Anti-Inflammatory Activities of Euglena gracilis Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cultures of Euglena gracilis
2.2. Preparation of Paramylon and Crude and Fractionated Extracts from Euglena gracilis Cells
2.3. Human Cell Culture Conditions
2.4. Cell Viability Assay
2.5. Enzyme-Linked Immunosorbent Assay
2.6. Assessment of Intracellular Glutathione Content
2.7. Detection of Intracellular Reactive Oxygen Species (ROS) Levels
2.8. Evaluation of Lipid Peroxidation
2.9. Statistical Analysis
3. Results
3.1. Analysis of Euglena gracilis Extracts and Biological Activities
3.2. Identification of Non-Cytotoxic Concentrations of Extracts from E. gracilis
3.3. Sub-Fractionated Extracts from E. gracilis Dampen LPS-Induced Inflammation
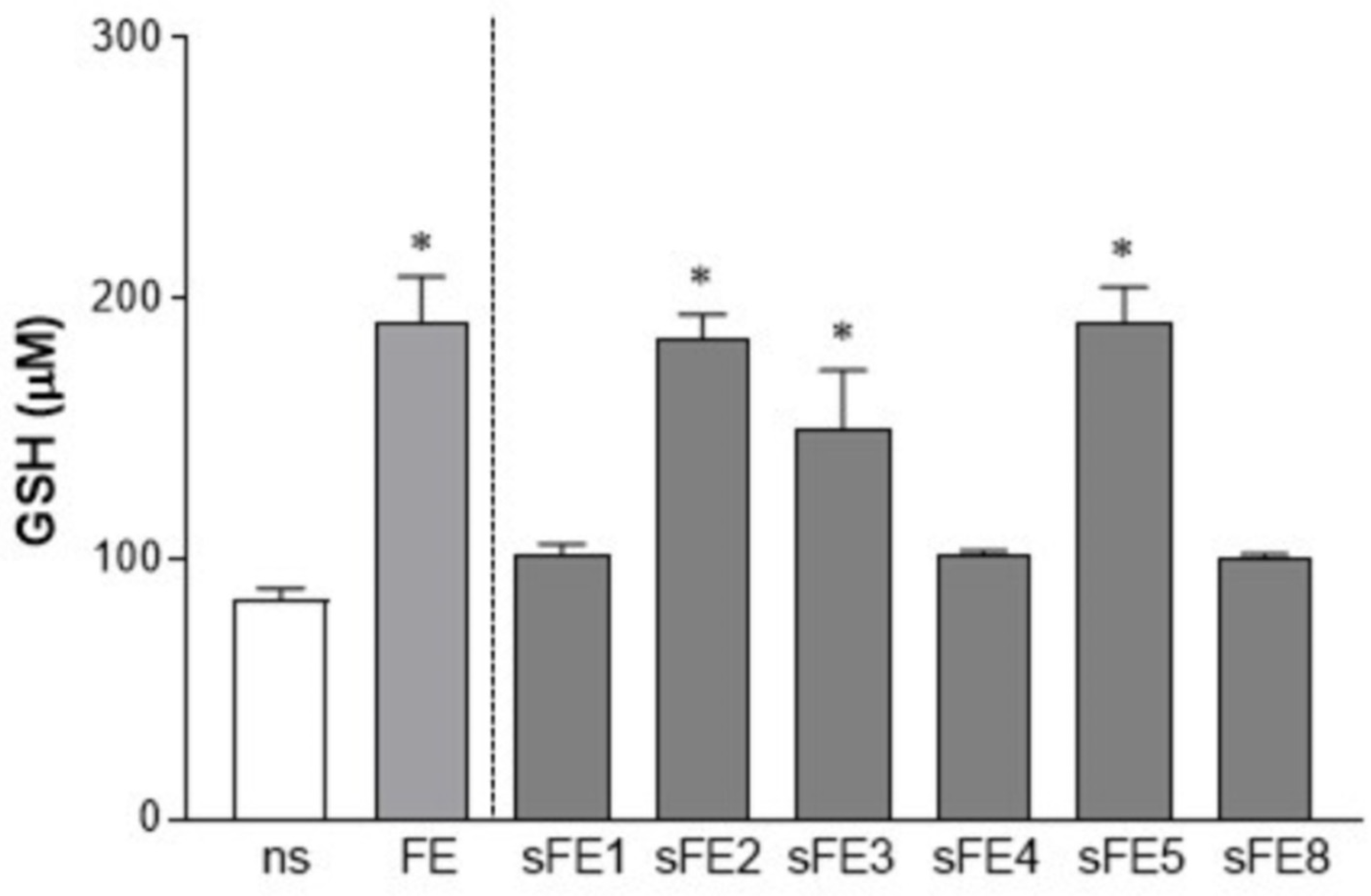
3.4. Extracts from E. gracilis Increase the Intracellular Reducing Potential
3.5. Extracts from E. gracilis Reduce Intracellular Reactive Oxygen Species Generated by LPS
3.6. Extracts from E. gracilis Prevent Lipid Peroxidation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buetow, D.E. The Biology of Euglena; Academic Press: New York, NY, USA, 1989; Volume I–IV. [Google Scholar]
- Enzing, A.C.; Ploeg, M.; Barbosa, M. Microalgae-based products for the food and feed sector: An outlook for Europe. JRC Sci. Policy Rep. 2014, 19–37. [Google Scholar] [CrossRef]
- Laza, M.E.P.; Errero, M.I.H.; Ifuentes, A.L.C. Innovative Natural Functional Ingredients from Microalgae. J. Agric. Food Chem. 2009, 57, 7159–7170. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Nutrition, Novel Foods and Food Allergens). Scientific Opinion on the safety of dried whole cell Euglena gracilis as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2020, 18, e06100. [Google Scholar] [CrossRef]
- Zeng, M.; Hao, W.; Zou, Y.; Shi, M.; Jiang, Y.; Xiao, P.; Lei, A.; Hu, Z.; Zhang, W.; Zhao, L.; et al. Fatty acid and metabolomic profiling approaches differentiate heterotrophic and mixotrophic culture conditions in a microalgal food supplement ‘Euglena’. BMC Biotechnol. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsanti, L.; Passarelli, V.; Evangelista, V.; Frassanito, A.M.; Gualtieri, P. Chemistry, physico-chemistry and applications linked to biological activities of b-glucans. Nat. Prod. Rep. 2011, 28, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Rischer, H.; Wiebe, M.G.; Wang, Y.; Seppa, T. Euglena gracilis growth and cell composition under different temperature, light and trophic conditions. PLoS ONE 2018, 13, e0195329. [Google Scholar] [CrossRef] [Green Version]
- Quesada, L.A.; Lustig, E.S.D.; Marechal, L.R.; Belocopitow, E. Antitumor activity of paramylon on sarcoma-180 in mice. Gan 1976, 67, 455–459. [Google Scholar]
- Kondo, Y.; Kato, A.; Hojo, H.; Nozoe, S.; Takeuchi, M.; Ochi, K. Cytokine-related immunopotentiating activities of paramylon, a beta-(1-->3)-D-glucan from Euglena gracilis. J. Pharm. Dyn. 1992, 15, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Sakagami, H.; Utsumi, A.; Fujinaga, S.; Takeda, M.; Asano, K.; Sugawara, I.; Ichikawa, S.; Kondo, H.; Mori, S.; et al. Anti-HIV (human immunodeficiency virus) activity of sulfated paramylon. Antiv. Res. 1993, 21, 1–14. [Google Scholar] [CrossRef]
- Sugiyama, A.; Suzuki, K.; Mitra, S.; Arashida, R.; Yoshida, E. Hepatoprotective effects of paramylon, a β-1, 3-D-Glucan Isolated from Euglena gracilis Z, on Acute Liver Injury Induced by Carbon Tetrachloride in Rats. J. Vet. Med. Sci. 2009, 71, 885–890. [Google Scholar] [CrossRef] [Green Version]
- Toliva, A.; Conforti, V.; Cordoba, O.; Flores, L. Chemical constituents and biological activity of Euglena gracilis extracts. J. Pharm. Res. 2013, 7, 209–214. [Google Scholar] [CrossRef]
- Watanabe, T.; Shimada, R.; Matsuyama, A.; Yuasa, M. Antitumor activity of the β-glucan paramylon from Euglena against preneoplastic colonic aberrant crypt. Food Funct. 2013, 4, 1685–1690. [Google Scholar] [CrossRef]
- Shimada, R.; Fujita, M.; Yuasa, M.; Sawamura, H.; Watanabe, T.; Nakashima, A.; Suzuki, K. Oral administration of green algae, Euglena gracilis, inhibits hyperglycemia in OLETF rats, a model of spontaneous type 2 diabetes. Food Funct. 2016, 7, 4655–4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russo, R.; Barsanti, L.; Evangelista, V.; Frassanito, A.M.; Longo, V.; Pucci, L.; Penno, G.; Gualtieri, P. Euglena gracilis paramylon activates human lymphocytes by upregulating pro- inflammatory factors. Food Sci. Nutr. 2016, 5, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, R.; Ishibashi-ohgo, N.; Atsuji, K.; Miwa, Y.; Iwata, O. Euglena extract suppresses adipocyte-differentiation in human adipose-derived stem cells. PLoS ONE 2018, 13, e0192404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, K.; Nakashima, A.; Igarashi, M.; Saito, K.; Konno, M.; Yamazaki, N.; Takimoto, H. Euglena gracilis Z and its carbohydrate storage substance relieve arthritis symptoms by modulating Th17 immunity. PLoS ONE 2018, 13, e0191462. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, A.; Hata, S.; Suzuki, K.; Yoshida, E.; Nakano, R. Oral administration of paramylon, a β-1,3-D-glucan isolated from Euglena gracilis Z inhibits development of atopic dermatitis-Like skin lesions in NC/Nga mice. J. Vet. Med. Sci. 2010, 72, 755–763. [Google Scholar] [CrossRef] [Green Version]
- Stienstra, R.; Duval, C.; Michael, M.; Kersten, S. PPARs, Obesity, and Inflammation. PPAR Res. 2007, 2007, 095974. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Obesity and Overweight. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 16 August 2021).
- Olefsky, J.M.; Glass, C.K. Macrophages, Inflammation, and Insulin Resistance. Annu. Rev. Physiol. 2010, 72, 219–246. [Google Scholar] [CrossRef]
- Brun, P.; Castagliuolo, I.; Leo, V.D.; Buda, A.; Pinzani, M.; Palu, G.; Martines, D. Increased intestinal permeability in obese mice: New evidence in the pathogenesis of nonalcoholic steatohepatitis. Am. J. Physiol. Grastrointest. Liver Physiol. 2007, 292, G518–G525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Amar, J.; Iglesias, M.A.; Poggi, M.; Knauf, C.; Bastelica, D.; Neyrinck, A.M.; Fava, F.; Tuohy, K.M.; Chabo, C.; et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007, 56, 1761–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergès, B.; Duvillard, L.; Lagrost, L.; Vachoux, C.; Garret, C.; Bouyer, K.; Courtney, M.; Pomié, C.; Burcelin, R. Changes in lipoprotein kinetics associated with type 2 diabetes affect the distribution of lipopolysaccharides among lipoproteins. J. Clin. Endocrinol. Metab. 2014, 99, E1245–E1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and oxidative stress in an obese state and the protective effects of gallic acid. Nutrients 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Loh, K.; Deng, H.; Fukushima, A.; Cai, X.; Boivin, B.; Galic, S.; Bruce, C.; Shields, B.J.; Skiba, B.; Ooms, L.M.; et al. Reactive Oxygen Species Enhance Insulin Sensitivity. Cell Metab. 2009, 10, 260–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cramer, M.; Myers, J. Growth and Photosynthetic Characteristics of Euglena graeilis. Arch. Mikrobiol. 1952, 17, 384–402. [Google Scholar] [CrossRef]
- Schwartzbach, S.D.; Shigeoka, S. Euglena: Biochemistry, Cell and Molecular Biology; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Method Enzymol. 1987, 148, 350–382. [Google Scholar]
- Yang, C.M.; Chang, K.W.; Yin, M.H.; Huang, H.M. Methods for the determination of the chlorophylls and their derivatives. Taiwania 1998, 43, 116–122. [Google Scholar]
- Hager, A.; Stransky, H. Das carotinoidmuster und die verbreitung des lichtinduzierten xanthophyllcyclus in verschiedenen algenklassen. V. Ein- zelne vertreter der Cryptophyceae, Euglenophyceae, Bacillariophyceae, Chrysophyceae und Phaeophyceae. Arch. Mikrobiol. 1970, 73, 77–89. [Google Scholar] [CrossRef]
- Heelis, D.V.; William Kernick, W.; Phillips, G.O.; Davies, K. Separation and identification of the carotenoid pigments of stigmata isolated from light-grown cells of Euglena gracilis strain Z. Arch. Microbiol. 1979, 121, 207–211. [Google Scholar] [CrossRef]
- Hynstovaa, V.; Sterbovaa, D.; Klejdusa, B.; Hedbavnya, J.; Huskaa, D.; Adam, V. Separation, identification and quantification of carotenoids and chlorophylls in dietary supplements containing Chlorella vulgaris and Spirulina platensis using High Performance Thin Layer Chromatography. J. Pharm. Biomed. Analysis 2018, 148, 108–118. [Google Scholar] [CrossRef]
- Krinski, N.I.; Gordon, A.; Stern, A.I. The appearance of neoxanthin during the regreening of dark-grown Euglena. Plant Physiol. 1964, 39, 441–445. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2007, 1, 3159–3165. [Google Scholar] [CrossRef]
- Brun, P.; Pathak, S.; Castagliuolo, I.; Palù, G.; Brun, P.; Zuin, M.; Cavazzana, R.; Martines, E. Helium generated cold plasma finely regulates activation of human fibroblast-like primary cells. PLoS ONE 2014, 9, e104397. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Zavala, J.S.; Ortiz-Cruz, M.A.; Mendoza-Hernández, G.; Moreno-Sánchez, R. Increased synthesis of α-tocopherol, paramylon and tyrosine by Euglena gracilis under conditions of high biomass production. J. Appl. Microbiol. 2010, 109, 2160–2172. [Google Scholar] [CrossRef] [PubMed]
- Piovan, A.; Filippini, R.; Corbioli, G.; Dalla Costa, V.; Giunco, E.M.V.; Burbello, G.; Pagetta, A.; Gisti, P.; Zusso, M. Carotenoid Extract Derived from Euglena gracilis Overcomes Lipopolysaccharide-Induced Neuroinflammation in Microglia: Role of NF-κB and Nrf2 Signaling Pathways. Mol. Neurobiol. 2021, 58, 3515–3528. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, V.; Chérouvrier, J.R.; Farhat, F.; Thiéry, V.; Piot, J.M.; Bérard, J.B.; Kaas, R.; Serive, B.; Patrice, T.; Cadoret, J.P.; et al. Study on the microalgal pigments extraction process: Performance of microwave assisted extraction. Process. Biochem. 2011, 46, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Amaro, H.M.; Fernandes, F.; Valentão, P.; Andrade, P.B.; Sousa-Pinto, I.; Malcata, F.X.; Guedes, A.C. Effect of solvent system on extractability of lipidic components of Scenedesmus obliquus (M2-1) and Gloeothece sp. on antioxidant scavenging capacity thereof. Mar. Drugs 2015, 13, 6453–6471. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.; Arora, S.; Lim, J.Y.; Lee, L.H.; Lim, L.H.K. The regulation of TNFα production after heat and endotoxin stimulation is dependent on Annexin-A1 and HSP70. Cell Stress Chaperones 2015, 20, 583–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-α Signaling in Macrophages. Crit. Rev. Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Saide, A.; Lauritano, C.; Ianora, A. Pheophorbide A: State of the art. Mar. Drugs 2020, 18, 257. [Google Scholar] [CrossRef]
- Islam, M.N.; Ishita, I.J.; Jin, S.E.; Choi, R.J.; Lee, C.M.; Kim, Y.S.; Jung, H.A.; Choi, J.S. Anti-inflammatory activity of edible brown alga Saccharina japonica and its constituents pheophorbide a and pheophytin a in LPS-stimulated RAW 264.7 macrophage cells. Food Chem. Toxicol. 2013, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Ditmars, W.E.; Van Winkle, Q. A comparative study of pheophytin a and pheophytin b monolayers. J. Phys. Chem. 1968, 72, 39–45. [Google Scholar] [CrossRef] [Green Version]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kankkunen, P.; Teirilä, L.; Rintahaka, J.; Alenius, H.; Wolff, H.; Matikainen, S. (1,3)-β-Glucans Activate Both Dectin-1 and NLRP3 Inflammasome in Human Macrophages. J. Immunol. 2010, 84, 6335–6342. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, F.; Hayashi, M.; Kondo, A. Comparative profiling analysis of central matabolites in Euglena gracilis under variuos cultivation conditions. Biosci. Biotechnol. Biochem. 2011, 75, 2253–2256. [Google Scholar] [CrossRef] [Green Version]
- Santiago-Vázquez, L.Z.; Mydlarz, L.D.; Pavlovich, J.G.; Jacobs, R.S. Identification of hydroxy fatty acids by liquid chromatography-atmospheric pressure chemical ionization mass spectroscopy in Euglena gracilis. J. Chromatogr. B 2004, 803, 233–236. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Tahir, N.; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A review of environmental contamination and health risk assessment of wastewater use for crop irrigation with a focus on low and high-income countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [Green Version]
- Bala, S.; Marcos, M.; Gattu, A.; Catalano, D.; Szabo, G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS ONE 2014, 9, e96864. [Google Scholar] [CrossRef] [PubMed]
- Ahola, A.J.; Lassenius, M.I.; Forsblom, C.; Harjutsalo, V.; Lehto, M.; Groop, P.H. Dietary patterns reflecting healthy food choices are associated with lower serum LPS activity. Sci. Rep. 2017, 7, 6511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shnyra, A.; Brewington, R.; Alipio, A.; Amura, C.; Morrison, D.C. Reprogramming of Lipopolysaccharide-Primed Macrophages Is Controlled by a Counterbalanced Production of IL-10 and IL-12. J. Immunol. 1998, 160, 3729–3736. Available online: http://www.jimmunol.org/content/160/8/3729 (accessed on 16 August 2021).
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen Peroxide Sensing and Signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
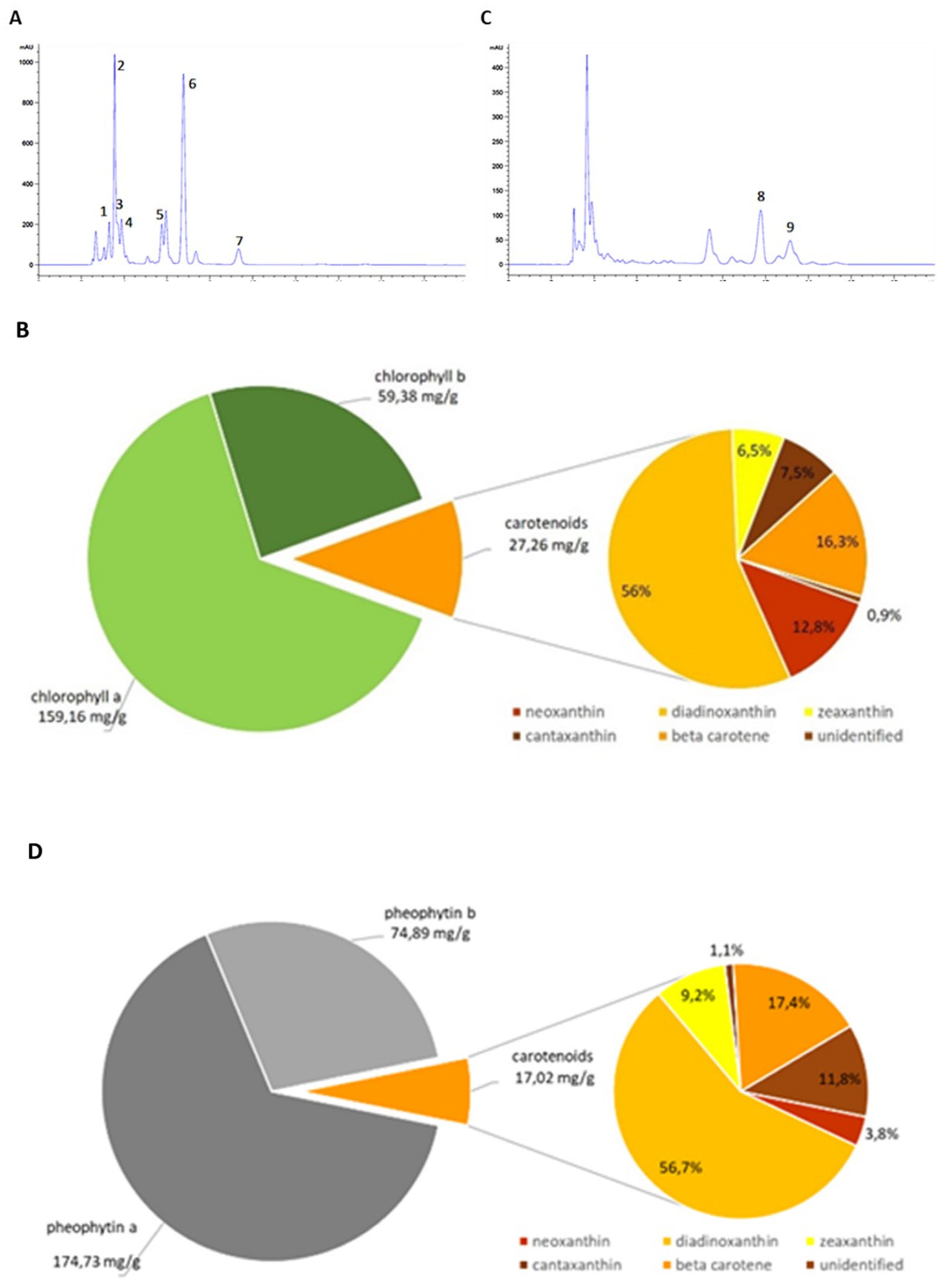





| Compounds | Rt (min) | Absorption Maxima (nm) | Rf |
|---|---|---|---|
| neoxanthin (1) | 3.3 | 435, 465 | 0.6 |
| diadinoxanthin (2) | 3.6 | 278, 445, 475 | 0.5 |
| zeaxanthin (3) | 3.7 | 275, 450, 476 | 0.6 |
| canthaxanthin (4) | 3.8 | 295, 475 | 0.65 |
| chlorophyll b (5) | 5.7 | 458, 617 | 0.7 |
| chlorophyll a (6) | 6.8 | 431, 663 | 0.68 |
| β-carotene (7) | 9.3 | 275, 451, 476 | 1 |
| pheophytin b (8) | 11.7 | 441, 650 | 0.75 |
| pheophytin a (9) | 13.1 | 410, 665 | 0.85 |
| pheophorbides | 3.6–3.8 | 441, 650; 410, 665 | 0.2 |
| Extracts from E. gracilis | Mφ IC50 | HT-29 IC50 |
|---|---|---|
| P | 30 | 45 |
| CE | 48 | 57 |
| FE | 47 | 59 |
| sFE1 | >100 | >100 |
| sFE2 | 31 | 39 |
| sFE3 | 33 | 41 |
| sFE4 | 50 | 55 |
| sFE5 | 47 | 58 |
| sFE6 | 38 | 46 |
| sFE7 | 37 | 42 |
| sFE8 | 36 | 44 |
| Extracts E. gracilis | TNF-α 24 h | TNF-α 4 days |
|---|---|---|
| FE | 1 | 2 |
| sFE1 | 0.1 | 0.1 |
| sFE2 | 1 | 1 |
| sFE3 | 10 | 10 |
| sFE4 | 2 | 2 |
| sFE5 | 5 | 5 |
| sFE8 | 1 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brun, P.; Piovan, A.; Caniato, R.; Dalla Costa, V.; Pauletto, A.; Filippini, R. Anti-Inflammatory Activities of Euglena gracilis Extracts. Microorganisms 2021, 9, 2058. https://doi.org/10.3390/microorganisms9102058
Brun P, Piovan A, Caniato R, Dalla Costa V, Pauletto A, Filippini R. Anti-Inflammatory Activities of Euglena gracilis Extracts. Microorganisms. 2021; 9(10):2058. https://doi.org/10.3390/microorganisms9102058
Chicago/Turabian StyleBrun, Paola, Anna Piovan, Rosy Caniato, Vanessa Dalla Costa, Anthony Pauletto, and Raffaella Filippini. 2021. "Anti-Inflammatory Activities of Euglena gracilis Extracts" Microorganisms 9, no. 10: 2058. https://doi.org/10.3390/microorganisms9102058
APA StyleBrun, P., Piovan, A., Caniato, R., Dalla Costa, V., Pauletto, A., & Filippini, R. (2021). Anti-Inflammatory Activities of Euglena gracilis Extracts. Microorganisms, 9(10), 2058. https://doi.org/10.3390/microorganisms9102058







