Co-Inoculation of Organic Potato with Fungi and Bacteria at High Disease Severity of Rhizoctonia solani and Streptomyces spp. Increases Beneficial Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Field Experiment
2.2. Weather Data during the Study Periods
2.3. Biofertilizer Treatments and Method of Inoculation
2.4. Seasonal Variation of Soil Microbial Characteristics
2.5. Sample Examination of Potato Tubers and Data Analysis
3. Results and Discussion
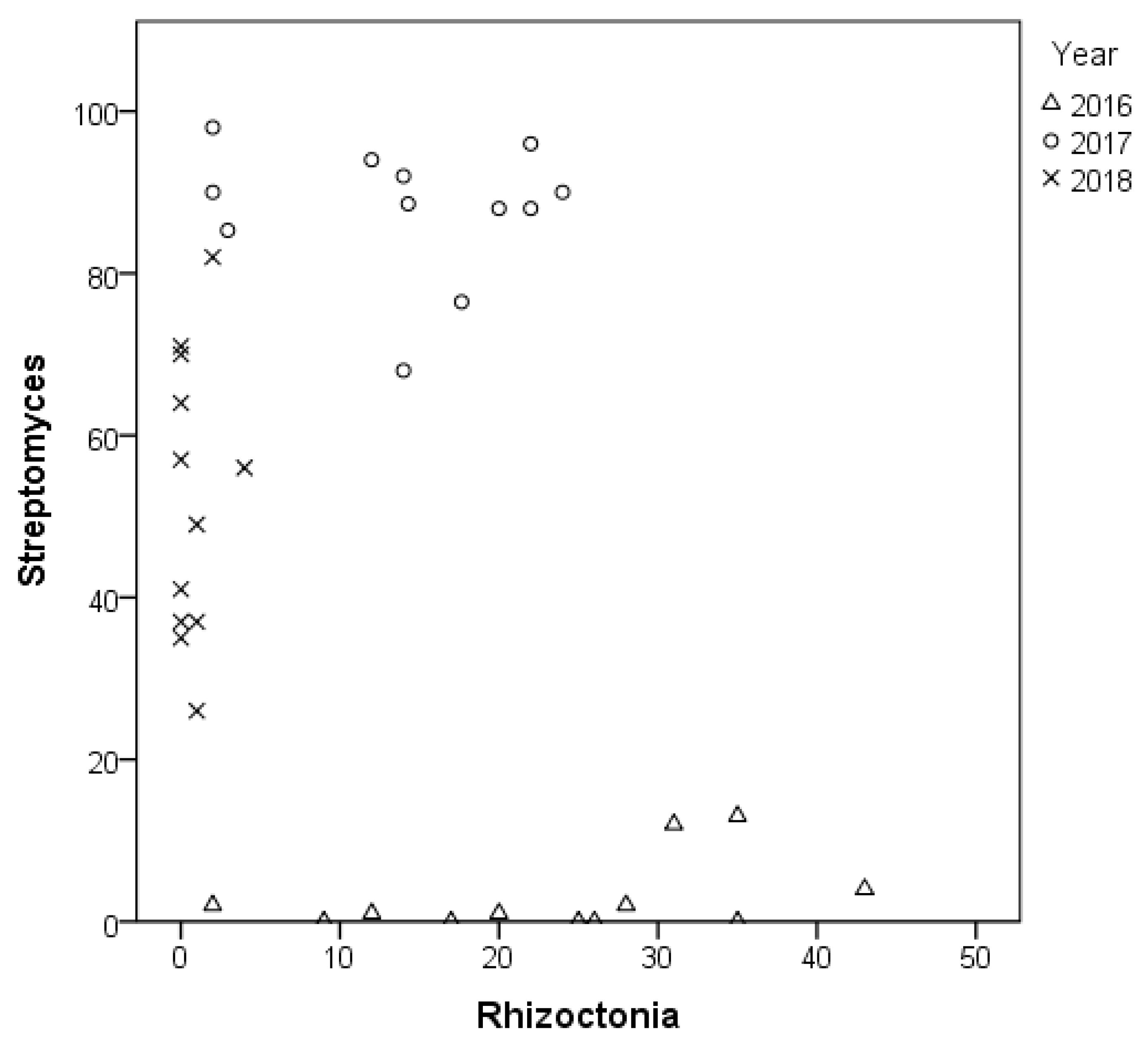
3.1. Streptomyces sp. Infection during the Three Consecutive Test Years
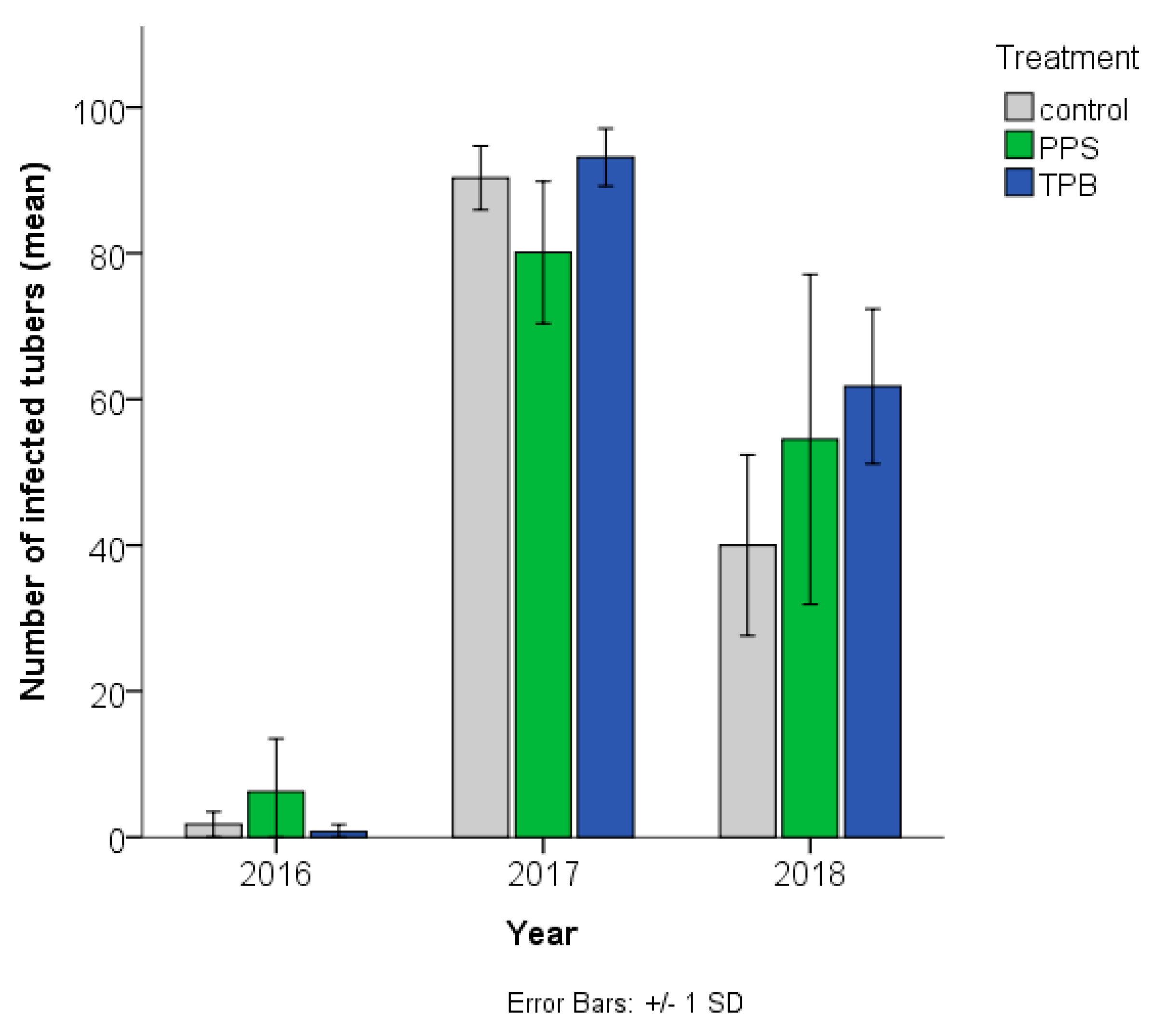
3.1.1. Number of Streptomyces sp. Infected Tubers
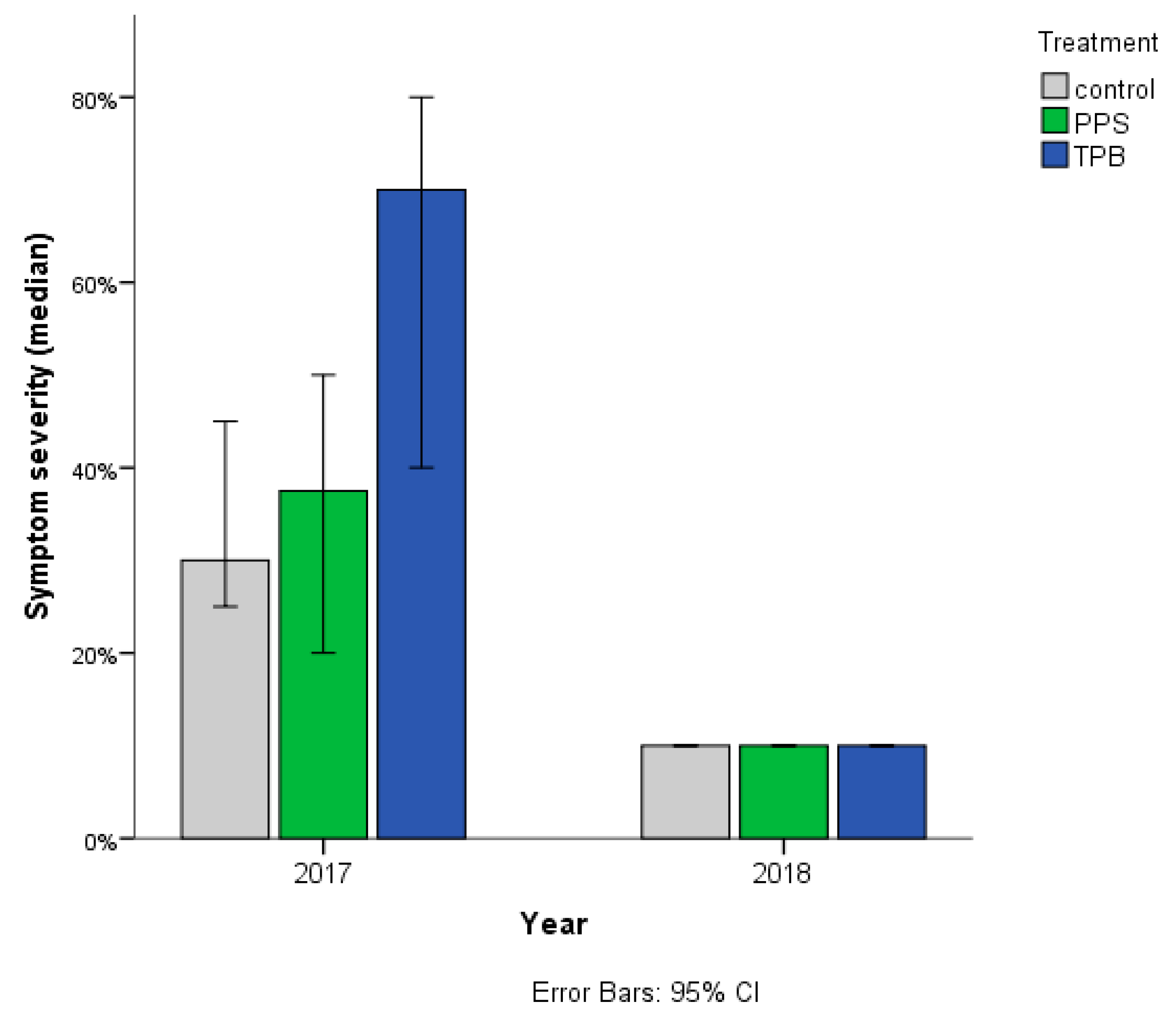
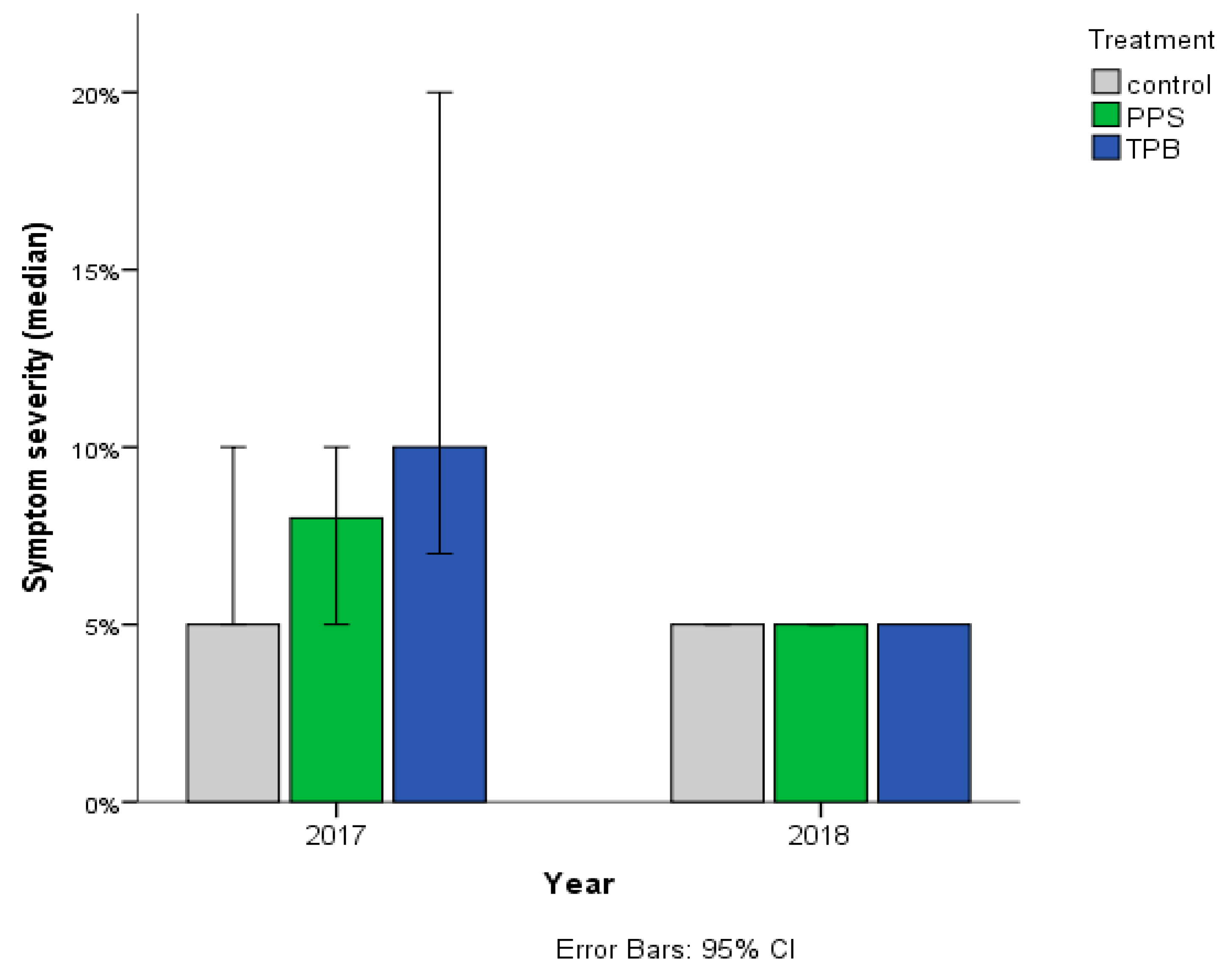
3.1.2. Streptomyces sp. Infection Symptom Severity
3.2. Rhizoctonia sp. Infection during the Three Consecutive Test Years
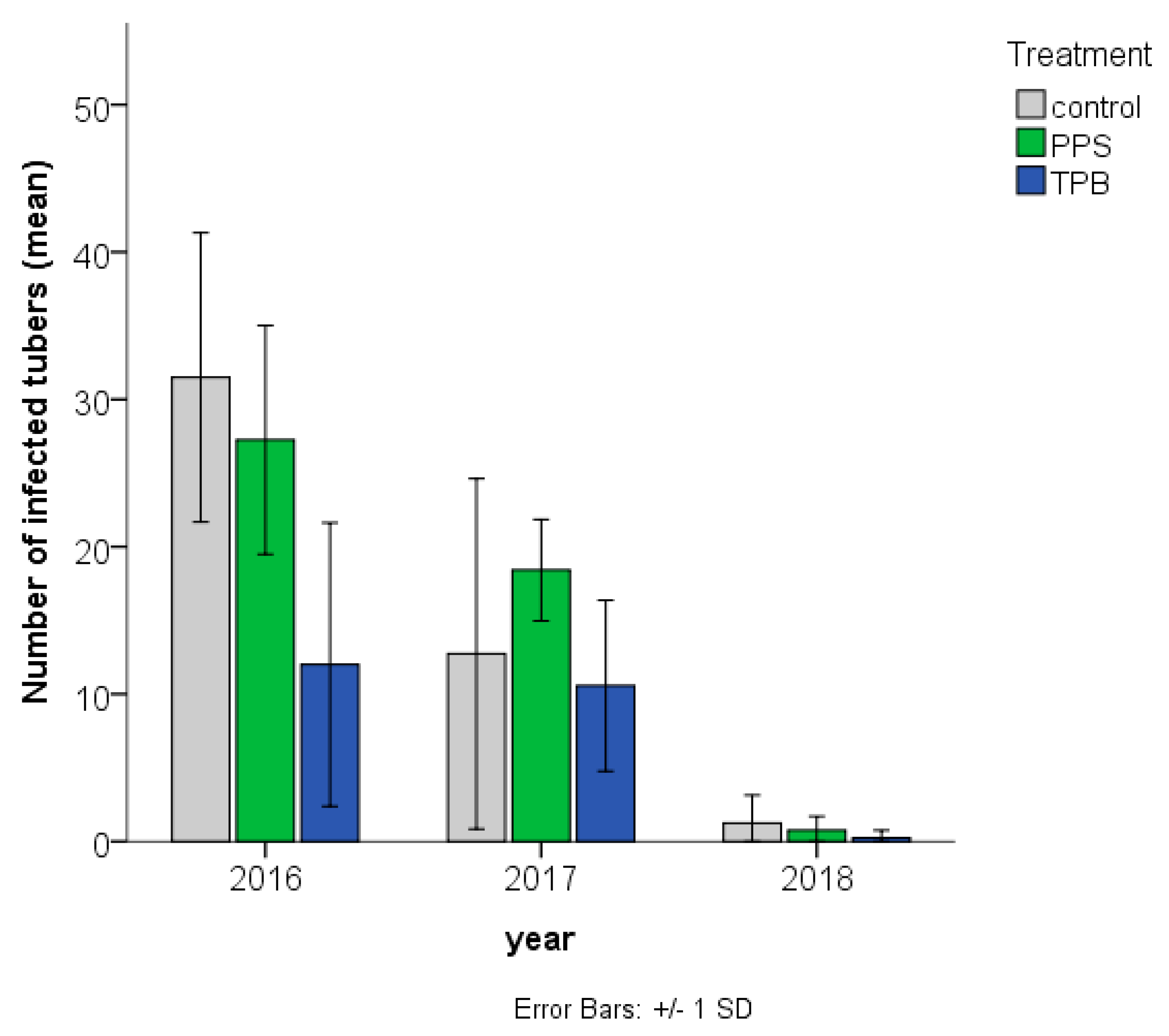
3.2.1. Number of Rhizoctonia-Infected Tubers
3.2.2. Rhizoctonia sp. Infection Symptom Severity
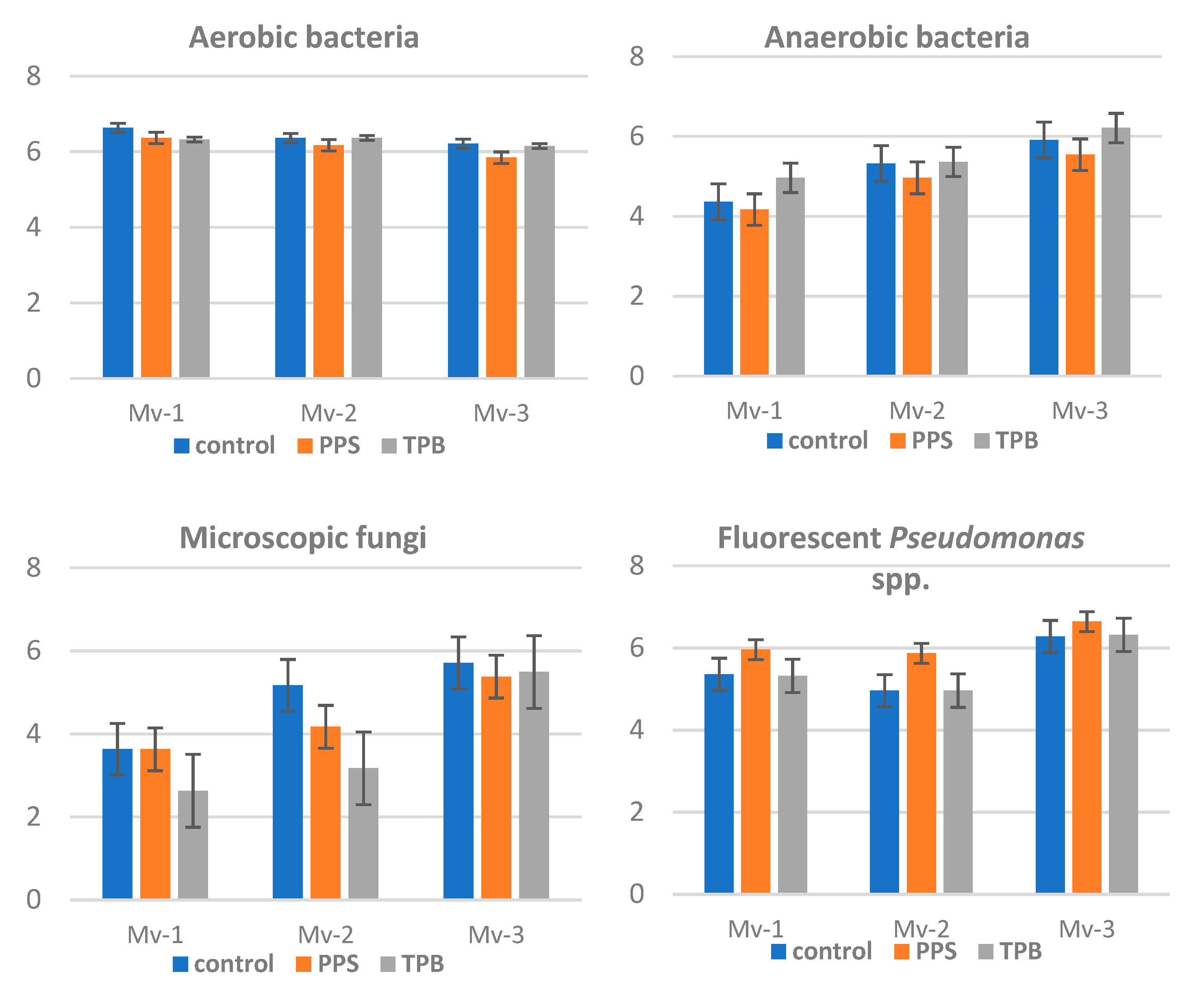
3.3. Seasonal Variability of Detectable Microorganisms
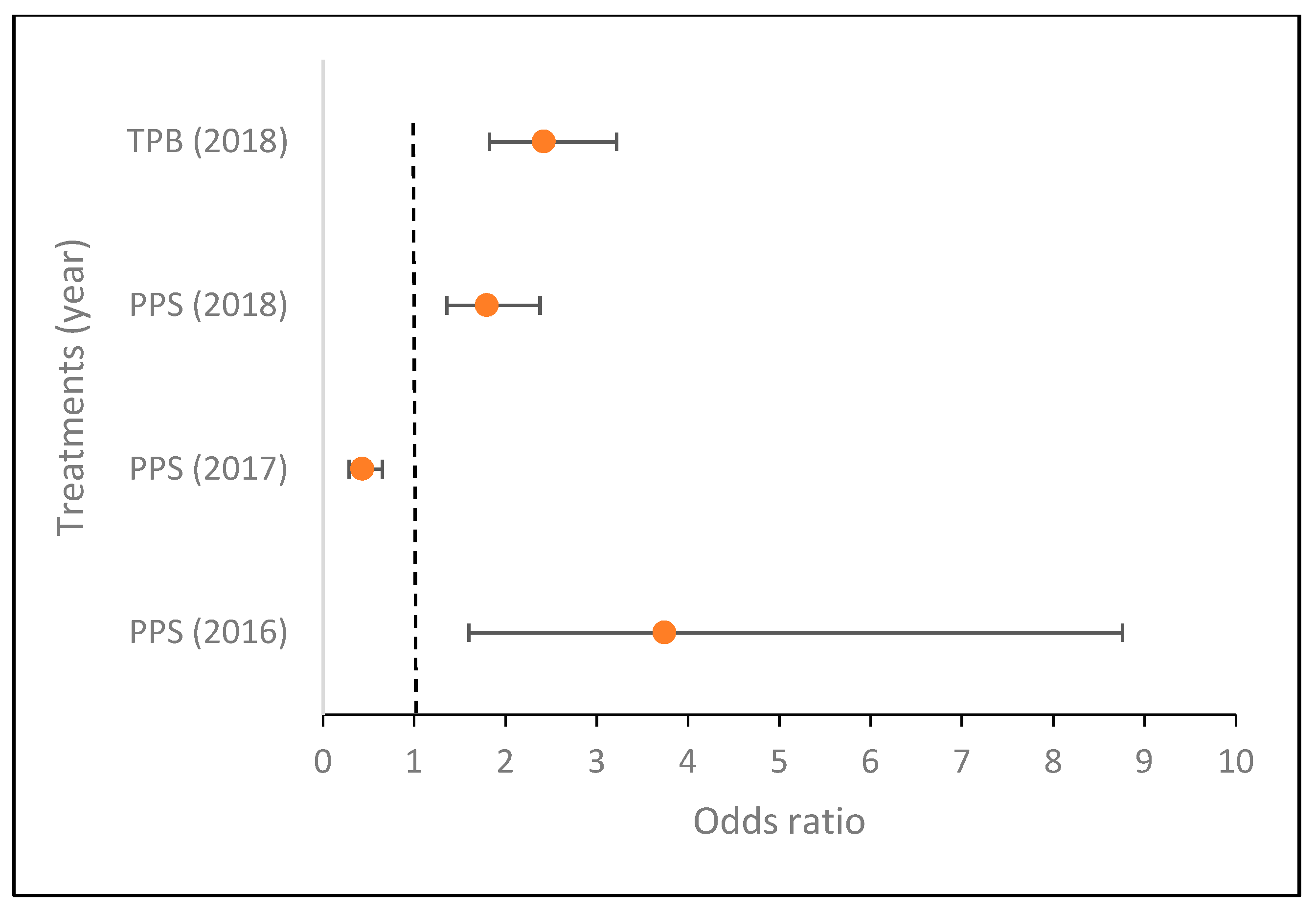
3.4. Comparison of the Effect of Microbial Inoculants on Tuber Infection
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloo, B.N.; Mbega, E.R.; Makumba, B.A. Rhizobacteria-based technology for sustainable cropping of potato (Solanum tuberosum L.). Potato Res. 2020, 63, 157–177. [Google Scholar] [CrossRef]
- Naqqash, T.; Hameed, S.; Imran, A.; Hanif, M.K.; Majeed, A.; van Elsas, J.D. Differential response of potato toward inoculation with taxonomically diverse plant growth promoting rhizobacteria. Front. Plant Sci. 2016, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Veerman, C.; Bastioli, C.; Biró, B.; Bouma, J.; Cienciala, E.; Emmett, B.; Frison, E.A.; Grand, A.; Hristov, L.; Kriaučiūnienė, Z.; et al. Caring for Soil Is Caring for Life—Ensure 75% of Soils Are Healthy by 2030 for Food, People, Nature and Climate; Independent Expert Report; European Commission, Publ. Office of European Union: Luxembourg, 2020; p. 82. [Google Scholar]
- Richardson, A.E.; Simpson, R.J. Soil microorganisms mediating phosphorus availability update on microbial phosphorus. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef]
- Foo, E.; Plett, J.M.; Lopez-Raez, J.A.; Reid, D. The role of plant hormones in plant-microbe symbioses. Front. Plant Sci. 2019, 10, 1391. [Google Scholar] [CrossRef]
- Kumar, A.; Kuzyakov, Y.; Pausch, J. Maize rhizosphere priming: Field estimates using 13C natural abundance. Plant Soil 2016, 409, 87–97. [Google Scholar] [CrossRef]
- Kays, S.J. The physiology of yield in the sweet potato. In Sweet Potato Products: A Natural Resource for the Tropics; CRC Press: Boca Raton, FL, USA, 2018; pp. 79–132. [Google Scholar]
- Shi, J.; Yu, X.; Zhang, M.; Lu, S.; Wu, W.; Wu, J.; Xu, J. Potential risks of copper, zinc, and cadmium pollution due to pig manure application in a soil–rice system under intensive farming: A case study of Nanhu, China. J. Environ. Qual. 2011, 40, 1695–1704. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, R.; Srednicka-Tober, D.; Hallmann, E.; Kopczynska, K.; Zarzynska, K. The impact of organic vs. conventional agricultural practices on selected quality features of eight potato cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, T.; Biró, B.; Mátrai, G.; Ulmer, Á.; Kotroczó, Z. Long-term effect of plant coal biochar on soil organic matter content and some agrochemical parameters. Kertgazdaság 2016, 48, 89–96. (In Hungarian) [Google Scholar]
- Kotroczó, Z.; Fekete, I. Significance of soil respiration from biological activity in the degradation processes of different types of organic matter. DRC Sustain. Future J. Environ. Agric. Energy 2020, 1, 171–179. [Google Scholar]
- Dudás, A.; Kotroczó, Z.; Vidéki, E.; Wass-Matics, H.; Kocsis, T.; Szalai, M.Z.; Végvári, G.; Biró, B. Fruit quality of tomato affected by single and combined bioeffectors in organically system. Pak. J. Agric. Sci. 2017, 54, 847–856. [Google Scholar]
- Dudás, A.; Szalai, Z.M.; Vidéki, E.; Wass-Matics, H.; Kocsis, T.; Végvári, G.; Kotroczó, Z.; Biró, B. Sporeforming bacillus bioeffectors for healthier fruit quality of tomato in pots and field. Appl. Ecol. Environm. Res. 2017, 15, 1399–1418. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Biofector. Available online: www.biofector.info (accessed on 29 July 2021).
- Li, H.; Qiu, Y.; Yao, T.; Ma, Y.; Zhang, H.; Yang, X. Effects of PGPR microbial inoculants on the growth and soil properties of Avena sativa, Medicago sativa, and Cucumis sativus seedlings. Soil Tillage Res. 2020, 199, 104577. [Google Scholar] [CrossRef]
- Hassen, A.I.; Bopape, F.L.; Sanger, L.K. Microbial inoculants as agents of growth promotion and abiotic stress tolerance in plants. In Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New Delhi, India, 2016; pp. 23–36. [Google Scholar]
- Matyjaszczyk, E. Plant protection means used in organic farming throughout the European Union. Pest Manag. Sci. 2018, 74, 505–510. [Google Scholar] [CrossRef]
- Diallo, S.; Crépin, A.; Barbey, C.; Orange, N.; Burini, J.F.; Latour, X. Mechanisms and recent advances in biological control mediated through the potato rhizosphere. FEMS Microbiol. Ecol. 2011, 75, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wang, W.; Ma, Y.; Liu, Y.; Ma, X.; An, L.; Feng, H. Prospect of beneficial microorganisms applied in potato cultivation for sustainable agriculture. Afr. J. Microbiol. Res. 2013, 7, 2150–2158. [Google Scholar]
- Fiers, M.; Chatot, C.; Edel-Hermann, V.; Hungrat, I.; Konate, A.Y.; Gautheron, N.; Guillery, E.; Alabouvette, C.; Steinberg, C. Diversity of microorganisms associated with atypical superficial blemishes of potato tubers and pathogenicity assessment. Eur. J. Plant Pathol. 2010, 128, 353–371. [Google Scholar] [CrossRef]
- Biró, B.; Kádár, I.; Lampis, S.; Gullner, G.; Kőmíves, T. Inside and outside rhizosphere parameters of barley and dose-dependent stress alleviation at some chronic metal exposures. Acta Phytopathol. Entomol. Hung. 2012, 47, 373–383. [Google Scholar] [CrossRef]
- Carvalhais, L.C.; Dennis, P.G.; Fan, B.; Fedoseyenko, D.; Kierul, K.; Becker, A.; Borriss, R. Linking plant nutritional status to plant-microbe interactions. PLoS ONE 2013, 8, e68555. [Google Scholar]
- Biró, B.; Dudás, A.; Wass-Matics, H.; Kocsis, T.; Pabar, S.; Tóth, E.; Kotroczó, Z. Improved soil and tomato quality by some biofertilizer products. Acta Agric. Debr. 2018, 150, 93–105. [Google Scholar] [CrossRef]
- Wanner, L.A.; Kirk, W.W. Streptomyces—From basic microbiology to role as a plant pathogen. Am. J. Potato Res. 2015, 92, 236–242. [Google Scholar] [CrossRef]
- Kirk, W.W.; Wharton, P.S. Fungal and bacterial disease aspects of potato production. In The Potato, Botany, Production and Uses; Navarre, R., Pavek, M., Eds.; CABI: Boston, MA, USA, 2014; pp. 167–201. [Google Scholar]
- Tariq, M.; Yasmin, S.; Hafeez, F.Y. Biological control of potato black scurf by rhizosphere associated bacteria. Braz. J. Microbiol. 2010, 41, 439–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brewer, M.T.; Larkin, R.P. Efficacy of several potential biocontrol organisms against Rhizoctonia solani on potato. Crop Prot. 2005, 24, 939–950. [Google Scholar] [CrossRef]
- Xie, J.; Jiang, D. New insights into mycoviruses and exploration for the biological control of crop fungal diseases. Annu. Rev. Phytopathol. 2014, 52, 45–68. [Google Scholar] [CrossRef] [Green Version]
- Sindhu, S.S.; Rakshiya, Y.S.; Sahu, G. Biological control of soilborne plant pathogens with rhizosphere bacteria. Pest. Technol. 2009, 3, 10–21. [Google Scholar]
- Abod, É.; Laslo, É.; Szentes, S.; Lányi, S.; Mara, G. Plant growth promoting bacteria: Strategies to improve wheat growth and development under sustainable agriculture. In Plant Growth Promoting Rhizobacteria for Agricultural Sustainability; Kumar, A., Meena, V., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Kocsis, T. Biochar and Bioeffector Combinations Affected on Biological Characteristics of Sandy Soils. Ph.D. Thesis, Doctoral School of Horticultural Sciences, Szent István University, Budapest, Hungary, 2018; 110p. (In Hungarian). [Google Scholar]
- Libisch, B.; Villányi, I.; Füzy, A.; Horváth, N.; Biró, B. Identification and characterization of bacterial strains capable to degrade aircraft deicing fluids at four degrees. J. Biotechnol. 2010, 1505, 259. [Google Scholar] [CrossRef]
- Cochran, W.G. Estimation of bacterial densities by means of the most probable number. Biometrics 1950, 6, 105–116. [Google Scholar] [CrossRef]
- Hilbe, J.M. Logistic Regression Models; Chapman and Hall/CRC: London, UK, 2009; 656p. [Google Scholar]
- Pohlert, T. The pairwise multiple comparison of mean ranks package (PMCMR). R Package 2019, 27, 9. [Google Scholar]
- BIOFECTOR 2012-2017 Funded by the European Commission within the 7th Framework Programme|Grant Agmt. No. 312117. Available online: www.biofector.info (accessed on 15 June 2021).
- Tsavkelova, E.A.; Klimova, S.Y.; Cherdyntseva, T.A.; Netrusov, A.I. Microbial producers of plant growth stimulators and their practical use: A review. Appl. Biochem. Microbiol. 2006, 42, 117–126. [Google Scholar] [CrossRef]
- Zechmeister-Boltenstern, S.; Keiblinger, K.M.; Mooshammer, M.; Peñuelas, J.; Richter, A.; Sardans, J.; Wanek, W. The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr. 2015, 85, 133–155. [Google Scholar] [CrossRef] [Green Version]
- Mensink, B.J.W.G.; Scheepmaker, J.W. How to evaluate the environmental safety of microbial plant protection products: A proposal. Biocontrol Sci. Technol. 2007, 17, 3–20. [Google Scholar] [CrossRef]
- Tein, B.; Kauer, K.; Eremeev, V.; Luik, A.; Selge, A.; Loit, E. Farming systems affect potato (Solanum tuberosum L.) tuber and soil quality. Field Crops Res. 2014, 156, 1–11. [Google Scholar] [CrossRef]
- Mie, A.; Andersen, H.R.; Gunnarsson, S.; Kahl, J.; Kesse-Guyot, E.; Rembiałkowska, E.; Quaglio, G.; Grandjean, P. Human health implications of organic food and organic agriculture: A comprehensive review. Environ. Health 2017, 16, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The effect on tuber quality of an organic versus a conventional cultivation system in the early crop potato. J. Food Compos. Anal. 2017, 62, 189–196. [Google Scholar] [CrossRef]

| Parameters | 2016 | 2017 | 2018 |
|---|---|---|---|
| pH(H₂O) | 7.20 | 7.82 | 7.59 |
| Dry matter content m/m% | 85.3 | 89.3 | n.d. |
| CaCO3 m/m% | 2.52 | 1.61 | 5.42 |
| Humus content m/m% | 3.54 | 3.30 | 2.92 |
| NO3 mg/kg | 230 | 532 | 296 |
| P2O5 mg/kg | 874 | 1280 | 645 |
| K2O mg/kg | 376 | 355 | 334 |
| Years | Month | Rainfall (mm) | Temperature (°C) | Irrigation (mm) |
|---|---|---|---|---|
| 2016 | April | 13.4 | 11.8 | 16 |
| May | 12.9 | 15.1 | - | |
| June | 7.1 | 19.9 | 20 | |
| July | 16 | 19.9 | - | |
| August | 0.9 | 9.9 | - | |
| 2017 | April | 55 | 9.1 | - |
| May | 45 | 16 | 22 | |
| June | 37 | 21.1 | 45 | |
| July | 37 | 21.4 | 18 | |
| August | 37 | n.d. | - | |
| 2018 | April | 14 | n.d. | - |
| May | 51 | n.d. | - | |
| June | 131 | 20.4 | - | |
| July | 43.5 | 22.4 | - | |
| August | 65.5 | 23.1 | - |
| Treatments (Origin) | Biofertilizer Strains in Inoculants | Microorganisms in Products (CFU mL−1) and Application Rates (mL/Tubers) |
|---|---|---|
| C (Control) | Without any microbial treatments | 100 mL water |
| PPS (Sapientia Hungarian University of Transylvania) | Pseudomonas protegens Pseudomonas jessenii Stenotrophomonasmaltophilia | Mix of strains in 100 mL suspension (CFU 1 × 108 mL−1) |
| TPB (MATE, Hungary) | Trichoderma atroviride Pseudomonas putida Bacillus subtilis | Mix of strains in 100 mL suspension (CFU 1 × 108) |
| Inoculants | Infection | 2016 | 2017 | 2018 |
|---|---|---|---|---|
| Rhizoctonia sp. incidence | high | medium | weak | |
| TPB | Rhizoctonia sp. | ↓ * | ↓ | 0 |
| PPS | Rhizoctonia sp. | ↓ | ↑ * | 0 |
| Streptomyces sp. incidence | weak | strong | large differences | |
| TPB | Streptomyces sp. | 0 | ↑ | ↑ * |
| PPS | Streptomyces sp. | ↑ * | ↓ * | ↑ * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papp, O.; Kocsis, T.; Biró, B.; Jung, T.; Ganszky, D.; Abod, É.; Tirczka, I.; Tóthné Bogdányi, F.; Drexler, D. Co-Inoculation of Organic Potato with Fungi and Bacteria at High Disease Severity of Rhizoctonia solani and Streptomyces spp. Increases Beneficial Effects. Microorganisms 2021, 9, 2028. https://doi.org/10.3390/microorganisms9102028
Papp O, Kocsis T, Biró B, Jung T, Ganszky D, Abod É, Tirczka I, Tóthné Bogdányi F, Drexler D. Co-Inoculation of Organic Potato with Fungi and Bacteria at High Disease Severity of Rhizoctonia solani and Streptomyces spp. Increases Beneficial Effects. Microorganisms. 2021; 9(10):2028. https://doi.org/10.3390/microorganisms9102028
Chicago/Turabian StylePapp, Orsolya, Tamás Kocsis, Borbála Biró, Timea Jung, Daniel Ganszky, Éva Abod, Imre Tirczka, Franciska Tóthné Bogdányi, and Dóra Drexler. 2021. "Co-Inoculation of Organic Potato with Fungi and Bacteria at High Disease Severity of Rhizoctonia solani and Streptomyces spp. Increases Beneficial Effects" Microorganisms 9, no. 10: 2028. https://doi.org/10.3390/microorganisms9102028







