Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Fusarium Inoculum and Inoculation
2.3. Pathogen Reisolation
2.4. DNA Extraction and qPCR
2.5. Mycotoxin Extraction and HPLC-MS
2.6. Statistical Analysis
3. Results
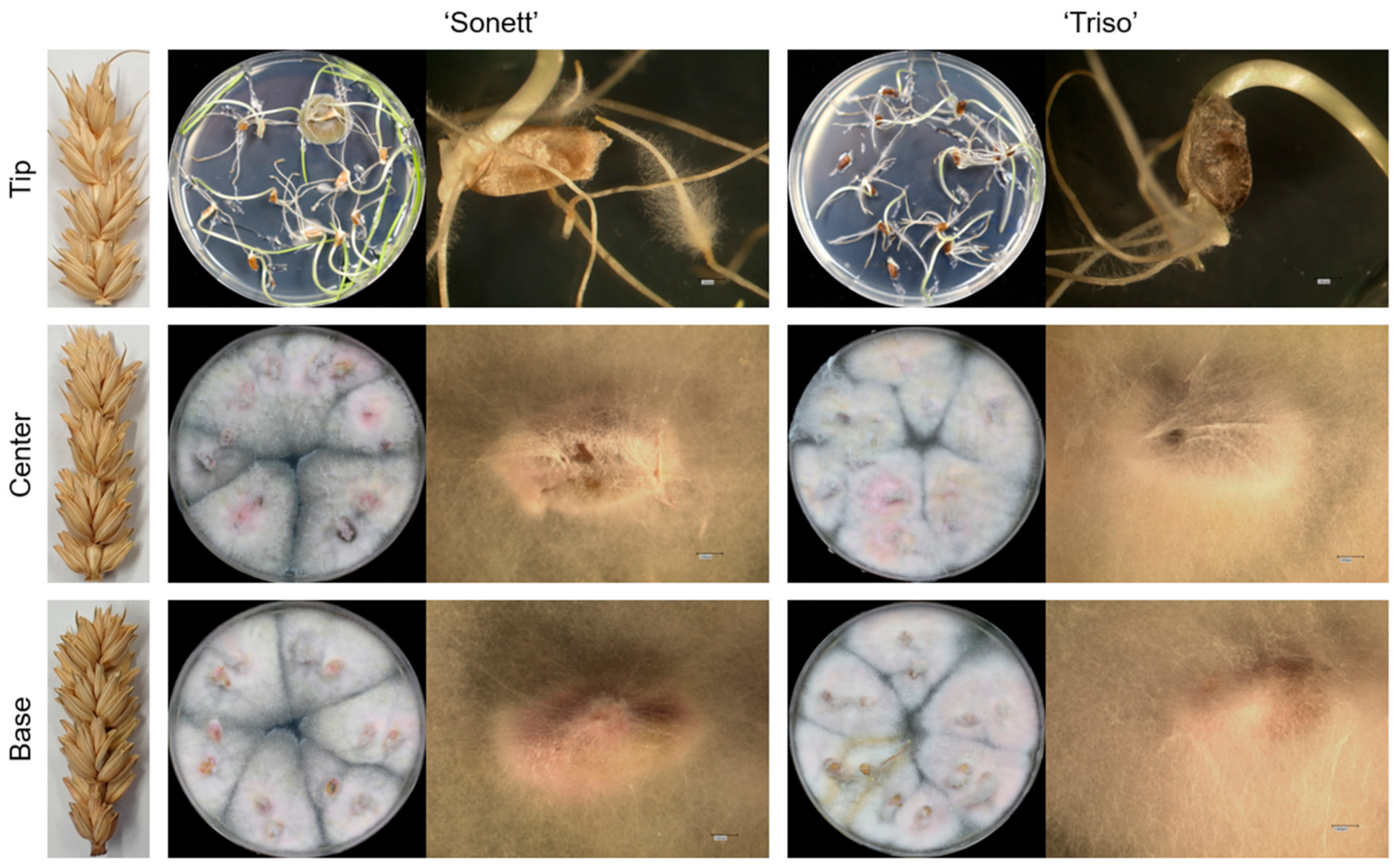
3.1. Pathogen Movement and Reisolation
3.2. Effect of Infection Timing on Fungal DNA Content in Wheat Kernels
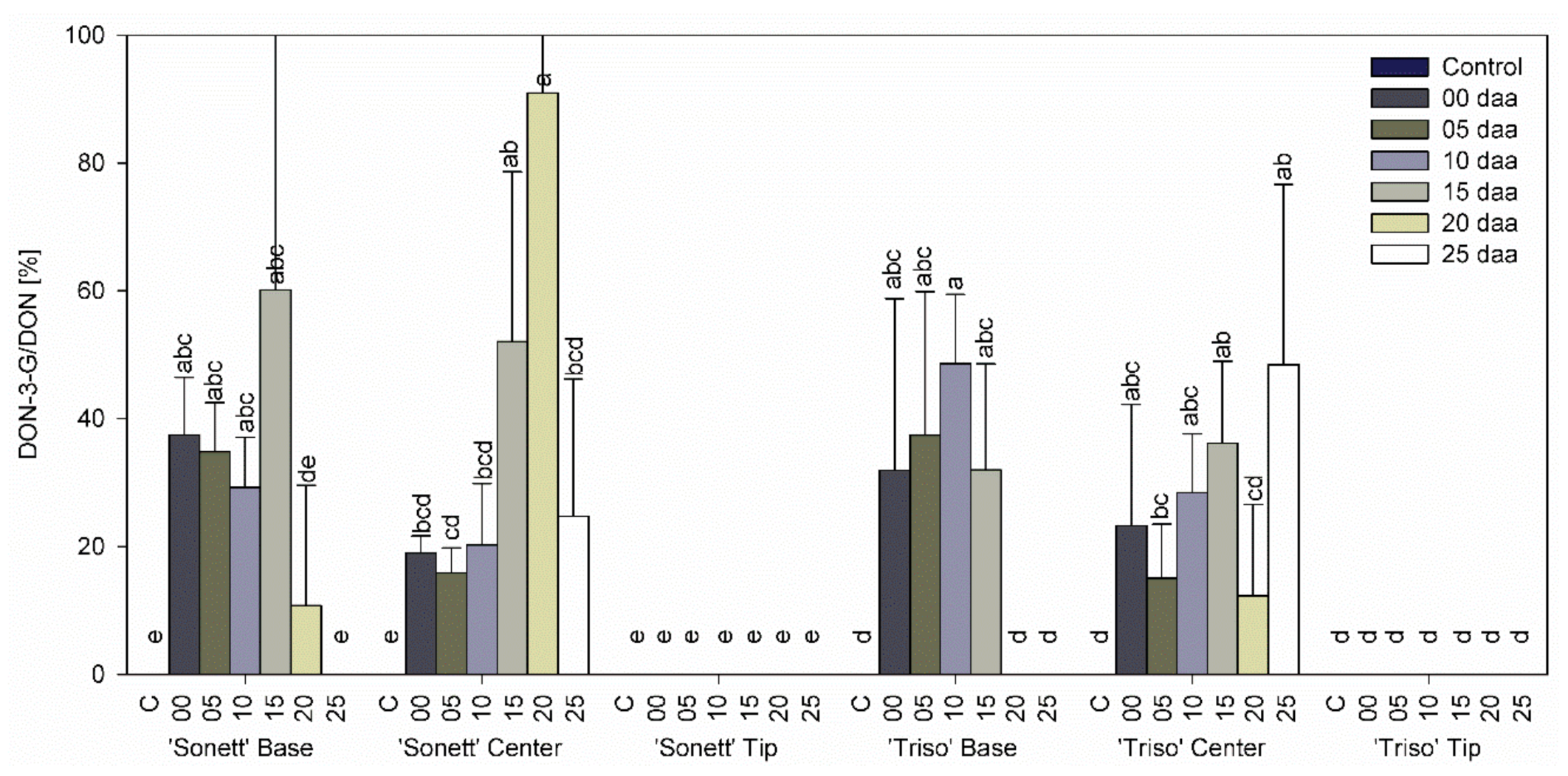
3.3. Effect of Infection Timing on DON, DON-3-G Content, and Detoxification Ratio in Wheat Kernels
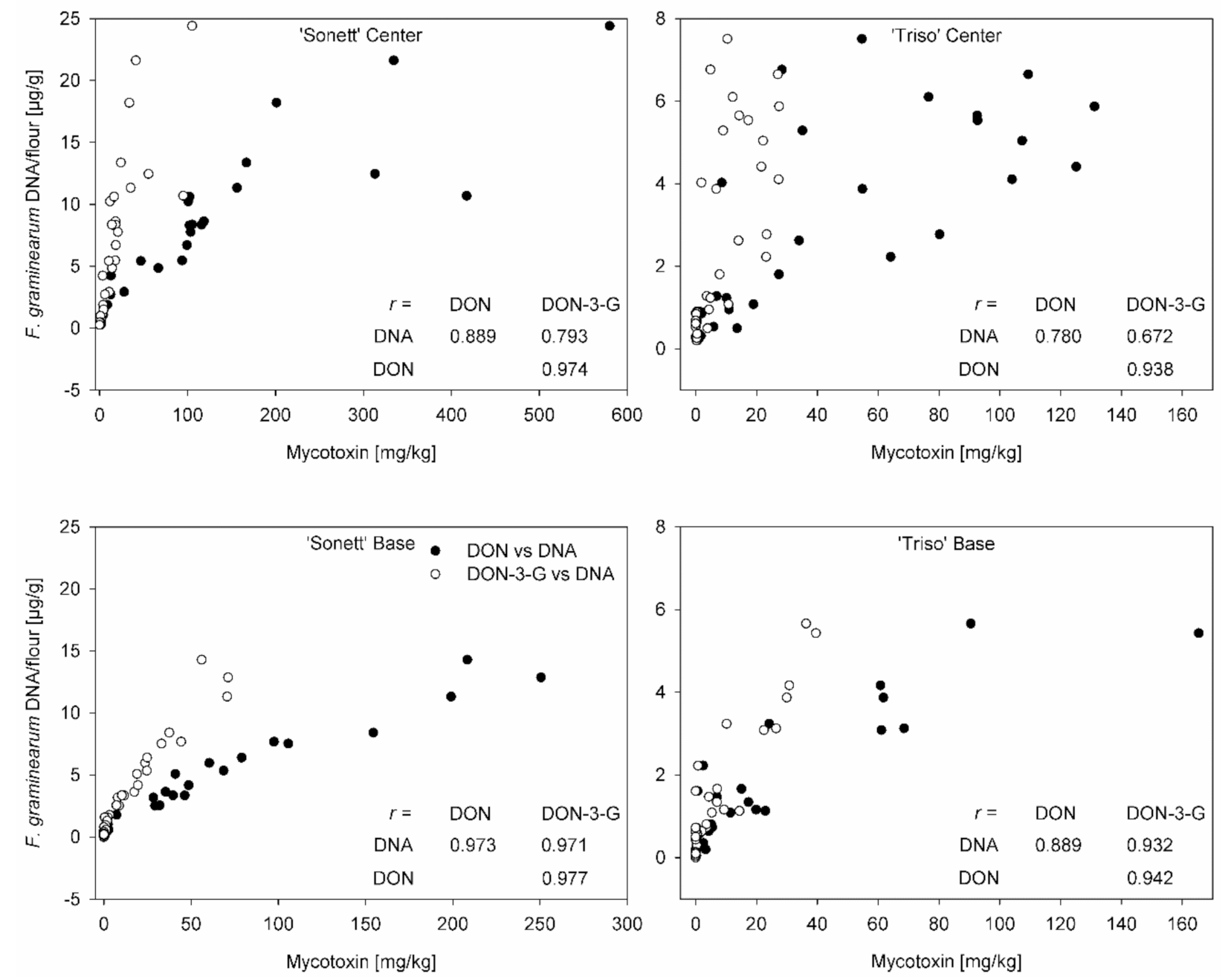
3.4. Correlation between Mycotoxin Content and Fungal DNA
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. Fusarium head blight disease cycle, symptoms, and impact on grain yield and quality frequency and magnitude of epidemics since 1997. Plant. Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef] [PubMed]
- Siou, D.; Gélisse, S.; Laval, V.; Repinçay, C.; Canalès, R.; Suffert, F.; Lannou, C. Effect of wheat spike infection timing on Fusarium head blight development and mycotoxin accumulation. Plant. Pathol. 2014, 63, 390–399. [Google Scholar] [CrossRef]
- Kubo, K.; Fujita, M.; Kawada, N.; Nakajima, T.; Nakamura, K.; Maejima, H.; Ushiyama, T.; Hatta, K.; Matsunaka, H. Minor differences in anther extrusion affect resistance to Fusarium head blight in wheat. J. Phytopathol. 2013, 161, 308–314. [Google Scholar] [CrossRef]
- Skinnes, H.; Semagn, K.; Tarkegne, Y.; Marøy, A.G.; Bjørnstad, Å. The inheritance of anther extrusion in hexaploid wheat and its relationship to Fusarium head blight resistance and deoxynivalenol content. Plant. Breed. 2010, 129, 149–155. [Google Scholar] [CrossRef]
- Strange, R.N.; Majer, J.R.; Smith, H. The isolation and identification of choline and betaine as the two major components in anthers and wheat germ that stimulate Fusarium graminearum in vitro. Physiol. Plant. Pathol. 1974, 4, 277–290. [Google Scholar] [CrossRef]
- Lu, Q.; Lillemo, M.; Skinnes, H.; He, X.; Shi, J.; Ji, F.; Dong, Y.; Bjørnstad, Å. Anther extrusion and plant height are associated with Type I resistance to Fusarium head blight in bread wheat line ‘Shanghai-3/Catbird’. Theor. Appl. Genet. 2013, 126, 317–334. [Google Scholar] [CrossRef]
- Xu, K.; He, X.; Dreisigacker, S.; He, Z.; Singh, P.K. Anther extrusion and its association with Fusarium head blight in CIMMYT wheat germplasm. Agronomy 2020, 10, 47. [Google Scholar] [CrossRef]
- Francl, L.; Shaner, G.; Bergstrom, G.; Gilbert, J.; Pedersen, W.; Dill-Macky, R.; Sweets, L.; Corwin, B.; Jin, Y.; Gallenberg, D.; et al. Daily inoculum levels of Gibberella zeae on wheat spikes. Plant. Dis. 1999, 83, 662–666. [Google Scholar] [CrossRef]
- Brown, N.A.; Urban, M.; van de Meene, A.M.L.; Hammond-Kosack, K.E. The infection biology of Fusarium graminearum: Defining the pathways of spikelet to spikelet colonisation in wheat ears. Fungal Biol. 2010, 114, 555–571. [Google Scholar] [CrossRef]
- Beccari, G.; Arellano, C.; Covarelli, L.; Tini, F.; Sulyok, M.; Cowger, C. Effect of wheat infection timing on Fusarium head blight causal agents and secondary metabolites in grain. Int. J. Food Microbiol. 2019, 290, 214–225. [Google Scholar] [CrossRef]
- Cowger, C.; Arrellano, C. Plump kernels with high deoxynivalenol linked to late Gibberella zeae infection and marginal disease conditions in winter wheat. Phytopathology 2010, 100, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Nakajima, T. Deoxynivalenol and nivalenol accumulation in wheat infected with Fusarium graminearum during grain development. Phytopathology 2010, 100, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Cowger, C.; Patton-Özkurt, J.; Brown-Guedira, G.; Perugini, L. Post-anthesis moisture increased Fusarium head blight and deoxynivalenol levels in North Carolina winter wheat. Phytopathology 2009, 99, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Cowger, C.; Arellano, C. Fusarium graminearum infection and deoxynivalenol concentrations during development of wheat spikes. Phytopathology 2013, 103, 460–471. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in cereal grains—An update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Bartók, T.; Mirocha, C.G.; Komoróczy, R. Nature of wheat resistance to Fusarium head blight and the role of deoxynivalenol for breeding. Plant. Breed. 1999, 118, 97–110. [Google Scholar] [CrossRef]
- Miller, J.D.; Arnison, P.G. Degradation of deoxynivalenol by suspension cultures of the Fusarium head blight resistant wheat cultivar Frontana. Can. J. Plant. Pathol. 1986, 8, 147–150. [Google Scholar] [CrossRef]
- Dall’Asta, C.; Berthiller, F.; Schuhmacher, R.; Adam, G.; Lemmens, M.; Krska, R. DON-Glycosides: Characterisation of synthesis products and screening for their occurrence in DON-treated wheat samples. Mycotoxin Res. 2005, 21, 123–127. [Google Scholar] [CrossRef]
- Winter, M.; Koopmann, B.; Döll, K.; Karlovsky, P.; Kropf, U.; Schlüter, K.; Von Tiedemann, A. Mechanisms regulating grain contamination with trichothecenes translocated from the stem base of wheat (Triticum aestivum) infected with Fusarium culmorum. Phytopathology 2013, 103, 682–689. [Google Scholar] [CrossRef]
- Kluger, B.; Bueschl, C.; Lemmens, M.; Michlmayr, H.; Malachova, A.; Koutnik, A.; Maloku, I.; Berthiller, F.; Adam, G.; Krska, R.; et al. Biotransformation of the mycotoxin deoxynivalenol in Fusarium resistant and susceptible near isogenic wheat lines. PLoS ONE 2015, 10, e0119656. [Google Scholar] [CrossRef]
- Lemmens, M.; Scholz, U.; Berthiller, F.; Dall’Asta, C.; Koutnik, A.; Schuhmacher, R.; Adam, G.; Buerstmayr, H.; Mesterházy, Á.; Krska, R.; et al. The ability to detoxify the mycotoxin deoxynivalenol colocalizes with a major quantitative trait locus for Fusarium head blight resistance in wheat. Mol. Plant.-Microbe Interact. 2005, 18, 1318–1324. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, M.; Steiner, B.; Sulyok, M.; Nicholson, P.; Mesterhazy, A.; Buerstmayr, H. Masked mycotoxins: Does breeding for enhanced Fusarium head blight resistance result in more deoxynivalenol-3-glucoside in new wheat varieties? World Mycotoxin J. 2016, 9, 741–754. [Google Scholar] [CrossRef]
- Anonymous. Keys to Soil Taxonomy, 12th ed.; Smith, D.W., Ed.; United States Department of Agriculture, Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Alisaac, E.; Behmann, J.; Rathgeb, A.; Karlovsky, P.; Dehne, H.-W.; Mahlein, A.-K. Assessment of Fusarium infection and mycotoxin contamination of wheat kernels and flour using hyperspectral imaging. Toxins 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, E.; Behmann, J.; Kuska, M.T.; Dehne, H.W.; Mahlein, A.K. Hyperspectral quantification of wheat resistance to Fusarium head blight: Comparison of two Fusarium species. Eur. J. Plant. Pathol. 2018, 152, 869–884. [Google Scholar] [CrossRef]
- Brandfass, C.; Karlovsky, P. Simultaneous detection of Fusarium culmorum and F. graminearum in plant material by duplex PCR with melting curve analysis. BMC Microbiol. 2006, 6, 4. [Google Scholar]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant. Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Beule, L.; Lehtsaar, E.; Rathgeb, A.; Karlovsky, P. Crop diseases and mycotoxin accumulation in temperate agroforestry systems. Sustainability 2019, 11, 2925. [Google Scholar] [CrossRef]
- Freeman, E.M. Minnesota Plant. Diseases (Geological and Natural History Survey of Minnesota). Botanical series 5; Board of Regents of the University of Minnesota: St. Paul, MN, USA, 1905. [Google Scholar]
- Atanasoff, D. Fusarium blight (scab) of wheat and other cereals. J. Agric. Res. 1920, 20, 1–32. [Google Scholar]
- Miller, S.S.; Chabot, D.M.P.; Ouellet, T.; Harris, L.J.; Fedak, G. Use of a Fusarium graminearum strain transformed with green fluorescent protein to study infection in wheat (Triticum aestivum). Can. J. Plant. Pathol. 2004, 26, 453–463. [Google Scholar] [CrossRef]
- Ribichich, K.F.; Lopez, S.E.; Vegetti, A.C. Histopathological spikelet changes produced by Fusarium graminearum in susceptible and resistant wheat cultivars. Plant. Dis. 2000, 84, 794–802. [Google Scholar] [CrossRef]
- Ilgen, P.; Hadeler, B.; Maier, F.J.; Schäfer, W. Developing kernel and rachis node induce the trichothecene pathway of Fusarium graminearum during wheat head infection. Mol. Plant.-Microbe Interact. 2009, 22, 899–908. [Google Scholar] [CrossRef] [PubMed]
- Argyris, J.; TeKrony, D.; Hershman, D.; VanSanford, D.; Hall, M.; Kennedy, B.; Rucker, M.; Edge, C. Fusarium head blight infection following point inoculation in the greenhouse compared with movement of Fusarium graminearum in seed and floral components. Crop. Sci. 2005, 45, 626–634. [Google Scholar] [CrossRef]
- Hallen-Adams, H.E.; Wenner, N.; Kuldau, G.A.; Trail, F. Deoxynivalenol biosynthesis-related gene expression during wheat kernel colonization by Fusarium graminearum. Phytopathology 2011, 101, 1091–1096. [Google Scholar] [CrossRef] [PubMed]
- Siou, D.; Gélisse, S.; Laval, V.; Suffert, F.; Lannou, C. Mutual exclusion between fungal species of the Fusarium head blight complex in a wheat spike. Appl. Environ. Microbiol. 2015, 81, 4682–4689. [Google Scholar] [CrossRef]
- Malbrán, I.; Mourelos, C.A.; Girotti, J.R.; Aulicino, M.B.; Balatti, P.A.; Lori, G.A. Aggressiveness variation of Fusarium graminearum isolates from Argentina following point inoculation of field grown wheat spikes. Crop. Prot. 2012, 42, 234–243. [Google Scholar] [CrossRef]
- Savard, M.E.; Sinha, R.C.; Lloyd Seaman, W.; Fedak, G. Sequential distribution of the mycotoxin deoxynivalenol in wheat spikes after inoculation with Fusarium graminearum. Can. J. Plant. Pathol. 2000, 22, 280–285. [Google Scholar] [CrossRef]
- Ha, X.; Koopmann, B.; von Tiedemann, A. Wheat blast and Fusarium head blight display contrasting interaction patterns on ears of wheat genotypes differing in resistance. Phytopathology 2016, 106, 270–281. [Google Scholar] [CrossRef][Green Version]
- Kang, Z.; Buchenauer, H. Immunocytochemical localization of Fusarium toxins in infected wheat spikes by Fusarium culmorum. Physiol. Mol. Plant. Pathol. 1999, 55, 275–288. [Google Scholar] [CrossRef]
- Beccari, G.; Prodi, A.; Pisi, A.; Nipoti, P.; Onofri, A.; Nicholson, P.; Pfohl, K. Development of three Fusarium crown rot causal agents and systemic translocation of deoxynivalenol following stem base infection of soft wheat. Plant. Pathol. 2018, 67, 1055–1065. [Google Scholar] [CrossRef]
- Boenisch, M.J.; Schäfer, W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant. Biol. 2011, 11, 110. [Google Scholar] [CrossRef]
- Berthiller, F.; Dall’Asta, C.; Schuhmacher, R.; Lemmens, M.; Adam, G.; Krska, A.R. Masked mycotoxins: Determination of a deoxynivalenol glucoside in artificially and naturally contaminated wheat by liquid chromatography-tandem mass spectrometry. J. Agric. Food Chem. 2005, 53, 3421–3425. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alisaac, E.; Rathgeb, A.; Karlovsky, P.; Mahlein, A.-K. Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes. Microorganisms 2021, 9, 79. https://doi.org/10.3390/microorganisms9010079
Alisaac E, Rathgeb A, Karlovsky P, Mahlein A-K. Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes. Microorganisms. 2021; 9(1):79. https://doi.org/10.3390/microorganisms9010079
Chicago/Turabian StyleAlisaac, Elias, Anna Rathgeb, Petr Karlovsky, and Anne-Katrin Mahlein. 2021. "Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes" Microorganisms 9, no. 1: 79. https://doi.org/10.3390/microorganisms9010079
APA StyleAlisaac, E., Rathgeb, A., Karlovsky, P., & Mahlein, A.-K. (2021). Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes. Microorganisms, 9(1), 79. https://doi.org/10.3390/microorganisms9010079





