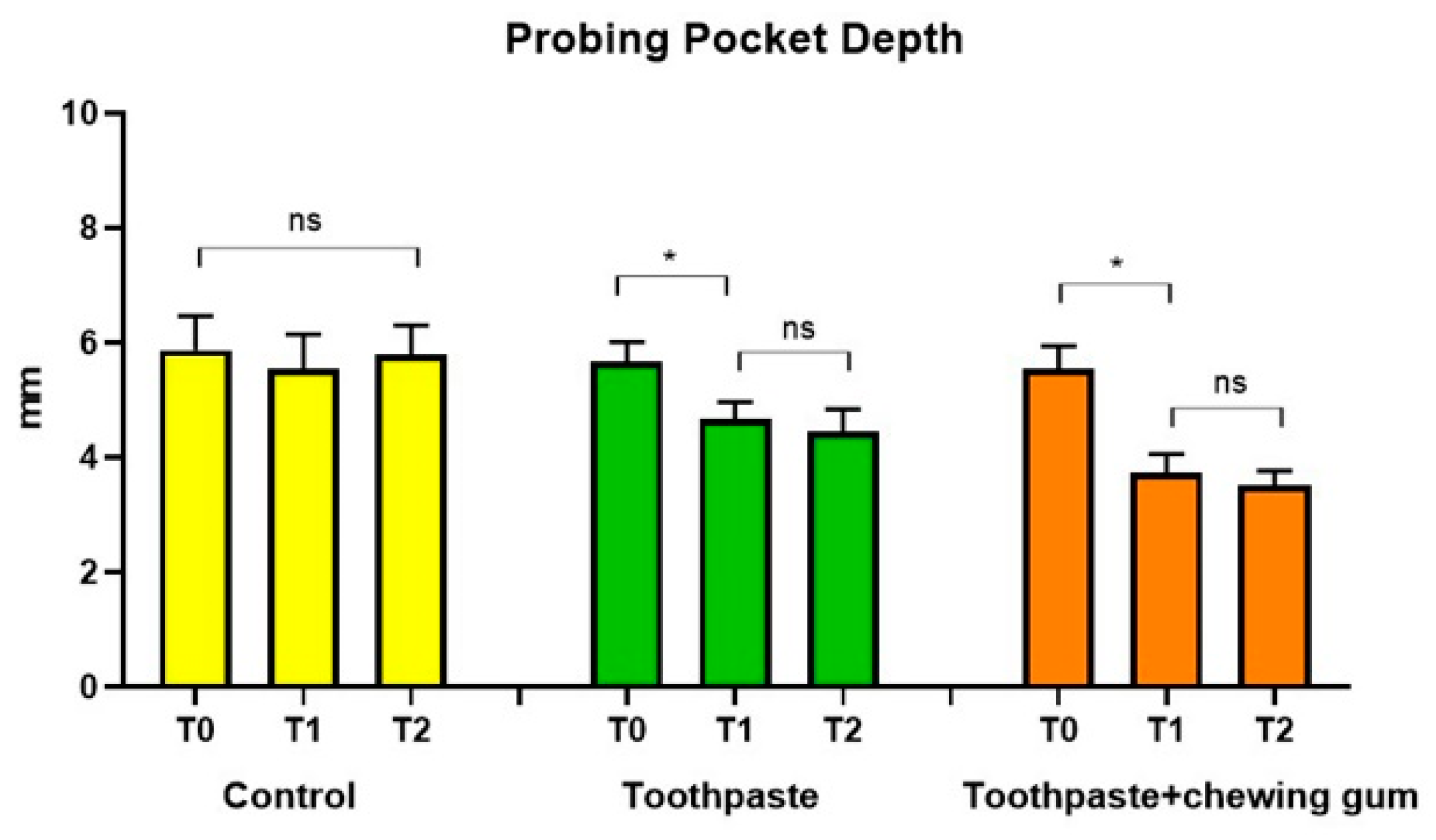
3.1.1. Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL)
3.1.2. Bleeding on Probing (BOP), Bleeding Score (BS), Sulcus Bleeding Index (SBI), Approximal Plaque Index (API), Plaque Index (PI) and Pathological Sites (PS)
3.1.3. Adherent Gingiva (AG) and Gingival Recession (GR)
3.2. Microbiological Parameters
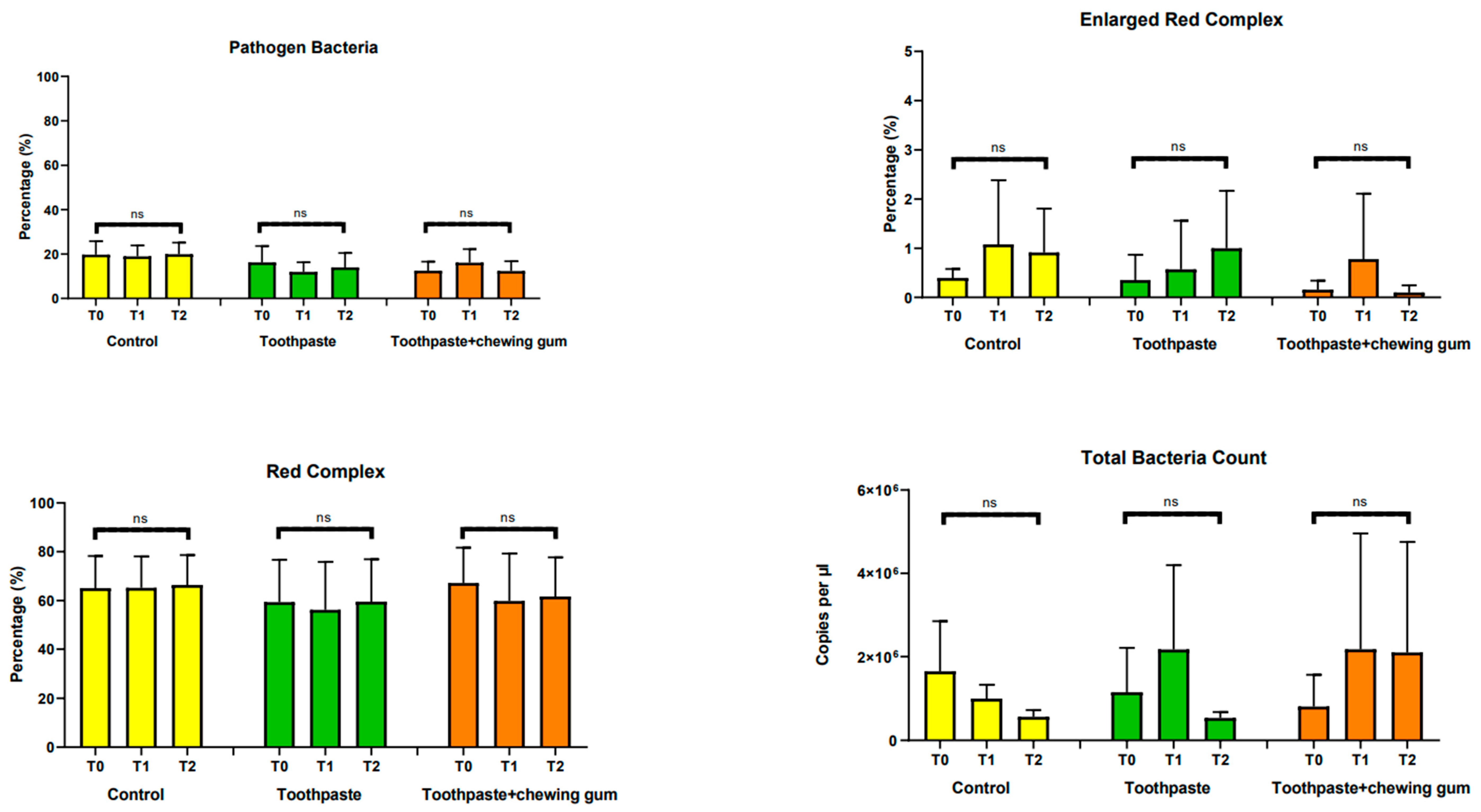
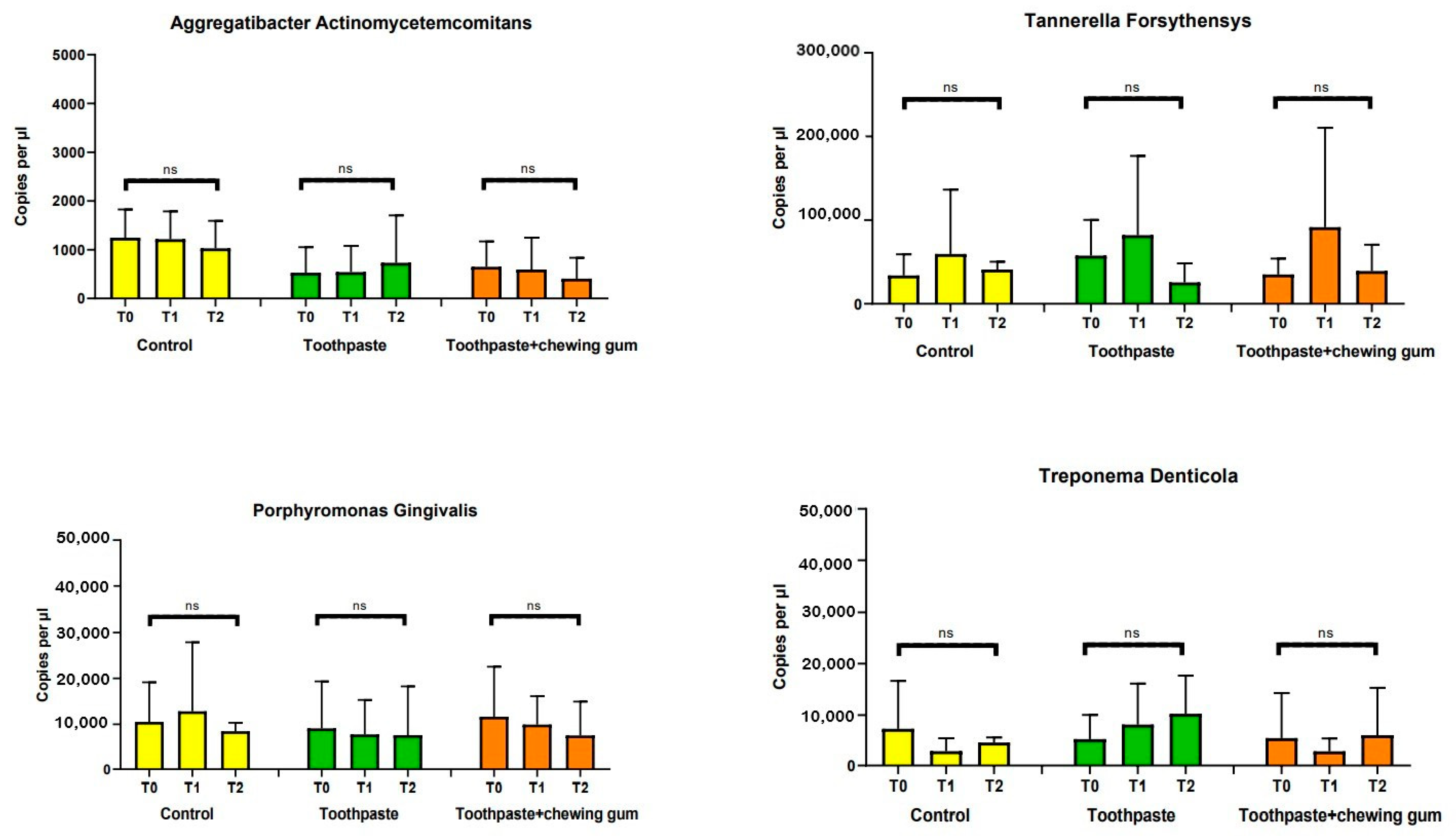
3.2.1. Pathogen Bacteria, Enlarged Red Complex, Red Complex, Total Bacteria Count, Aggregatibacter Actinomycetemcomitans, Tannerella Forsythia, Porphyromonas Gingivalis and Troponema Denticola
3.2.2. Orange Complex, Prevotella Intermedia and Fusobacterium Nucleatum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baelum, V.; Lopez, R. Periodontal disease epidemiology—Learned and unlearned? Periodontology 2000 2013, 62, 37–58. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinane, D.F.; Chestnutt, I.G. Smoking and periodontal disease. Crit. Rev. Oral Biol. Med. 2000, 11, 356–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilton, J.M.; Griffiths, G.S.; Curtis, M.A.; Maiden, M.F.; Gillett, I.R.; Wilson, D.T.; Sterne, J.A.; Johnson, N.W. Detection of high-risk groups and individuals for periodontal diseases. Systemic predisposition and markers of general health. J. Clin. Periodontol. 1988, 15, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Barr, C.; Lopez, M.R.; Rua-Dobles, A. Periodontal changes by HIV serostatus in a cohort of homosexual and bisexual men. J. Clin. Periodontol. 1992, 19, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Shapira, L.; Wilensky, A.; Kinane, D.F. Effect of genetic variability on the inflammatory response to periodontal infection. J. Clin. Periodontol. 2005, 32, 72–86. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Berezow, A.B.; Darveau, R.P. Microbial shift and periodontitis. Periodontology 2000 2011, 55, 36–47. [Google Scholar] [CrossRef]
- Mombelli, A. Microbial colonization of the periodontal pocket and its significance for periodontal therapy. Periodontology 2000 2018, 76, 85–96. [Google Scholar] [CrossRef]
- Invernici, M.M.; Salvador, S.L.; Silva, P.; Soares, M.; Casarin, R.; Palioto, D.B.; Souza, S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef] [Green Version]
- Francino, M. Antibiotics and the human gut microbiome: Dysbioses and accumulation of resistances. Front. Microbiol. 2016, 6, 1543–1545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joint FAO/WHO Working Group. Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food; WHO: London, UK, 2002. [Google Scholar]
- İnce, G.; Gürsoy, H.; İpçi, Ş.D.; Cakar, G.; Emekli-Alturfan, E.; Yılmaz, S. Clinical and Biochemical Evaluation of Lozenges Containing Lactobacillus reuteri as an Adjunct to Non-Surgical Periodontal Therapy in Chronic Periodontitis. J. Periodontol. 2015, 86, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.P.; Gujjari, S.K.; Chandrasekhar, V.S. Evaluation of the effect of probiotic (inersan®) alone, combination of probiotic with doxycycline and doxycycline alone on aggressive periodontitis—A clinical and microbiological study. J. Clin. Diagn. Res. 2013, 7, 595–600. [Google Scholar] [PubMed]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Yilmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1- year follow- up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo- controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef] [Green Version]
- Vivekananda, M.R.; Vandana, K.L.; Bhat, K.G. Effect of the probiotic Lactobacilli reuteri (Prodentis) in the management of periodontal disease: A preliminary randomized clinical trial. J. Oral Microbiol. 2010, 2, 5344. [Google Scholar] [CrossRef]
- Ainamo, J.; Bay, I. Problems and proposals for recording gingivitis and plaque. Int. Dent. J. 1975, 25, 229–235. [Google Scholar]
- Mühlemann, H.R.; Son, S. Gingival sulcus bleeding—A leading symptom in initial gingivitis. Helv. Odontol. Acta 1971, 15, 107–113. [Google Scholar]
- Lange, D.E.; Plagmann, H.C.; Eenboom, A.; Promesberger, A. Klinische Bewertungsverahren zur Objektivierung der Mundhygiene [Clinical methods for the objective evaluation of oral hygiene]. Dtsch. Zahnarztl. Z. 1977, 32, 44–47. (In Germany) [Google Scholar]
- Martelli, F.S.; Fanti, E.; Rosati, C.; Martelli, M.; Bacci, G.; Martelli, M.L.; Medico, E. Long-term efficacy of microbiology-driven periodontal laser-assisted therapy. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 423–431. [Google Scholar] [CrossRef]
- Boyeena, L.; Koduganti, R.R.; Panthula, V.R.; Jammula, S.P. Comparison of efficacy of probiotics versus tetracycline fibers as adjuvants to scaling and root planing. J. Indian Soc. Periodontol. 2019, 23, 539–544. [Google Scholar] [PubMed]
- Charan, J.; Biswas, T. How to calculate sample size for different study designs in medical research? Indian J. Psychol. Med. 2013, 35, 121–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scribante, A.; Poggio, C.; Gallo, S.; Riva, P.; Cuocci, A.; Carbone, M.; Arciola, C.R.; Colombo, M. In Vitro Re-Hardening of Bleached Enamel Using Mineralizing Pastes: Toward Preventing Bacterial Colonization. Materials 2020, 13, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sfondrini, M.F.; Debiaggi, M.; Zara, F.; Brerra, R.; Comelli, M.; Bianchi, M.; Pollone, S.R.; Scribante, A. Influence of lingual bracket position on microbial and periodontal parameters in vivo. J. Appl. Oral Sci. 2012, 20, 357–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutovac, M.; Popova, O.V.; Macanovic, G.; Kristina, R.; Lutovac, B.; Ketin, S.; Biocanin, R. Testing the Effect of Aggressive Beverage on the Damage of Enamel Structure. Open Access Maced. J. Med. Sci. 2017, 5, 987–993. [Google Scholar] [CrossRef] [Green Version]
- Colombo, M.; Poggio, C.; Lasagna, A.; Chiesa, M.; Scribante, A. Vickers Micro-Hardness of New Restorative CAD/CAM Dental Materials: Evaluation and Comparison after Exposure to Acidic Drink. Materials 2019, 12, 1246. [Google Scholar] [CrossRef] [Green Version]
- Deng, Z.L.; Szafrański, S.P.; Jarek, M.; Bhuju, S.; Wagner-Döbler, I. Dysbiosis in chronic periodontitis: Key microbial players and interactions with the human host. Sci. Rep. 2017, 7, 3703–3715. [Google Scholar] [CrossRef] [Green Version]
- Ikram, S.; Hassan, N.; Raffat, M.A.; Mirza, S.; Akram, Z. Systematic review and meta- analysis of double- blind, placebo- controlled, randomized clinical trials using probiotics in chronic periodontitis. J. Investig. Clin. Dent. 2018, 9, e12338. [Google Scholar] [CrossRef]
- Ikram, S.; Hassan, N.; Baig, S.; Borges, K.J.J.; Raffat, M.A.; Akram, Z. Effect of local probiotic (Lactobacillus reuteri) vs systemic antibiotic therapy as an adjunct to non-surgical periodontal treatment in chronic periodontitis. J. Investig. Clin. Dent. 2019, 10, e12393. [Google Scholar] [CrossRef]
- Szkaradkiewicz, A.K.; Stopa, J.; Karpiński, T.M. Effect of oral administration involving a probiotic strain of lactobacillus reuteri on pro- inflammatory cytokine response in patients with chronic periodontitis. Arch. Immunol. Ther. Exp. 2014, 62, 495–500. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.; Nambiar, S.; Kamath, D.; Suman, E.; Unnikrishnan, B.; Desai, A.; Mahajan, S.; Dhawan, K.K. Comparative Evaluation of Plaque Inhibitory and Antimicrobial Efficacy of Probiotic and Chlorhexidine Oral Rinses in Orthodontic Patients: A Randomized Clinical Trial. Int. J. Dent. 2019, 2019, 1964158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peña, M.; Barallat, L.; Vilarrasa, J.; Vicario, M.; Violant, D.; Nart, J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: A triple-blind randomized clinical trial. Clin. Oral Investig. 2019, 23, 1673–1683. [Google Scholar] [CrossRef] [PubMed]





| Product | Description | Ingredients | Manufacturer | Code |
|---|---|---|---|---|
| Biorepair Peribioma | Toothpaste | Aqua, Zinc Hydroxyapatite *, Sorbitol, Glycerin, Hydrated Silica, Silica, Cocamidopropyl Betaine, Cellulose Gum, Aroma, Pistacia Lentiscus (Mastic) Gum Oil, Ascorbic Acid, Tocopheryl Acetate, Retynil Palmitate, Sodium Hyaluronate, Hamamelis Virginiana Leaf Extract, Spirulina Platensis Extract, Calendula Officinalis Flower Extract, Eucaliptus Globulus Leaf Oil, Bifidobacterium *, Lactobacillus *, Sodium Myristoyl Sarcosinate, Sodium Methyl Cocoyl Taurate, Phenoxyethanol, Benzyl Alcohol, Sodium Benzoate, Sodium Saccharin, Potassium Sorbate, Maltodextrin, Citric Acid, Helianthus Annuus Seed Oil, BHT, Limonene, Eugenol, CI 77891, CI 73360. * microRepair®BIOMA | Coswell SPA, 40050 Funo di Argelato, Bologna, Italy | GA1504900 |
| Biorepair Peribioma | Chewing gum | Gum base (aroma; emulsifier: soya lecithin; sweetener: acesulfame, sucralose; antioxidant: tocopherols); bulking agents: isomalt, sorbitol; microRepair® (calcium salts of orthophosphoric acid) *; Probiotics * [L. reuteri (SGL 01), L. salivarius (SGL 03), L. plantarum (SGL 07), support: corn maltodextrin, anti-caking agent: silicium dioxide]; Aroma; Vitamin C (ascorbate calcium); colorant foods (radish concentrate and sweet potato); sweeteners: sucralose, acesulfame K; Vitamin D (Cholecalciferol). * microRepair®BIOMA | Coswell SPA, 40050 Funo di Argelato, Bologna, Italy | GA1536200 |
| Curasept Regenerative Treatment 0.20% | Toothpaste | Purified Water, Sorbitol, Hydrated Silica, PEG-32, Cocamidopropyl Betaine, Xylitol, Cellulose Gum, Aroma, Sodium Hyaluronate, Ascorbic Acid, Chlorhexidine, Digluconate, Sodium Metabisulfite, Sodium Citrate, Titanium Dioxide (C.I. 77891), Sodium Benzoate, Sodium Saccharin, Citric Acid, C.I. 17200, C.I. 42090. | Curasept SPA, 21047 Saronno, Varese, Italy | 190011661 |
| Appointment | Procedures |
|---|---|
| Baseline (T0) |
|
| After 3 months (T1) After 6 months (T2) |
|
| Group | Time | PPD | CAL | BOP | BS | SBI | API | PI | AG | GR | PS |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||
| (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | ||
| Control | T0 | 5.88 (1.26) a | 5.83 (1.87) a | 66.25 (17.23) a | 1.78 (0.80) a | 1.96 (0.80) a | 79.75 (21.61) a | 70.00 (26.56) a | 3.85 (0.67) a | 2.48 (1.28) a | 74.00 (10.46) a |
| T1 | 5.55 (1.27) a | 5.66 (1.80) a | 61.25 (18.16) a | 1.66 (0.75) a | 1.82 (0.73) a | 78.00 (20.03) a | 66.50 (22.72) a | 3.65 (0.68) a | 2.40 (1.18) a | 67.50 (13.33) a | |
| T2 | 5.80 (1.08) a | 5.57 (1.72) a | 64.00 (14.01) a | 1.70 (0.57) a | 2.00 (0.44) a | 81.00 (20.17) a | 67.00 (22.33) a | 3.75 (0.62) a | 2.44 (1.19) a | 67.50 (8.66) a | |
| Toothpaste | T0 | 5.67 (0.74) a | 5.64 (2.27) a | 67.00 (24.94) a | 1.64 (0.76) a | 1.71 (1.06) a | 72.25 (20.42) a | 68,50 (22.48) a | 3.73 (0.51) a | 2.46 (0.92) a | 81.50 (4.89) a |
| T1 | 4.67 (0.64) b | 4.74 (2.45) b | 39.00 (17.59) b | 0.89 (0.32) b | 0.85 (0.87) b | 53.25 (19.42) b | 42.25 (12.82) b | 3.73 (0.52) a | 2.57 (1.06) a | 48.00 (38.06) b | |
| T2 | 4.46 (0.84) b | 4.44 (2.14) b | 33.00 (20.39) b | 0.70 (0.26) b | 0.76 (0.74) b | 48.75 (12.13) b | 34.15 (14.08) b | 3.72 (0.52) a | 2.60 (1.08) a | 46.00 (39.52) b | |
| Toothpaste + Chewing Gum | T0 | 5.57 (0.85) a | 5.36 (1.46) a | 66.15 (34.89) a | 1.59 (1.10) a | 1.56 (1.05) a | 74.83 (27.38) a | 70.50 (20.38) a | 3.76 (0.43) a | 2.38 (1.14) a | 81.75 (4.94) a |
| T1 | 3.74 (0.69) c | 3.76 (1.35) c | 39.90 (29.23) b | 1.06 (0.82) b | 0.71 (0.87) b | 57.75 (35.67) b | 40.50 (18.20) b | 3.74 (0.43) a | 2.51 (1.05) a | 51.30 (25.10) b | |
| T2 | 3.52 (0.53) c | 3.46 (0.94) c | 21.50 (17.55) c | 0.44 (0.72) c | 0.26 (0.40) c | 30.75 (39.01) c | 28.50 (17.85) c | 3.69 (0.42) a | 2.63 (0.87) a | 23.40 (19.48) c |
| Group | Time | Total Bacteria Count | AAE | PG | TF | TD | PI | FN |
|---|---|---|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | Mean | Mean | Mean | ||
| (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | (SD) | ||
| Control | T0 | 1648650 | 1247.48 | 10530.55 | 34012.51 | 7339.82 | 11018 | 17607.3 |
| (2571189.00) a | (1238.52) a | (18424.41) a | (54134.03) a | (19922.95) a | (10208.32) a | (25342.18) a | ||
| T1 | 999745.2 | 1216.92 | 12836.1 | 59654.57 | 2973.42 | 8326 | 17834.3 | |
| (705812.30) a | (1219.51) a | (32125.70) a | (164197.50) a | (5358.644) a | (5063.76) a | (20783.60) a | ||
| T2 | 561150 | 1030.08 | 8479 | 40990 | 4651 | 8830 | 16298.82 | |
| (349477.80) a | (1202.40) a | (3974.44) a | (19938.19) a | (2158.87) a | (5617.44) a | (17212.96) a | ||
| Toothpaste | T0 | 1150665 | 528 | 9107.65 | 57690.4 | 5318 | 9720.1 | 19381.2 |
| (2270115.00) a | (1121.42) a | (21882.53) a | (90873,28) a | (10086.63) a | (2405.66) a | (10360.37) a | ||
| T1 | 2173295 | 540.75 | 7780 | 81998.28 | 8153 | 9652.2 | 17719.25 | |
| (4325099.00) a | (1151.19) a | (16100.69) a | (202365.80) a | (16980.35) a | (4670.72) a | (14680.71) a | ||
| T2 | 535470 | 734.5 | 7625.75 | 25656.4 | 10244.63 | 3536 | 7843.6 | |
| (306466.90) a | (2076.09) a | (22714.00) a | (48302.74) a | (15860.15) a | (5931.72) b | (5509.43) b | ||
| Toothpaste + Chewing Gum | T0 | 808115 | 650.25 | 11644.1 | 35091.9 | 5521.75 | 7476.55 | 18053 |
| (1619913.00) a | (1114.43) a | (23306.05) a | (40463.06) a | (18720.46) a | (4787.82) a | (10931.75) a | ||
| T1 | 2171589 | 595.95 | 9939.8 | 91622.76 | 2942.15 | 8406 | 16734.2 | |
| (5938379.00) a | (1387.69) a | (13193.90) a | (253652.70) a | (5375.99) a | (5333.22) a | (9249.37) a | ||
| T2 | 2097731 | 406.2 | 7553.641 | 3929080 | 6065.13 | 2520.5 | 7211.78 | |
| (5655579.00) a | (919.53) a | (15781.77) a | (66828.91) a | (19738.92) a | (2435.87) b | (5971.25) b |
| Group | Time | Pathogen Bacteria | Enlarged Red Complex | Red Complex | Orange Complex |
|---|---|---|---|---|---|
| Mean | Mean | Mean | Mean | ||
| (SD) | (SD) | (SD) | (SD) | ||
| Control | T0 | 19.71 | 0.4 | 65.08 | 53.27 |
| (12.93) a | (0.39) a | (28.13) a | (35.14) a | ||
| T1 | 18.92 | 1.08 | 65.19 | 51.38 | |
| (10.64) a | (2.79) a | (27.52) a | (33.83) a | ||
| T2 | 19.90 | 0.91 | 66.38 | 52.69 | |
| (11.20) a | (1.91) a | (26.10) a | (34.08) a | ||
| Toothpaste | T0 | 16.23 | 0.35 | 59.32 | 51.88 |
| (15.75) a | (1.10) a | (37.05) a | (30.52) a | ||
| T1 | 11.97 | 0.57 | 56.19 | 49.39 | |
| (9.22) a | (2.11) a | (41.82) a | (40.66) a | ||
| T2 | 13.99 | 1 | 59.51 | 24.87 | |
| (13.79) a | (2.49) a | (37.14) a | (24.99) b | ||
| Toothpaste + Chewing Gum | T0 | 12.49 | 0.16 | 67.19 | 42.58 |
| (8.73) a | (0.39) a | (30.91) a | (31.07) a | ||
| T1 | 16.12 | 0.78 | 59.8 | 44.42 | |
| (13.10) a | (2.85) a | (41.47) a | (39.57) a | ||
| T2 | 12.31 | 0.1 | 61.66 | 16.34 | |
| (9.50) a | (0.31) a | (34.32) a | (22.13) b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butera, A.; Gallo, S.; Maiorani, C.; Molino, D.; Chiesa, A.; Preda, C.; Esposito, F.; Scribante, A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms 2021, 9, 69. https://doi.org/10.3390/microorganisms9010069
Butera A, Gallo S, Maiorani C, Molino D, Chiesa A, Preda C, Esposito F, Scribante A. Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms. 2021; 9(1):69. https://doi.org/10.3390/microorganisms9010069
Chicago/Turabian StyleButera, Andrea, Simone Gallo, Carolina Maiorani, Domenico Molino, Alessandro Chiesa, Camilla Preda, Francesca Esposito, and Andrea Scribante. 2021. "Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters" Microorganisms 9, no. 1: 69. https://doi.org/10.3390/microorganisms9010069
APA StyleButera, A., Gallo, S., Maiorani, C., Molino, D., Chiesa, A., Preda, C., Esposito, F., & Scribante, A. (2021). Probiotic Alternative to Chlorhexidine in Periodontal Therapy: Evaluation of Clinical and Microbiological Parameters. Microorganisms, 9(1), 69. https://doi.org/10.3390/microorganisms9010069







