National Monitoring of Mosquito Populations and Molecular Analysis of Flavivirus in the Republic of Korea in 2020
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Mosquito Population Surveillance
2.3. Mosquito Species Identification
2.4. Molecular Detection of Flavivirus in Mosquitoes
2.5. RNA Sequencing and Phylogenetic Analysis
2.6. Virus Isolation and Purification
2.7. Geographical and Statistical Analyses
3. Results
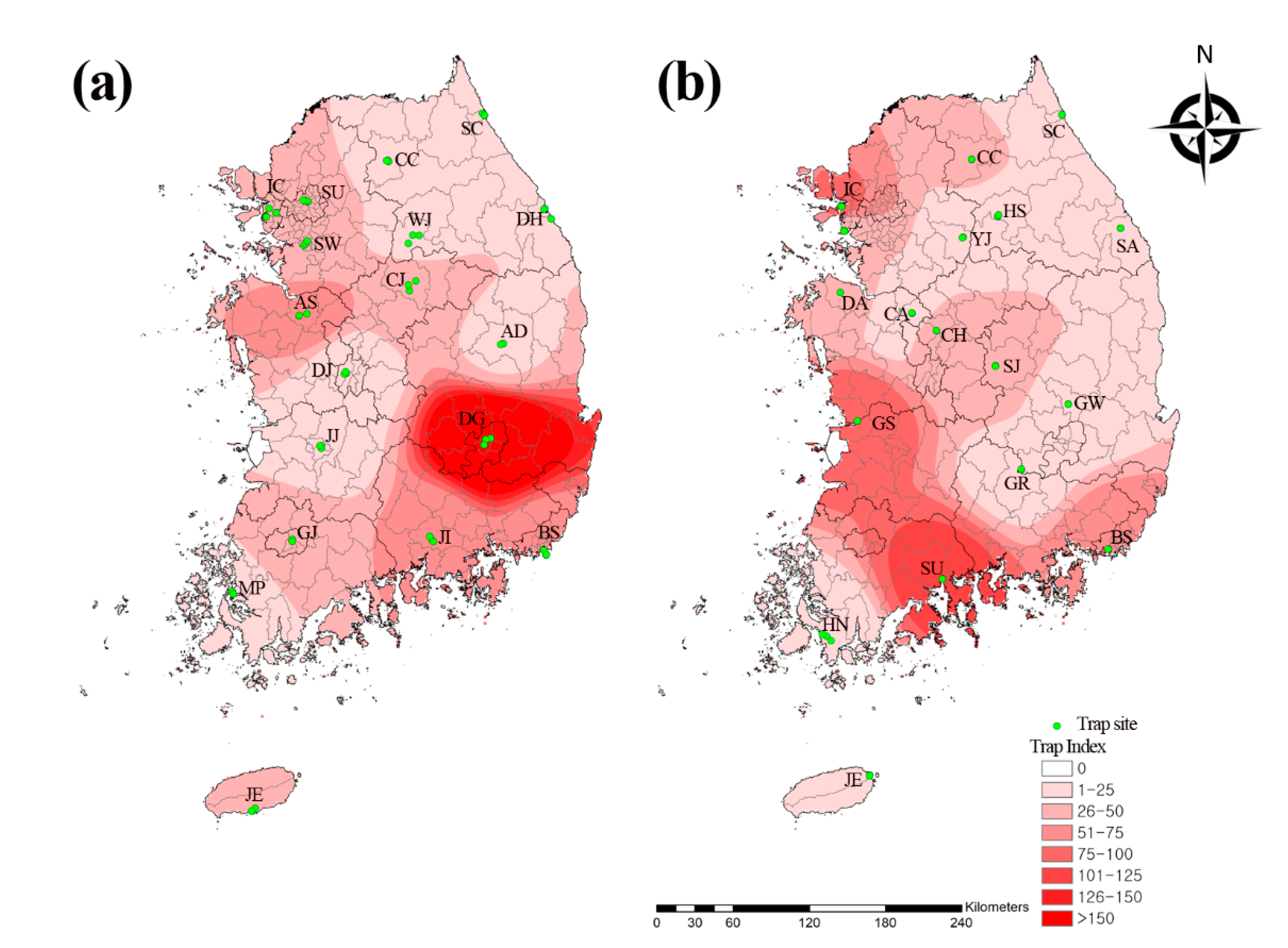
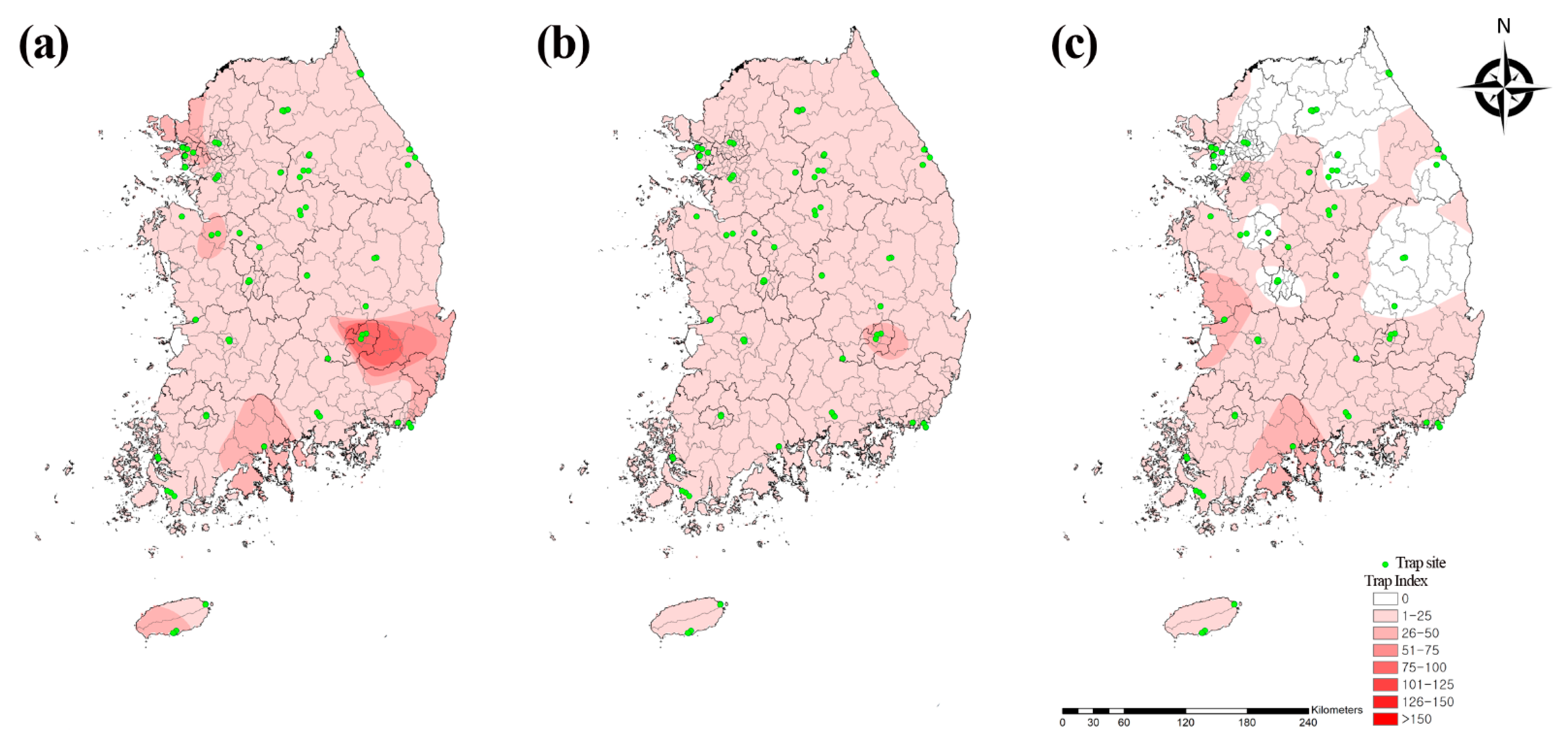
3.1. Prevalence of Mosquito Populations
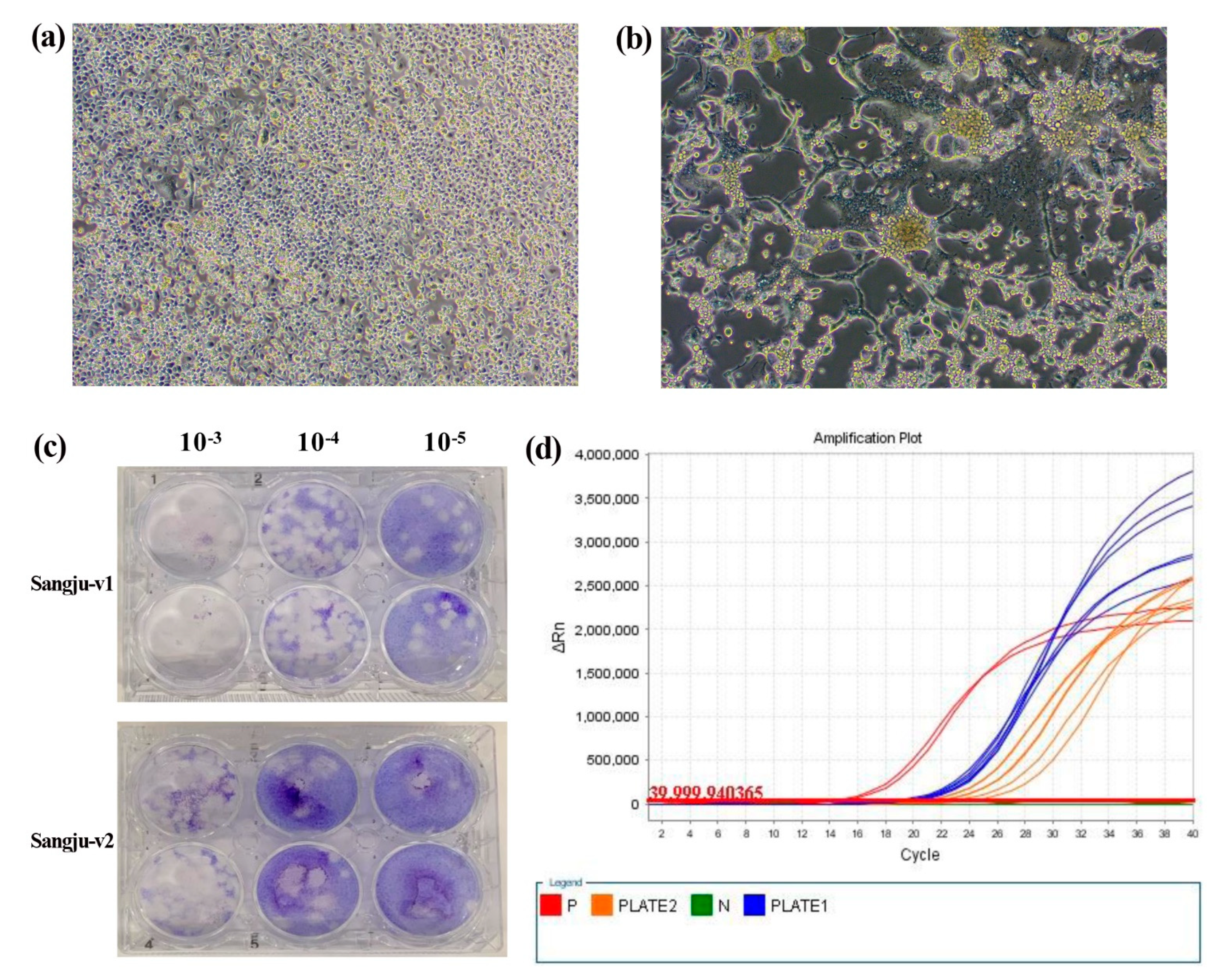
3.2. Virus Isolation and Purification
3.3. Molecular and Phylogenetic Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Mosquito-Borne Diseases. 2019. Available online: http://www.who.int/neglected_diseases/vector_ecology/mosquito-borne-diseases/en (accessed on 1 May 2021).
- Lee, D.K. Ecological characteristics and current status of infectious disease vectors in South Korea. J. Korean Med. Assoc. 2017, 60, 458–467. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Gubler, D.J.; Petersen, L.R. Emerging flaviviruses: The spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat. Med. 2004, 10, S98–S109. [Google Scholar] [CrossRef]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korea Diseases Control and Prevention Agency. Infectious Disease Portal. The Results of the National Infectious Disease Surveillance. 2020. Available online: http://kdca.go.kr/npt/biz/npp/ist/bass/bassDissStatsMain.do (accessed on 1 May 2021).
- Bae, W.; Kim, J.H.; Kim, J.; Lee, J.; Hwang, E.S. Changes of epidemiological characteristics of Japanese encephalitis viral infection and birds as a potential viral transmitter in Korea. J. Korean Med. Sci. 2018, 33, e70. [Google Scholar] [CrossRef] [PubMed]
- Bahk, Y.Y.; Park, S.H.; Kim-Jeon, M.D.; Oh, S.S.; Jung, H.; Jun, H.; Kim, K.A.; Park, J.M.; Ahn, S.K.; Lee, J.; et al. Monitoring culicine mosquitoes (Diptera: Culicidae) as a vector of flavivirus in Incheon metropolitan city and Hwaseong-Si, Gyeonggi-Do, Korea, during 2019. Korean J. Parasitol. 2020, 58, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Hwang, M.J.; Kim, H.C.; Klein, T.A.; Chong, S.T.; Sim, K.; Chung, Y.; Cheong, H.K. Comparison of climatic factors on mosquito abundance at US Army Garrison Humphreys, Republic of Korea. PLoS ONE 2020, 15, e0240363. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.G.; Yang, S.C. Introduction of regional center for vector surveillance against climate change. Korea KDCA Public Health Wkly. Rep. 2014, 7, 936–938. [Google Scholar]
- Foley, D.H.; Klein, T.A.; Kim, H.C.; Kim, M.S.; Wilkerson, R.C.; Harrison, G.; Rueda, L.M.; Lee, W.J. Synchronous peaks in trap catches of malaria-infected mosquito species at Daeseongdong, a border village between North and South Korea. J. Vector. Ecol. 2012, 37, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Mosquito Species Composition and Plasmodium vivax Infection Rates on Baengnyeong-do (Island), Republic of Korea. Available online: https://www.parasitol.kr/journal/view.php?number=1527 (accessed on 1 May 2021).
- Ree, H.I. Taxonomic review and revised keys of the Korean mosquitoes (Diptera: Culicidae). Korean J. Entomol. 2003, 33, 39–52. [Google Scholar] [CrossRef]
- Kim, H.; Cha, G.W.; Jeong, Y.E.; Lee, W.G.; Chang, K.S.; Roh, J.Y.; Yang, S.C.; Park, M.Y.; Park, C.; Shin, E.H. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS ONE 2015, 10, e0116547. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.S.; Kim, G.H.; Ha, Y.R.; Jeong, E.K.; Kim, H.C.; Klein, T.A.; Shin, S.H.; Kim, E.J.; Jegal, S.; Chung, S.J.; et al. Monitoring and control of Aedes albopictus, a vector of Zika virus, near residences of imported Zika virus patients during 2016 in South Korea. Am. J. Trop. Med. Hyg. 2018, 98, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Lee, E.J.; Lee, W.G.; Cho, S.H. Geographical distribution of Aedes albopictus around urban areas in Korea. Korea KDCA Public Health Wkly. Rep. 2018, 11, 463–468. [Google Scholar]
- Kim, T.K.; Jang, C.W.; Seo, M.G.; Kim, H.; Lee, H.I. Monitoring of Japanese encephalitis vector mosquitoes (Culex tritaeniorhynchus) in Korea, 2020. Korea KDCA Public Health Wkly. Rep. 2021, 14, 800–811. [Google Scholar]
- Chen, W.J.; Dong, C.F.; Chiou, L.Y.; Chuang, W.L. Potential role of Armigeres subalbatus (Diptera: Culicidae) in the transmission of Japanese encephalitis virus in the absence of rice culture on Liu-chiu islet, Taiwan. J. Med. Entomol. 2000, 37, 108–113. [Google Scholar] [CrossRef]
- Li, C.X.; Guo, X.X.; Deng, Y.Q.; Liu, Q.M.; Xing, D.; Sun, A.J.; Wu, Q.; Dong, Y.D.; Zhang, Y.M.; Zhang, H.D.; et al. Susceptibility of Armigeres subalbatus Coquillett (Diptera: Culicidae) to Zika virus through oral and urine infection. PLoS Negl. Trop. Dis. 2020, 14, e0008450. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Ferrandino, F.J. Horizontal and Vertical Transmission of West Nile Virus by Aedes vexans (Diptera: Culicidae). J. Med. Entomol. 2020, 57, 1614–1618. [Google Scholar] [CrossRef]
- Gendernalik, A.; Weger-Lucarelli, J.; Garcia Luna, S.M.; Fauver, J.R.; Rückert, C.; Murrieta, R.A.; Bergren, N.; Samaras, D.; Nguyen, C.; Kading, R.C.; et al. American Aedes vexans mosquitoes are competent vectors of Zika virus. Am. J. Trop. Med. Hyg. 2017, 96, 1338–1340. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, A.F.; Ritchie, S.A.; Mackenzie, J.S. Ecology and geographical expansion of Japanese encephalitis virus. Annu. Rev. Entomol. 2009, 54, 17–35. [Google Scholar] [CrossRef] [Green Version]
- Woo, J.H.; Jeong, Y.E.; Jo, J.E.; Shim, S.M.; Ryou, J.; Kim, K.C.; Lee, W.J.; Lee, J.Y. Genetic characterization of Japanese encephalitis virus genotype 5 isolated from patient, South Korea, 2015. Emerg. Infect. Dis. 2020, 26, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Phenotypic and genotypic characteristics of Japanese encephalitis attenuated live vaccine virus SA14-14-2 and their stabilities. Vaccine 2010, 28, 3635–3641. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Mizusawa, K.; Saugstad, E.S. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae). Contrib. Amer. Entomol. Inst. 1979, 16, 1–987. [Google Scholar]
- Takhampunya, R.; Kim, H.C.; Tippayachai, B.; Kengluecha, A.; Klein, T.A.; Lee, W.J.; Grieco, J.; Evans, B.P. Emergence of Japanese encephalitis virus genotype V in the Republic of Korea. Virol. J. 2011, 8, 449. [Google Scholar] [CrossRef] [Green Version]
- Seo, H.J.; Kim, H.C.; Klein, T.A.; Ramey, A.M.; Lee, J.H.; Kyung, S.G.; Park, J.Y.; Cho, Y.S.; Cho, I.S.; Yeh, J.Y. Molecular detection and genotyping of Japanese encephalitis virus in mosquitoes during a 2010 outbreak in the Republic of Korea. PLoS ONE 2013, 8, e55165. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Lee, E.; Choi, W.; Han, M.G. Laboratory-based diagnosis of Japanese encephalitis in Korea, 2018. Korea KDCA Public Health Wkly. Rep. 2019, 12, 1260–1266. [Google Scholar]
- Jegal, S.; Jun, H.; Kim-Jeon, M.D.; Park, S.H.; Ahn, S.K.; Lee, J.; Gong, Y.W.; Joo, K.; Kwon, M.J.; Roh, J.Y.; et al. Three-year surveillance of culicine mosquitoes (Diptera: Culicidae) for flavivirus infections in Incheon metropolitan city and Hwaseong-si of Gyeonggi-do province, Republic of Korea. Acta. Trop. 2020, 202, 105258. [Google Scholar] [CrossRef] [PubMed]
- Nga, P.T.; Parquet, M.D.C.; Cuong, V.D.; Ma, S.P.; Hasebe, F.; Inoue, S.; Makino, Y.; Takagi, M.; Nam, V.S.; Morita, K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: Implications for frequent introductions of JEV from Southeast Asia to East Asia. J. Gen. Virol. 2004, 85, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liu, H.; Li, X.; Fu, S.; Cao, L.; Shao, N.; Zhang, W.; Wang, Q.; Lu, Z.; Lei, W.; et al. Changing geographic distribution of Japanese encephalitis virus genotypes, 1935-2017. Vector Borne Zoonotic. Dis. 2019, 19, 35–44. [Google Scholar] [CrossRef]
- Yun, S.M.; Cho, J.E.; Ju, Y.R.; Kim, S.Y.; Ryou, J.; Han, M.G.; Choi, W.Y.; Jeong, Y.E. Molecular epidemiology of Japanese encephalitis virus circulating in South Korea, 1983-2005. Virol. J. 2010, 7, 127. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Fu, S.; Gao, X.; Li, M.; Cui, S.; Li, X.; Cao, Y.; Lei, W.; Lu, Z.; He, Y.; et al. Low protective efficacy of the current Japanese encephalitis vaccine against the emerging genotype 5 Japanese encephalitis virus. PLoS Negl. Trop. Dis. 2016, 10, e0004686. [Google Scholar] [CrossRef] [PubMed]
- Li, M.H.; Fu, S.H.; Chen, W.X.; Wang, H.Y.; Cao, Y.X.; Liang, G.D. Molecular characterization of full-length genome of Japanese encephalitis virus genotype V isolated from Tibet, China. Biomed. Environ. Sci. 2014, 27, 231–239. [Google Scholar]


| Regions | No. of Collected Mosquitoes | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cx.pip | Ae. albop | Cx. tri | Ar. sub | Ae. vex | Anopheles spp. | Och. kor | Cx. ori | Och. dor | Man. uni | Cx. ina | Och. tog | Cx. bit | Ae. lin | Coq. och | Cx. vag | Och. hat | Tri. bam | Ae. albos | Och. jap | Ae. eso | Ae. fla | Och. ore | Others | Total | % | TI | |
| Urban areas | |||||||||||||||||||||||||||
| Seoul | 883 | 563 | 1 | 112 | 17 | 4 | 269 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1850 | 5.1 | 36.3 |
| Incheon | 2095 | 173 | 0 | 0 | 33 | 10 | 63 | 1 | 5 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2383 | 6.5 | 46.7 |
| Sokcho | 229 | 79 | 0 | 220 | 12 | 8 | 26 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 575 | 1.6 | 11.3 |
| Donghae | 636 | 196 | 65 | 208 | 4 | 2 | 57 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1175 | 3.2 | 23.0 |
| Suwon | 1116 | 33 | 8 | 143 | 64 | 26 | 84 | 7 | 0 | 1 | 8 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1492 | 4.1 | 29.3 |
| Chuncheon | 148 | 334 | 0 | 240 | 48 | 98 | 72 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 945 | 2.6 | 18.5 |
| Wonju | 66 | 110 | 0 | 226 | 287 | 13 | 103 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 807 | 2.2 | 15.8 |
| Chungju | 268 | 158 | 33 | 1250 | 201 | 2 | 316 | 59 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2289 | 6.3 | 44.9 |
| Asan | 1358 | 949 | 3 | 218 | 191 | 25 | 40 | 419 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3203 | 8.8 | 62.8 |
| Andong | 638 | 325 | 0 | 56 | 2 | 10 | 15 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1049 | 2.9 | 20.6 |
| Daegu | 4109 | 1411 | 25 | 4002 | 35 | 69 | 204 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9855 | 27 | 193.2 |
| Daejeon | 247 | 5 | 1 | 61 | 17 | 131 | 9 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 5 | 1 | 0 | 0 | 0 | 0 | 479 | 1.3 | 9.4 |
| Gwangju | 1208 | 321 | 364 | 20 | 28 | 36 | 68 | 1 | 1 | 55 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2104 | 5.8 | 41.3 |
| Jeonju | 493 | 38 | 26 | 31 | 23 | 19 | 17 | 4 | 0 | 21 | 8 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 681 | 1.9 | 13.4 |
| Mokpo | 265 | 161 | 18 | 189 | 0 | 26 | 0 | 0 | 8 | 0 | 0 | 5 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 674 | 1.8 | 13.2 |
| Jinju | 802 | 1275 | 10 | 123 | 113 | 21 | 341 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2697 | 7.4 | 52.9 |
| Busan | 1950 | 360 | 6 | 195 | 2 | 0 | 11 | 0 | 0 | 0 | 0 | 212 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2736 | 7.5 | 53.6 |
| Jeju | 1318 | 87 | 7 | 117 | 0 | 2 | 9 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1543 | 4.2 | 30.3 |
| Subtotal | 17,829 | 6578 | 567 | 7411 | 1077 | 502 | 1704 | 506 | 14 | 77 | 17 | 222 | 11 | 0 | 1 | 3 | 8 | 2 | 6 | 1 | 0 | 0 | 0 | 1 | 36,537 | 100 | 39.8 |
| Migratory bird habitats | |||||||||||||||||||||||||||
| Samcheok | 3 | 85 | 0 | 61 | 1 | 1 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 159 | 0.5 | 3.1 |
| Incheon | 3030 | 13 | 53 | 2 | 578 | 210 | 8 | 4 | 1293 | 4 | 101 | 5 | 6 | 47 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5360 | 17.5 | 52.5 |
| Hoengseong | 102 | 167 | 1 | 219 | 143 | 1 | 5 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 640 | 2.1 | 12.5 |
| Sokcho | 166 | 27 | 0 | 1 | 10 | 2 | 5 | 2 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 224 | 0.7 | 4.4 |
| Yeoju | 94 | 120 | 67 | 68 | 489 | 80 | 23 | 12 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 954 | 3.1 | 18.7 |
| Chuncheon | 114 | 499 | 0 | 435 | 134 | 42 | 146 | 34 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1408 | 4.6 | 27.6 |
| Cheonan | 39 | 23 | 0 | 19 | 56 | 10 | 4 | 10 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 169 | 0.6 | 3.3 |
| Cheongju | 534 | 118 | 134 | 326 | 374 | 36 | 162 | 87 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1774 | 5.8 | 34.8 |
| Dangjin | 274 | 165 | 50 | 85 | 911 | 74 | 33 | 175 | 0 | 3 | 0 | 0 | 2 | 0 | 0 | 15 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1787 | 5.8 | 35.0 |
| Sangju | 149 | 174 | 10 | 251 | 1380 | 71 | 16 | 395 | 0 | 2 | 0 | 0 | 23 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 1 | 0 | 1 | 0 | 2478 | 8.1 | 48.6 |
| Gunwi | 82 | 90 | 0 | 73 | 10 | 15 | 49 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 340 | 1.1 | 6.7 |
| Goryeong | 38 | 151 | 48 | 147 | 90 | 145 | 18 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 22 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 672 | 2.2 | 13.2 |
| Gunsan | 436 | 22 | 2316 | 14 | 380 | 211 | 122 | 60 | 1 | 438 | 361 | 1 | 49 | 23 | 50 | 18 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 4503 | 14.7 | 88.3 |
| Suncheon | 1631 | 166 | 2161 | 28 | 539 | 856 | 27 | 10 | 5 | 43 | 0 | 0 | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5472 | 17.8 | 107.3 |
| Busan | 836 | 28 | 788 | 15 | 1215 | 428 | 19 | 41 | 0 | 131 | 0 | 1 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3509 | 11.4 | 68.8 |
| Haenam | 13 | 295 | 19 | 11 | 3 | 22 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 363 | 1.2 | 7.1 |
| Jeju | 25 | 68 | 7 | 139 | 3 | 576 | 31 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 854 | 2.8 | 16.7 |
| Subtotal | 7566 | 2211 | 5654 | 1894 | 6316 | 2780 | 671 | 864 | 1310 | 621 | 464 | 15 | 91 | 72 | 63 | 34 | 22 | 10 | 0 | 4 | 1 | 1 | 1 | 1 | 30,666 | 100 | 33.4 |
| Total | 25,395 | 8789 | 6221 | 9305 | 7393 | 3282 | 2375 | 1370 | 1324 | 698 | 481 | 237 | 102 | 72 | 64 | 37 | 30 | 12 | 6 | 5 | 1 | 1 | 1 | 2 | 67,203 | - | 36.6 |
| Species | March | April | May | June | July | August | September | October | November | No. of Mosquitoes | TI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | 1st wk | 3rd wk | Collected (%) | Tested | Pools | Positive Pools (MIR) | ||
| Cx. pip | 263 | 316 | 189 | 618 | 701 | 2013 | 3096 | 3960 | 3583 | 1369 | 1560 | 1188 | 1731 | 2144 | 1319 | 371 | 974 | 25,395 (37.8) | 25,209 | 1618 | 0 | 13.8 |
| Ae. albop | 5 | 0 | 42 | 1 | 77 | 228 | 289 | 444 | 988 | 1652 | 1281 | 1423 | 1640 | 474 | 216 | 24 | 5 | 8789 (13.1) | 8747 | 671 | 0 | 4.8 |
| Cx. tri | 5 | 25 | 0 | 4 | 14 | 73 | 31 | 148 | 348 | 460 | 774 | 3530 | 525 | 97 | 140 | 47 | 0 | 6221 (9.3) | 6217 | 356 | 0 | 3.4 |
| Cx. ori | 5 | 2 | 5 | 15 | 1 | 21 | 82 | 102 | 101 | 112 | 597 | 208 | 113 | 2 | 3 | 0 | 1 | 1370 (2.0) | 1370 | 207 | 7 (0.5) | 0.7 |
| Others | 6 | 12 | 42 | 362 | 949 | 3692 | 3227 | 2465 | 2540 | 2216 | 2897 | 2821 | 2513 | 1297 | 212 | 118 | 59 | 25,428 (37.8) | 22,119 | 2101 | 0 | 13.8 |
| Total | 284 | 355 | 278 | 1000 | 1742 | 6027 | 6725 | 7119 | 7560 | 5809 | 7109 | 9170 | 6522 | 4014 | 1890 | 560 | 1039 | 67,203 | 63,662 | 4953 | 7 (0.01) | 36.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, M.-G.; Lee, H.S.; Yang, S.-C.; Noh, B.-E.; Kim, T.-K.; Lee, W.-G.; Lee, H.I. National Monitoring of Mosquito Populations and Molecular Analysis of Flavivirus in the Republic of Korea in 2020. Microorganisms 2021, 9, 2085. https://doi.org/10.3390/microorganisms9102085
Seo M-G, Lee HS, Yang S-C, Noh B-E, Kim T-K, Lee W-G, Lee HI. National Monitoring of Mosquito Populations and Molecular Analysis of Flavivirus in the Republic of Korea in 2020. Microorganisms. 2021; 9(10):2085. https://doi.org/10.3390/microorganisms9102085
Chicago/Turabian StyleSeo, Min-Goo, Hak Seon Lee, Sung-Chan Yang, Byung-Eon Noh, Tae-Kyu Kim, Wook-Gyo Lee, and Hee Il Lee. 2021. "National Monitoring of Mosquito Populations and Molecular Analysis of Flavivirus in the Republic of Korea in 2020" Microorganisms 9, no. 10: 2085. https://doi.org/10.3390/microorganisms9102085






