Novel and Conventional Isolation Techniques to Obtain Planctomycetes from Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Isolation
2.2. Phylogenetic Inference of Isolates
3. Results and Discussion
3.1. Macroalgae as Source for Planctomycetes
3.2. Isolation of Planctomycetes from the Sea Water Column and Marine Invertebrates and Sediments
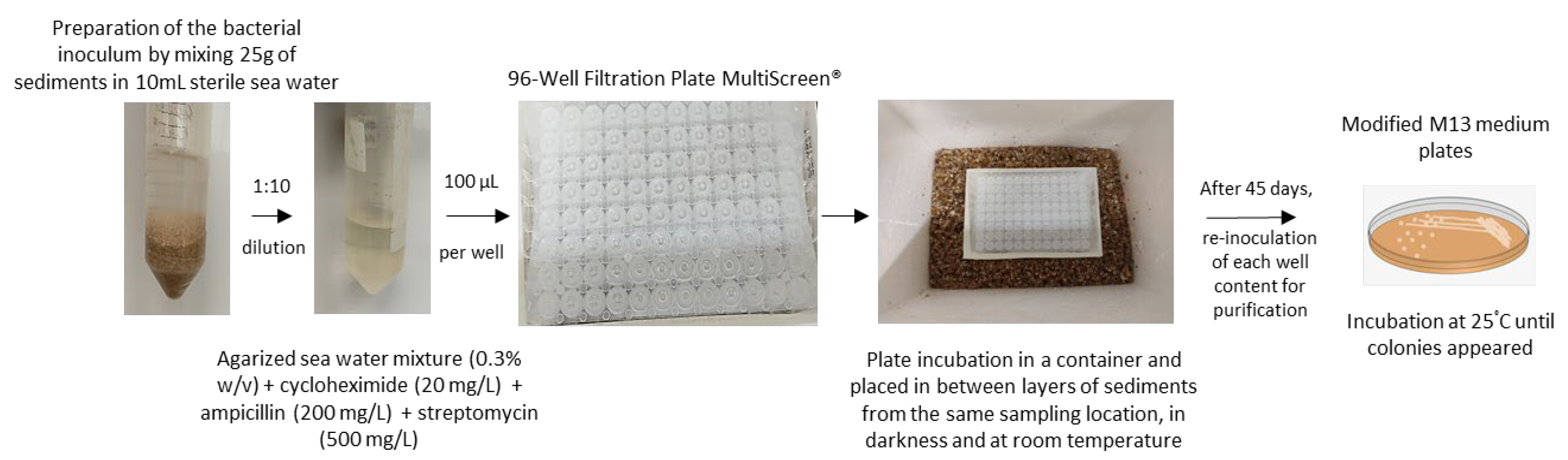
3.3. iChip Based In-Situ Culturing System for the Isolation of Planctomycetes from Marine Sediments
3.4. Ecology of the Isolated Planctomycetal Species
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiegand, S.; Jogler, M.; Jogler, C. On the maverick Planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef] [Green Version]
- Lage, O.M.; van Niftrik, L.; Jogler, C.; Devos, D.P. Planctomycetes. In Encyclopedia of Microbiology, 4th ed.; Schmidt, T.M., Ed.; Academic Press: Oxford, UK, 2019; pp. 614–626. [Google Scholar]
- Bondoso, J.; Balague, V.; Gasol, J.M.; Lage, O.M. Community composition of the Planctomycetes associated with different macroalgae. FEMS Microbiol. Ecol. 2014, 88, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondoso, J.; Godoy-Vitorino, F.; Balague, V.; Gasol, J.M.; Harder, J.; Lage, O.M. Epiphytic Planctomycetes communities associated with three main groups of macroalgae. FEMS Microbiol. Ecol. 2017, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumi, H.; Sagulenko, E.; Webb, R.I.; Fuerst, J.A. Isolation and diversity of planctomycetes from the sponge Niphates sp., seawater, and sediment of Moreton Bay, Australia. Antonie Van Leeuwenhoek 2013, 104, 533–546. [Google Scholar] [CrossRef]
- Lage, O.M.; Bondoso, J. Planctomycetes and macroalgae, a striking association. Front. Microbiol. 2014, 5, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lage, O.M.; Bondoso, J. Planctomycetes diversity associated with macroalgae. FEMS Microbiol. Ecol. 2011, 78, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallscheuer, N.; Wiegand, S.; Kohn, T.; Boedeker, C.; Jeske, O.; Rast, P.; Muller, R.W.; Brummer, F.; Heuer, A.; Jetten, M.S.M.; et al. Cultivation-Independent Analysis of the Bacterial Community Associated With the Calcareous Sponge Clathrina clathrus and Isolation of Poriferisphaera corsica Gen. Nov., Sp. Nov., Belonging to the Barely Studied Class Phycisphaerae in the Phylum Planctomycetes. Front. Microbiol. 2020, 11, 602250. [Google Scholar] [CrossRef]
- Kohn, T.; Wiegand, S.; Boedeker, C.; Rast, P.; Heuer, A.; Jetten, M.S.M.; Schuler, M.; Becker, S.; Rohde, C.; Muller, R.W.; et al. Planctopirus ephydatiae, a novel Planctomycete isolated from a freshwater sponge. Syst. Appl. Microbiol. 2020, 43, 126022. [Google Scholar] [CrossRef]
- Bengtsson, M.M.; Ovreas, L. Planctomycetes dominate biofilms on surfaces of the kelp Laminaria hyperborea. BMC Microbiol. 2010, 10, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kohn, T.; Heuer, A.; Jogler, M.; Vollmers, J.; Boedeker, C.; Bunk, B.; Rast, P.; Borchert, D.; Glockner, I.; Freese, H.M.; et al. Fuerstia marisgermanicae gen. nov., sp. nov., an Unusual Member of the Phylum Planctomycetes from the German Wadden Sea. Front. Microbiol. 2016, 7, 2079. [Google Scholar] [CrossRef] [Green Version]
- Dedysh, S.N.; Ivanova, A.A. Planctomycetes in boreal and subarctic wetlands: Diversity patterns and potential ecological functions. FEMS Microbiol. Ecol. 2019, 95. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.A.; Beletsky, A.V.; Rakitin, A.L.; Kadnikov, V.V.; Philippov, D.A.; Mardanov, A.V.; Ravin, N.V.; Dedysh, S.N. Closely Located but Totally Distinct: Highly Contrasting Prokaryotic Diversity Patterns in Raised Bogs and Eutrophic Fens. Microorganisms 2020, 8, 484. [Google Scholar] [CrossRef] [Green Version]
- Ivanova, A.A.; Kulichevskaya, I.S.; Merkel, A.Y.; Toshchakov, S.V.; Dedysh, S.N. High Diversity of Planctomycetes in Soils of Two Lichen-Dominated Sub-Arctic Ecosystems of Northwestern Siberia. Front. Microbiol. 2016, 7, 2065. [Google Scholar] [CrossRef] [Green Version]
- Elcheninov, A.G.; Podosokorskaya, O.A.; Kovaleva, O.L.; Novikov, A.A.; Toshchakov, S.V.; Bonch-Osmolovskaya, E.A.; Kublanov, I.V. Thermogemmata fonticola gen. nov., sp. nov., the first thermophilic planctomycete of the order Gemmatales from a Kamchatka hot spring. Syst. Appl. Microbiol. 2020, 44, 126157. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Youssef, N.H.; Luo, Q.; Najar, F.Z.; Roe, B.A.; Sisk, T.M.; Buhring, S.I.; Hinrichs, K.U.; Krumholz, L.R. Phylogenetic and metabolic diversity of Planctomycetes from anaerobic, sulfide- and sulfur-rich Zodletone Spring, Oklahoma. Appl. Environ. Microbiol. 2007, 73, 4707–4716. [Google Scholar] [CrossRef] [Green Version]
- Jogler, C.; Wiegand, S.; Boedeker, C.; Heuer, A.; Peeters, S.H.; Jogler, M.; Jetten, M.S.M.; Rohde, M.; Kallscheuer, N. Tautonia plasticadhaerens sp. nov., a novel species in the family Isosphaeraceae isolated from an alga in a hydrothermal area of the Eolian Archipelago. Antonie Van Leeuwenhoek 2020, 113, 1889–1900. [Google Scholar] [CrossRef]
- Giovannoni, S.J.; Schabtach, E.; Castenholz, R.W. Isosphaera pallida, gen. and comb. nov., a gliding, budding eubacterium from hot springs. Arch. Microbiol. 1987, 147, 276–284. [Google Scholar] [CrossRef]
- Kovaleva, O.L.; Merkel, A.Y.; Novikov, A.A.; Baslerov, R.V.; Toshchakov, S.V.; Bonch-Osmolovskaya, E.A. Tepidisphaera mucosa gen. nov., sp. nov., a moderately thermophilic member of the class Phycisphaerae in the phylum Planctomycetes, and proposal of a new family, Tepidisphaeraceae fam. nov., and a new order, Tepidisphaerales ord. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Slobodkina, G.B.; Kovaleva, O.L.; Miroshnichenko, M.L.; Slobodkin, A.I.; Kolganova, T.V.; Novikov, A.A.; van Heerden, E.; Bonch-Osmolovskaya, E.A. Thermogutta terrifontis gen. nov., sp. nov. and Thermogutta hypogea sp. nov., thermophilic anaerobic representatives of the phylum Planctomycetes. Int. J. Syst. Evol. Microbiol. 2015, 65, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, Y.; Kurahashi, M.; Sakiyama, Y.; Ohuchi, M.; Yokota, A.; Harayama, S. Phycisphaera mikurensis gen. nov., sp. nov., isolated from a marine alga, and proposal of Phycisphaeraceae fam. nov., Phycisphaerales ord. nov. and Phycisphaerae classis nov. in the phylum Planctomycetes. J. Gen. Appl. Microbiol. 2009, 55, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Krieg, N.R.; Staley, J.T.; Brown, D.R.; Hedlund, B.P.; Paster, B.J.; Ward, N.L.; Ludwig, W.; Whitman, W.B. Phylum XXV. Planctomycetes Garrity and Holt 2001 137 emend. Ward. In Bergey’s Manual of Systematic Bacteriology: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes; Springer: New York, NY, USA, 2010; Volume 4. [Google Scholar]
- Jetten, M.S.M.; Op den Camp, H.J.M.; Kuenen, J.G.; Strous, M. Description of the order brocadiales. In Bergey’s Manual of Systematic Bacteriology: The Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes; Springer: New York, NY, USA, 2010; Volume 4, pp. 596–603. [Google Scholar]
- Wiegand, S.; Jogler, M.; Boedeker, C.; Pinto, D.; Vollmers, J.; Rivas-Marin, E.; Kohn, T.; Peeters, S.H.; Heuer, A.; Rast, P.; et al. Cultivation and functional characterization of 79 planctomycetes uncovers their unique biology. Nat. Microbiol. 2020, 5, 126–140. [Google Scholar] [CrossRef]
- Rivas-Marin, E.; Canosa, I.; Devos, D.P. Evolutionary Cell Biology of Division Mode in the Bacterial Planctomycetes-Verrucomicrobia- Chlamydiae Superphylum. Front. Microbiol. 2016, 7, 1964. [Google Scholar] [CrossRef] [Green Version]
- van Niftrik, L.; Jetten, M.S. Anaerobic ammonium-oxidizing bacteria: Unique microorganisms with exceptional properties. Microbiol. Mol. Biol. Rev. 2012, 76, 585–596. [Google Scholar] [CrossRef] [Green Version]
- Kallscheuer, N.; Moreira, C.; Airs, R.; Llewellyn, C.A.; Wiegand, S.; Jogler, C.; Lage, O.M. Pink- and orange-pigmented Planctomycetes produce saproxanthin-type carotenoids including a rare C45 carotenoid. Environ. Microbiol. Rep. 2019, 11, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boedeker, C.; Schuler, M.; Reintjes, G.; Jeske, O.; van Teeseling, M.C.; Jogler, M.; Rast, P.; Borchert, D.; Devos, D.P.; Kucklick, M.; et al. Determining the bacterial cell biology of Planctomycetes. Nat. Commun. 2017, 8, 14853. [Google Scholar] [CrossRef] [PubMed]
- Shiratori, T.; Suzuki, S.; Kakizawa, Y.; Ishida, K.I. Phagocytosis-like cell engulfment by a planctomycete bacterium. Nat. Commun. 2019, 10, 5529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Graca, A.P.; Calisto, R.; Lage, O.M. Planctomycetes as Novel Source of Bioactive Molecules. Front. Microbiol. 2016, 7, 1241. [Google Scholar] [CrossRef] [PubMed]
- Jeske, O.; Jogler, M.; Petersen, J.; Sikorski, J.; Jogler, C. From genome mining to phenotypic microarrays: Planctomycetes as source for novel bioactive molecules. Antonie Van Leeuwenhoek 2013, 104, 551–567. [Google Scholar] [CrossRef] [PubMed]
- da Conceicao Marinho, M.; Lage, O.M.; Sousa, C.D.; Catita, J.; Antunes, S.C. Assessment of Rhodopirellula rubra as a supplementary and nutritional food source to the microcrustacean Daphnia magna. Antonie Van Leeuwenhoek 2019, 112, 1231–1243. [Google Scholar] [CrossRef]
- Marinho, M.C.; Lage, O.M.; Catita, J.; Antunes, S.C. Adequacy of planctomycetes as supplementary food source for Daphnia magna. Antonie Van Leeuwenhoek 2018, 111, 825–840. [Google Scholar] [CrossRef] [PubMed]
- Belova, S.E.; Saltykova, V.A.; Dedysh, S.N. Antimicrobial Activity of a Novel Freshwater Planctomycete Lacipirellula parvula PX69T. Microbiology 2020, 89, 503–509. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Jeske, O.; Sandargo, B.; Boedeker, C.; Wiegand, S.; Bartling, P.; Jogler, M.; Rohde, M.; Petersen, J.; Medema, M.H.; et al. The planctomycete Stieleria maiorica Mal15(T) employs stieleriacines to alter the species composition in marine biofilms. Commun. Biol. 2020, 3, 303. [Google Scholar] [CrossRef] [PubMed]
- Jeske, O.; Surup, F.; Ketteniss, M.; Rast, P.; Forster, B.; Jogler, M.; Wink, J.; Jogler, C. Developing Techniques for the Utilization of Planctomycetes As Producers of Bioactive Molecules. Front. Microbiol. 2016, 7, 1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calisto, R.; Sæbø, E.F.; Storesund, J.E.; Øvreås, L.; Herfindal, L.; Lage, O.M. Anticancer Activity in Planctomycetes. Front. Mar. Sci 2019, 5, 499. [Google Scholar] [CrossRef]
- Schlesner, H. The Development of Media Suitable for the Microorganisms Morphologically Resembling Planctomyces spp., Pirellula spp., and other Planctomycetales from Various Aquatic Habitats Using Dilute Media. Syst. Appl. Microbiol. 1994, 17, 135–145. [Google Scholar] [CrossRef]
- Dedysh, S.N.; Henke, P.; Ivanova, A.A.; Kulichevskaya, I.S.; Philippov, D.A.; Meier-Kolthoff, J.P.; Goker, M.; Huang, S.; Overmann, J. 100-year-old enigma solved: Identification, genomic characterization and biogeography of the yet uncultured Planctomyces bekefii. Environ. Microbiol. 2020, 22, 198–211. [Google Scholar] [CrossRef]
- Lage, O.M.; Bondoso, J. Bringing Planctomycetes into pure culture. Front. Microbiol. 2012, 3, 405. [Google Scholar] [CrossRef] [Green Version]
- Winkelmann, N.; Harder, J. An improved isolation method for attached-living Planctomycetes of the genus Rhodopirellula. J. Microbiol. Methods 2009, 77, 276–284. [Google Scholar] [CrossRef]
- Nichols, D.; Cahoon, N.; Trakhtenberg, E.M.; Pham, L.; Mehta, A.; Belanger, A.; Kanigan, T.; Lewis, K.; Epstein, S.S. Use of ichip for high-throughput in situ cultivation of "uncultivable" microbial species. Appl. Environ. Microbiol. 2010, 76, 2445–2450. [Google Scholar] [CrossRef] [Green Version]
- Kaeberlein, T.; Lewis, K.; Epstein, S.S. Isolating "uncultivable" microorganisms in pure culture in a simulated natural environment. Science 2002, 296, 1127–1129. [Google Scholar] [CrossRef] [Green Version]
- Bollmann, A.; Lewis, K.; Epstein, S.S. Incubation of environmental samples in a diffusion chamber increases the diversity of recovered isolates. Appl. Environ. Microbiol. 2007, 73, 6386–6390. [Google Scholar] [CrossRef] [Green Version]
- Berdy, B.; Spoering, A.L.; Ling, L.L.; Epstein, S.S. In situ cultivation of previously uncultivable microorganisms using the ichip. Nat. Protoc. 2017, 12, 2232–2242. [Google Scholar] [CrossRef]
- Liu, H.; Xue, R.; Wang, Y.; Stirling, E.; Ye, S.; Xu, J.; Ma, B. FACS-iChip: A high-efficiency iChip system for microbial ‘dark matter’ mining. Mar. Life Sci. Technol. 2021, 3, 162–168. [Google Scholar] [CrossRef]
- Sherpa, R.T.; Reese, C.J.; Montazeri Aliabadi, H. Application of iChip to Grow "Uncultivable" Microorganisms and its Impact on Antibiotic Discovery. J. Pharm. Pharm. Sci. 2015, 18, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Lodhi, A.F.; Zhang, Y.; Adil, M.; Deng, Y. Antibiotic discovery: Combining isolation chip (iChip) technology and co-culture technique. Appl. Microbiol. Biotechnol. 2018, 102, 7333–7341. [Google Scholar] [CrossRef] [PubMed]
- Piddock, L.J. Teixobactin, the first of a new class of antibiotics discovered by iChip technology? J. Antimicrob. Chemother. 2015, 70, 2679–2680. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.L.; Schneider, T.; Peoples, A.J.; Spoering, A.L.; Engels, I.; Conlon, B.P.; Mueller, A.; Schaberle, T.F.; Hughes, D.E.; Epstein, S.; et al. A new antibiotic kills pathogens without detectable resistance. Nature 2015, 517, 455–459. [Google Scholar] [CrossRef]
- Bondoso, J.; Albuquerque, L.; Nobre, M.F.; Lobo-da-Cunha, A.; da Costa, M.S.; Lage, O.M. Aquisphaera giovannonii gen. nov., sp. nov., a planctomycete isolated from a freshwater aquarium. Int. J. Syst. Evol. Microbiol. 2011, 61, 2844–2850. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA gene sequences and whole-genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Yarza, P.; Yilmaz, P.; Pruesse, E.; Glockner, F.O.; Ludwig, W.; Schleifer, K.H.; Whitman, W.B.; Euzeby, J.; Amann, R.; Rossello-Mora, R. Uniting the classification of cultured and uncultured bacteria and archaea using 16S rRNA gene sequences. Nat. Rev. Microbiol. 2014, 12, 635–645. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Dedysh, S.N.; Kulichevskaya, I.S.; Beletsky, A.V.; Ivanova, A.A.; Rijpstra, W.I.C.; Damste, J.S.S.; Mardanov, A.V.; Ravin, N.V. Lacipirellula parvula gen. nov., sp. nov., representing a lineage of planctomycetes widespread in low-oxygen habitats, description of the family Lacipirellulaceae fam. nov. and proposal of the orders Pirellulales ord. nov., Gemmatales ord. nov. and Isosphaerales ord. nov. Syst. Appl. Microbiol. 2020, 43, 126050. [Google Scholar] [CrossRef]
- Vitorino, I.; Albuquerque, L.; Wiegand, S.; Kallscheuer, N.; da Costa, M.S.; Lobo-Da-Cunha, A.; Jogler, C.; Lage, O.M. Alenimonas chondri sp. nov., a novel planctomycete isolated from the biofilm of the red alga Chondrus crispus. Syst. Appl. Microbiol. 2020, 43, 126083, correction in 2021, 44, 126219. [Google Scholar] [CrossRef] [PubMed]
- Lage, O.M.; Bondoso, J.; Viana, F. Isolation and characterization of Planctomycetes from the sediments of a fish farm wastewater treatment tank. Arch. Microbiol. 2012, 194, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Godinho, O.; Calisto, R.; Ovreas, L.; Quinteira, S.; Lage, O.M. Antibiotic susceptibility of marine Planctomycetes. Antonie Van Leeuwenhoek 2019, 112, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Kumar, D.; Jagadeeshwari, U.; Sreya, P.K.; Shabbir, A.; Sasikala, C.; Ramana, C.V. Crateriforma spongiae sp. nov., isolated from a marine sponge and emended description of the genus "Crateriforma". Antonie Van Leeuwenhoek 2021, 114, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kallscheuer, N.; Wiegand, S.; Boedeker, C.; Peeters, S.H.; Jogler, M.; Rast, P.; Heuer, A.; Jetten, M.S.M.; Rohde, M.; Jogler, C. Aureliella helgolandensis gen. nov., sp. nov., a novel Planctomycete isolated from a jellyfish at the shore of the island Helgoland. Antonie Van Leeuwenhoek 2020, 113, 1839–1849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fuerst, J.A.; Sambhi, S.K.; Paynter, J.L.; Hawkins, J.A.; Atherton, J.G. Isolation of a bacterium resembling Pirellula species from primary tissue culture of the giant tiger prawn (Penaeus monodon). Appl. Environ. Microbiol. 1991, 57, 3127–3134. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.F.; Xu, J.K.; Chen, Y.W.; Ding, W.Y.; Shao, A.Q.; Liang, X.; Zhu, Y.T.; Yang, J.L. Characterization of Gut Microbiome in the Mussel Mytilus galloprovincialis in Response to Thermal Stress. Front. Physiol. 2019, 10, 1086. [Google Scholar] [CrossRef] [Green Version]
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Jogler, M.; Wiegand, S.; Peeters, S.H.; Heuer, A.; Boedeker, C.; Jetten, M.S.M.; Rohde, M.; Jogler, C. Rubinisphaera italica sp. nov. isolated from a hydrothermal area in the Tyrrhenian Sea close to the volcanic island Panarea. Antonie Van Leeuwenhoek 2020, 113, 1727–1736. [Google Scholar] [CrossRef] [Green Version]
- Storesund, J.E.; Ovreas, L. Diversity of Planctomycetes in iron-hydroxide deposits from the Arctic Mid Ocean Ridge (AMOR) and description of Bythopirellula goksoyri gen. nov., sp. nov., a novel Planctomycete from deep sea iron-hydroxide deposits. Antonie Van Leeuwenhoek 2013, 104, 569–584. [Google Scholar] [CrossRef]
- Schlesner, H. Planctomyces brasiliensis sp. nov., a Halotolerant Bacterium from a Salt Pit. Syst. Appl. Microbiol. 1989, 12, 159–161. [Google Scholar] [CrossRef]
- Yoon, J.; Matsuo, Y.; Kasai, H.; Lee, M.-K. Phylogenetic and Taxonomic Analyses of Rhodopirellula caenicola Sp. Nov., a New Marine Planctomycetes Species Isolated from Iron Sand. J. Phylogen. Evol. Biol. 2015, 03. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Wiegand, S.; Jogler, M.; Boedeker, C.; Peeters, S.H.; Rast, P.; Heuer, A.; Jetten, M.S.M.; Rohde, M.; Jogler, C. Rhodopirellula heiligendammensis sp. nov., Rhodopirellula pilleata sp. nov., and Rhodopirellula solitaria sp. nov. isolated from natural or artificial marine surfaces in Northern Germany and California, USA, and emended description of the genus Rhodopirellula. Antonie Van Leeuwenhoek 2020, 113, 1737–1750. [Google Scholar] [CrossRef] [PubMed]
- Bondoso, J.; Albuquerque, L.; Lobo-da-Cunha, A.; da Costa, M.S.; Harder, J.; Lage, O.M. Rhodopirellula lusitana sp. nov. and Rhodopirellula rubra sp. nov., isolated from the surface of macroalgae. Syst. Appl. Microbiol. 2014, 37, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Roh, S.W.; Lee, H.W.; Yim, K.J.; Shin, N.R.; Lee, J.; Whon, T.W.; Lim, N.L.; Kim, D.; Bae, J.W. Rhodopirellula rosea sp. nov., a novel bacterium isolated from an ark clam Scapharca broughtonii. J. Microbiol. 2013, 51, 301–304. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Boedeker, C.; Heuer, A.; Rast, P.; Peeters, S.H.; Jetten, M.S.M.; Kaster, A.K.; Rohde, M.; Kallscheuer, N.; et al. Additions to the genus Gimesia: Description of Gimesia alba sp. nov., Gimesia algae sp. nov., Gimesia aquarii sp. nov., Gimesia aquatilis sp. nov., Gimesia fumaroli sp. nov. and Gimesia panareensis sp. nov., isolated from aquatic habitats of the Northern Hemisphere. Antonie Van Leeuwenhoek 2020, 113, 1999–2018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gaurav, K.; Pk, S.; A, S.; Uppada, J.; Ch, S.; Ch, V.R. Gimesia chilikensis sp. nov., a haloalkali-tolerant planctomycete isolated from Chilika lagoon and emended description of the genus Gimesia. Int. J. Syst. Evol. Microbiol. 2020, 70, 3647–3655. [Google Scholar] [CrossRef]


| Date | Source | Media | Antibiotic Treatment | Methodology | Location | |
|---|---|---|---|---|---|---|
| October 2018 | Chondrus crispus | 1:10 M13, M13 and ASM | Ampicillin+ streptomycin or none | Pieces of the thallus/ biofilm from the macroalgae surface | Traditional | Luz beach |
| Ulva sp. | ||||||
| Porphyra dioica | ||||||
| Fucus sp. | ||||||
| Sea water | Water filtration (0.22 µm pore) | |||||
| March 2020 | Actinia equina | M14 and M13+ NAG | Ampicillin+ streptomycin or vancomycin or imipenem or ciprofloxacin | Body maceration | Traditional | Memória beach |
| Mytilus edulis | Biofilm of the shell | |||||
| Sediments | Enrichment in liquid medium | |||||
| Sea water | Water filtration (0.22 µm pore) | |||||
| October 2020 | Mytilus edulis | M13 and NAGM | Ampicillin+ streptomycin | Biofilm of the shell | Traditional | Memória beach |
| Ulva sp. | Pieces of the thallus/ biofilm from the macroalgae surface | |||||
| Codium sp. | ||||||
| Porphyra dioica | ||||||
| Corallina sp. | ||||||
| Sea water | Water filtration (0.22 µm pore) | |||||
| Actinia equina | Body maceration | |||||
| Sediments | M13+ NAG | Enrichment in liquid medium | ||||
| Sediments | Natural medium | In-situ iChip based culturing system | Novel | |||
| Reagents | Units per Liter | |||||
|---|---|---|---|---|---|---|
| M13 Medium a | 1:10 M13 Medium | Ammonium Sulfate Medium (ASM) | N-acetylglucosamine Medium (NAGM) | M14 Medium a | M13+ NAG Medium | |
| Peptone | 0.25 g | 0.025 g | - | - | 1 g | 0.25 g |
| Yeast extract | 0.25 g | 0.025 g | - | - | 1 g | 0.25 g |
| 0.1 mM HCl-Tris buffer, pH 7.5 | 50 mL | 50 mL | 50 mL | 50 mL | 50 mL | 50 mL |
| Natural sea water filtrated trough a 0.22µm filter | 900 mL | 909 mL | 900 mL | 880 mL | 880 mL | 900 mL |
| Deionized water | 10 mL | 10 mL | 10 mL | - | - | 10 mL |
| Glucose solution in deionized water (2.5%) 1 | 10 mL | 1 mL | 10 mL | - | 40 mL | - |
| Vitamins solution 1,2 | 10 mL | 10 mL | 10 mL | 10 mL | 10 mL | 10 mL |
| Hutner’s solution 1,3 | 20 mL | 20 mL | 20 mL | 20 mL | 20 mL | 20 mL |
| Ammonium sulfate (NH4SO4) | - | - | 10 g | - | - | - |
| N-acetylglucosamine solution in deionized water (5%) 1 | - | - | - | 40 mL | - | 10 mL |
| Isolate Designation | Beach of Sampling | Date of Sampling In-Situ | Source * | Medium of Isolation ** | 16S rRNA Gene Similarity with the Closest Species Type Strain |
|---|---|---|---|---|---|
| LzU2 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® + Ant | 99.4% Rhodopirellula baltica |
| LzU3 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® + Ant | 99.8% Rhodopirellula baltica |
| LzU6 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® + Ant | 99.8 % Rhodopirellula baltica |
| LzU8 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® + Ant | 99.8% Rhodopirellula baltica |
| LzU9 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® + Ant | 99.8% Rhodopirellula baltica |
| LzU15 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® | 100.0% Rhodopirellula baltica |
| LzU16 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® | 99.4% Rhodopirellula baltica |
| LzU18 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® | 100.0% Rhodopirellula baltica |
| LzU19 | Luz | 10/2018 | P.Ulv | 1:10 M13+ Pevaril® | 99.8% Rhodopirellula baltica |
| LzU20 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 100.0% Rhodopirellula baltica |
| LzU21 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.7% Rhodopirellula baltica |
| LzU22 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.8% Rhodopirellula baltica |
| LzU23 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.7% Rhodopirellula baltica |
| LzU24 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.9% Rhodopirellula baltica |
| LzU25 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.9% Rhodopirellula baltica |
| LzU27 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.7% Rhodopirellula baltica |
| LzU29 | Luz | 10/2018 | P.Ulv | ASM + Pevaril® | 99.6% Rhodopirellula baltica |
| LzP3 | Luz | 10/2018 | P. Por | ASM + Pevaril® | 99.6% Rhodopirellula baltica |
| LzP6 | Luz | 10/2018 | P. Por | 1:10 M13+ Pevaril® + Ant | 99.2% Rhodopirellula baltica |
| LzP7 | Luz | 10/2018 | P. Por | 1:10 M13+ Pevaril® + Ant | 99.5% Rhodopirellula baltica |
| LzP8 | Luz | 10/2018 | P. Por | 1:10 M13+Pevaril® + Ant | 99.2% Rhodopirellula baltica |
| LzF4 | Luz | 10/2018 | P. Fuc | ASM + Pevaril® | 100.0% Rhodopirellula lusitana |
| LzA1 | Luz | 10/2018 | Sea water | M13+ Pevaril® + Ant | 99.5% Novipirellula caenicola |
| LzC1 | Luz | 10/2018 | SC.Cho | M13+ Pevaril® + Ant | 100.0% Alienimonas chondri |
| LzC2 | Luz | 10/2018 | SC.Cho | M13+ Pevaril® | 100.0% Alienimonas chondri |
| PMO112_11.1 | Memória | 03/2020 | SC.Myt | M14+ Pevaril® + Van | 100.0% Rubinisphaera brasiliensis |
| PMO137_2 | Memória | 03/2020 | Sediments | M13+NAG+ Pevaril® + Van | 99.9% Novipirellula rosea |
| PMO137_6 | Memória | 03/2020 | Sediments | M13+NAG+ Pevaril® + Van | 99.9% Novipirellula rosea |
| PMO137_9 | Memória | 03/2020 | Sediments | M13+NAG+ Pevaril® + Van | 99.9% Novipirellula rosea |
| PMO137_10 | Memória | 03/2020 | Sediments | M13+NAG+ Pevaril® + Van | 99.9% Novipirellula rosea |
| PMO137_3 | Memória | 03/2020 | Sediments | M13+NAG+ Pevaril® + Van | 99.9% Gimesia chilikensis |
| MEMO3_6 | Memória | 10/2020 | SC.Myt | M13+ cycloheximide + Ant | 99.8 % Rhodopirellula baltica |
| MEMO3_5 | Memória | 10/2020 | SC.Myt | M13+ cycloheximide + Ant | 100.0% Rhodopirellula baltica |
| MEMO3_5.2 | Memória | 10/2020 | SC.Myt | M13+ cycloheximide + Ant | 99.9% Rhodopirellula baltica |
| MEMO3_10.2 | Memória | 10/2020 | SC.Myt | M13+ cycloheximide + Ant | 99.9% Rhodopirellula baltica |
| MEMO17_8 | Memória | 10/2020 | P.Ulv | NAGM + cycloheximide + Ant | 99.7% Novipirellula caenicola |
| MEMO_26.1 | Memória | 10/2020 | Sc.Cor | NAGM + cycloheximide + Ant | 99.9% Rhodopirellula baltica |
| ICM_H5 | Memória | 10/2020 | Sediments from iChip | Natural medium + cycloheximide + Ant | 99.7 % Novipirellula caenicola |
| ICM_G4 | Memória | 10/2020 | Sediments from iChip | Natural medium + cycloheximide + Ant | 99.7 % Novipirellula caenicola |
| ICM_H10 | Memória | 10/2020 | Sediments from iChip | Natural medium + cycloheximide + Ant | 96.7% Rubinisphaera italica |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vitorino, I.; Santos, J.D.N.; Godinho, O.; Vicente, F.; Vasconcelos, V.; Lage, O.M. Novel and Conventional Isolation Techniques to Obtain Planctomycetes from Marine Environments. Microorganisms 2021, 9, 2078. https://doi.org/10.3390/microorganisms9102078
Vitorino I, Santos JDN, Godinho O, Vicente F, Vasconcelos V, Lage OM. Novel and Conventional Isolation Techniques to Obtain Planctomycetes from Marine Environments. Microorganisms. 2021; 9(10):2078. https://doi.org/10.3390/microorganisms9102078
Chicago/Turabian StyleVitorino, Inês, José Diogo Neves Santos, Ofélia Godinho, Francisca Vicente, Vítor Vasconcelos, and Olga Maria Lage. 2021. "Novel and Conventional Isolation Techniques to Obtain Planctomycetes from Marine Environments" Microorganisms 9, no. 10: 2078. https://doi.org/10.3390/microorganisms9102078
APA StyleVitorino, I., Santos, J. D. N., Godinho, O., Vicente, F., Vasconcelos, V., & Lage, O. M. (2021). Novel and Conventional Isolation Techniques to Obtain Planctomycetes from Marine Environments. Microorganisms, 9(10), 2078. https://doi.org/10.3390/microorganisms9102078










