The Influence of Artificial Fusarium Infection on Oat Grain Quality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Artificial Inoculation
2.3. Determination of Starch
2.4. Determination of TDF
2.5. Determination of β-D-glucans
2.6. Determination of Lipids and Fatty Acids
2.7. Quantification of DON
2.7.1. Preparation of Standard Solution of DON
2.7.2. Preparation of the Sample
2.7.3. Clean-Up on Immunoaffinity Columns (IAC)
2.7.4. HPLC Procedures
2.8. Statistical Evaluation
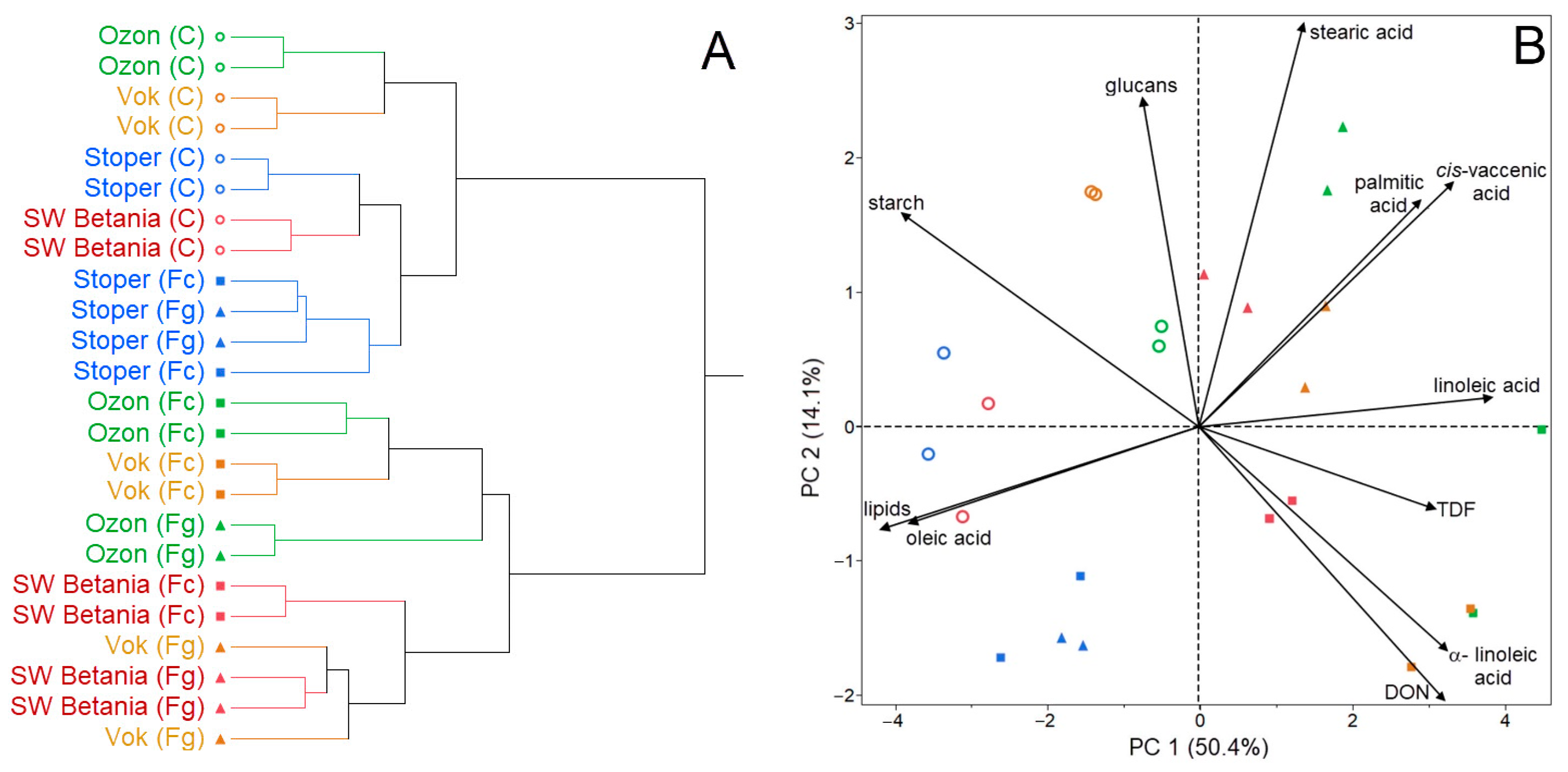
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Marshall, A.; Cowan, S.; Edwards, S.; Griffiths, I.; Howarth, C.; Langdon, T.; White, E. Crops That Feed the World 9. Oats—A Cereal Crop for Human and Livestock Feed with Industrial Applications. Food Sec. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Fan, M.; Marshall, W.; Daugaard, D.; Brown, R.C. Steam Activation of Chars Produced from Oat Hulls and Corn Stover. Bioresour. Technol. 2004, 93, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional Advantages of Oats and Opportunities for Its Processing as Value Added Foods—A Review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef] [Green Version]
- Kouřimská, L.; Sabolová, M.; Horčička, P.; Rys, S.; Božik, M. Lipid Content, Fatty Acid Profile, and Nutritional Value of New Oat Cultivars. J. Cereal. Sci. 2018, 84, 44–48. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Sulyok, M.; Musa, T.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Voegele, R.T.; Vogelgsang, S. Can Plant Phenolic Compounds Reduce Fusarium Growth and Mycotoxin Production in Cereals? Food Addit Contam Part A Chem Anal Control. Expo Risk Assess 2018, 35, 2455–2470. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Martín, J.; Rubiales, D.; Prats, E. Resistance to Powdery Mildew (Blumeria Graminis f.Sp. Avenae) in Oat Seedlings and Adult Plants. Plant Pathol. 2011, 60, 846–856. [Google Scholar] [CrossRef] [Green Version]
- Ostry, V. Alternaria Mycotoxins: An Overview of Chemical Characterization, Producers, Toxicity, Analysis and Occurrence in Foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Oviedo, M.S.; Sturm, M.E.; Reynoso, M.M.; Chulze, S.N.; Ramirez, M.L. Toxigenic Profile and AFLP Variability of Alternaria Alternata and Alternaria Infectoria Occurring on Wheat. Braz. J. Microbiol. 2013, 44, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazareno, E.S.; Li, F.; Smith, M.; Park, R.F.; Kianian, S.F.; Figueroa, M. Puccinia Coronata f. Sp. Avenae: A Threat to Global Oat Production. Mol. Plant Pathol. 2018, 19, 1047–1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdrassulova, Z.T.; Kuzhantaeva, Z.Z.; Anuarova, L.E. Biological Specifics of Some Species of Fungi on Seeds of Grain Crops. Life Sci. J. 2014, 11, 79–82. [Google Scholar] [CrossRef]
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium Ear Blight (Scab) in Small Grain Cereals—A Review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Tekle, S.; Dill-Macky, R.; Skinnes, H.; Tronsmo, A.M.; Bjørnstad, Å. Infection Process of Fusarium Graminearum in Oats (Avena Sativa L.). Eur. J. Plant Pathol. 2012, 132, 431–442. [Google Scholar] [CrossRef]
- De Lucca, A.J. Hongos patógenos communes en la Agricultura y la Medicina. Rev. Iberoam. Micol. 2007, 24, 3–13. [Google Scholar] [CrossRef]
- Becher, R.; Miedaner, T.; Wirsel, S.G.R. Biology, Diversity, and Management of FHB-Causing Fusarium Species in Small-Grain Cereals. In Biology, Diversity and Management of FHB-Causing Fusarium Species in Small-Grain Cereals. Agricultural Applications. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Kempken, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 11, pp. 199–241. [Google Scholar] [CrossRef]
- Xu, X.M.; Nicholson, P.; Thomsett, M.; Simpson, D.; Cooke, B.; Doohan, F.; Brennan, J.; Monaghan, S.; Moretti, A. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected Shifts in Fusarium Species’ Composition on Cereal Grain in Northern Europe Due to Climatic Change. Food Addit. Contam. Part A 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Stępień, L. The Use of Fusarium Secondary Metabolite Biosynthetic Genes in Chemotypic and Phylogenetic Studies. Crit. Rev. Microbiol. 2014, 40, 176–185. [Google Scholar] [CrossRef]
- Yang, W.; Yu, M.; Fu, J.; Bao, W.; Wang, D.; Hao, L.; Yao, P.; Nüssler, A.K.; Yan, H.; Liu, L. Deoxynivalenol Induced Oxidative Stress and Genotoxicity in Human Peripheral Blood Lymphocytes. Food Chem. Toxicol. 2013, 64, 383–396. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [Green Version]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Gunnaiah, R.; Kushalappa, A.C. Metabolomics Deciphers the Host Resistance Mechanisms in Wheat Cultivar Sumai-3, against Trichothecene Producing and Non-Producing Isolates of Fusarium Graminearum. Plant Physiol. Biochem. 2014, 83, 40–50. [Google Scholar] [CrossRef]
- Rashid, A. Defense Responses of Plant Cell Wall Non-Catalytic Proteins against Pathogens. Physiol. Mol. Plant Pathol. 2016, 94, 38–46. [Google Scholar] [CrossRef]
- Kachroo, A.; Kachroo, P. Fatty Acid–Derived Signals in Plant Defense. Annu. Rev. Phytopathol. 2009, 47, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Shim, W.-B.; Göbel, C.; Kunze, S.; Feussner, I.; Meeley, R.; Balint-Kurti, P.; Kolomiets, M. Disruption of a Maize 9-Lipoxygenase Results in Increased Resistance to Fungal Pathogens and Reduced Levels of Contamination with Mycotoxin Fumonisin. Mol. Plant-Microbe Interact. 2007, 20, 922–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Havrlentová, M.; Gregusová, V.; Šliková, S.; Nemeček, P.; Hudcovicová, M.; Kuzmová, D. Relationship between the Content of β-D-Glucans and Infection with Fusarium Pathogens in Oat (Avena Sativa L.) Plants. Plants 2020, 9, 1776. [Google Scholar] [CrossRef]
- Šliková, S.; Hozlár, P.; Gregová, E. Ovos: Infekcia Hubami Fusarium Spp. V Poľných Podmienkach; Edičné Stredisko NPPC; Výskumný Ústav Pôdoznalectva a Ochrany Pôdy: Bratislava, Slovakia, 2017; p. 91. [Google Scholar]
- Tvarůžek, L.; Matušinský, P.; Vyšohlídová, M. Metodika pro zakládání a hodnocení pokusů s umělou inokulací obilnin fuzáriózami klasů. 2012. Available online: https://www.vukrom.cz/userfiles/files/vysledky_vyzkumu/Metodiky/2012_Metodika_pro_zakladani_a_hodnoceni_pokusu_s_umelou_inokulaci_obilnin_fuzariozami_klasu.pdf (accessed on 15 August 2021).
- Nirenberg, H. Untersuchungen über die morphologische und biologische Differenzierung in der Fusarium Sektion Liseola. Mitt. Aus Der Biol. Bundesanst. Für Land Und Forstwirtsch. 1976, 169, 1–117. [Google Scholar]
- Lee, S.C.; Prosky, L.; Vries, J.W.D. Determination of Total, Soluble, and Insoluble Dietary Fiber in Foods—Enzymatic-Gravimetric Method, MES-TRIS Buffer: Collaborative Study. J. AOAC Int. 1992, 75, 395–416. [Google Scholar] [CrossRef]
- Prosky, L.; Asp, N.-G.; Schweizer, T.F.; Devries, J.W.; Furda, I. Determination of Insoluble and Soluble Dietary Fiber in Foods and Food Products: Collaborative Study. J. AOAC Int. 1992, 75, 360–367. [Google Scholar] [CrossRef]
- McCleary, B.V.; Codd, R. Measurement of (1 → 3) (1 → 4)-β-D-Glucan in Barley and Oats: A Streamlined Enzymic Procedure. J. Sci. Food Agric. 1991, 55, 303–312. [Google Scholar] [CrossRef]
- Christopherson, S.W.; Glass, R.L. Preparation of Milk Fat Methyl Esters by Alcoholysis in an Essentially Nonalcoholic Solution. J. Dairy Sci. 1969, 52, 1289–1290. [Google Scholar] [CrossRef]
- Sterna, V.; Zute, S.; Brunava, L. Oat Grain Composition and Its Nutrition Benefice. Agric. Agric. Sci. Procedia 2016, 8, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pawelzik, E.; Weinert, J.; Wolf, G.A. Impact of Fusarium culmorum on the polysaccharides of wheat flour. J. Agric. Food Chem. 2005, 53, 5818–5823. [Google Scholar] [CrossRef]
- Rakić, S.; Janković, S.; Marčetić, M.; Živković, D.; Kuzevski, J. The Impact of Storage on the Primary and Secondary Metabolites, Antioxidant Activity and Digestibility of Oat Grains (Avena Sativa). J. Funct. Foods 2014, 7, 373–380. [Google Scholar] [CrossRef]
- Gudmundsson, M.; Eliasson, A. Some Physico-Chemical Properties of Oat Starches Extracted from Varieties with Different Oil Content. Acta Agric. Scand. 1989, 39, 101–111. [Google Scholar] [CrossRef]
- Sowa, S.M.; White, P.J. Characterization of Starch Isolated from Oat Groats with Different Amounts of Lipid. Cereal. Chem. 1992, 69, 521–527. [Google Scholar]
- Galdeano, M.C.; Grossmann, M.V.E.; Mali, S.; Bello-Perez, L.A.; Garcia, M.A.; Zamudio-Flores, P.B. Effects of Production Process and Plasticizers on Stability of Films and Sheets of Oat Starch. Mater. Sci. Eng. C 2009, 29, 492–498. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Shelenga, T.V.; Konarev, A.V.; Horeva, V.I.; Shavarda, A.L.; Blinova, E.V.; Gnutikov, A.A. Biochemical Aspects of Interactions between Fungi and Plants: A Case Study of Fusarium in Oats. Sel’skokhozyaistvennaya Biol. 2019, 54, 575–588. [Google Scholar] [CrossRef]
- Flander, L.; Salmenkallio-Marttila, M.; Suortti, T.; Autio, K. Optimization of Ingredients and Baking Process for Improved Wholemeal Oat Bread Quality. LWT—Food Sci. Technol. 2007, 40, 860–870. [Google Scholar] [CrossRef]
- Schauer, N.; Fernie, A.R. Plant Metabolomics: Towards Biological Function and Mechanism. Trends Plant Sci. 2006, 11, 508–516. [Google Scholar] [CrossRef]
- Schulze-Lefert, P. Knocking on the Heaven’s Wall: Pathogenesis of and Resistance to Biotrophic Fungi at the Cell Wall. Curr. Opin. Plant Biol. 2004, 7, 377–383. [Google Scholar] [CrossRef]
- Martin, C.; Schöneberg, T.; Vogelgsang, S.; Morisoli, R.; Bertossa, M.; Mauch-Mani, B.; Mascher, F. Resistance against Fusarium Graminearum and the Relationship to β-Glucan Content in Barley Grains. Eur. J. Plant Pathol. 2018, 152, 621–634. [Google Scholar] [CrossRef]
- Kofuji, K.; Aoki, A.; Tsubaki, K.; Konishi, M.; Isobe, T.; Murata, Y. Antioxidant Activity of β-Glucan. ISRN Pharm. 2012, 2012, 125864. [Google Scholar] [CrossRef] [Green Version]
- Šliková, S.; Havrlentová, M.; Hauptvogel, P.; Mendel, Ľ.; Gregová, E.; Šudyová, V. β-D-Glucan Content of Wheat Kernels after Inoculation with Fusarium Culmorum Sacc. Acta Agron. Hung. 2012, 60, 377–384. [Google Scholar] [CrossRef]
- Capouchová, I.; Kouřimská, L.; Pazderu, K.; Škvorová, P.; Božik, M.; Konvalina, P.; Dvořák, P.; Dvořāček, V. Fatty Acid Profile of New Oat Cultivars Grown via Organic and Conventional Farming. J. Cereal Sci. 2021, 98, 103180. [Google Scholar] [CrossRef]
- Boedi, S.; Berger, H.; Sieber, C.; Münsterkötter, M.; Maloku, I.; Warth, B.; Sulyok, M.; Lemmens, M.; Schuhmacher, R.; Güldener, U.; et al. Comparison of Fusarium Graminearum Transcriptomes on Living or Dead Wheat Differentiates Substrate-Responsive and Defense-Responsive Genes. Front. Microbiol. 2016, 7, 1113. [Google Scholar] [CrossRef] [PubMed]
- Kock, J.L.F.; Strauss, C.J.; Pohl, C.H.; Nigam, S. The Distribution of 3-Hydroxy Oxylipins in Fungi. Prostaglandins Prostaglandins Other Lipid Mediat. 2003, 71, 85–96. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Production of Eicosanoids and Other Oxylipins by Pathogenic Eukaryotic Microbes. Clin. Microbiol. Rev. 2003, 16, 517–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pestka, J. Toxicological Mechanisms and Potential Health Effects of Deoxynivalenol and Nivalenol. World Mycotoxin J. 2010, 3, 323–347. [Google Scholar] [CrossRef]
- Cutler, H.G. Trichothecenes and Their Role in the Expression of Plant Disease. In Biotechnology for Crop Protection; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1988; Volume 379, pp. 50–72. [Google Scholar] [CrossRef]
- Jansen, C.; von Wettstein, D.; Schäfer, W.; Kogel, K.-H.; Felk, A.; Maier, F.J. Infection Patterns in Barley and Wheat Spikes Inoculated with Wild-Type and Trichodiene Synthase Gene Disrupted Fusarium Graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boenisch, M.J.; Schäfer, W. Fusarium Graminearum forms Mycotoxin Producing Infection Structures on Wheat. BMC Plant Biol. 2011, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Ben Halima, N.; Ben Saad, R.; Khemakhem, B.; Fendri, I.; Abdelkafi, S. Oat (Avena Sativa L.): Oil and Nutriment Compounds Valorization for Potential Use in Industrial Applications. J. Oleo Sci. 2015, 64, 915–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aro, H.; Järvenpää, E.; Mäkinen, J.; Lauraeus, M.; Huopalahti, R.; Hietaniemi, V. The Utilization of Oat Polar Lipids Produced by Supercritical Fluid Technologies in the Encapsulation of Probiotics. LWT—Food Sci. Technol. 2013, 53, 540–546. [Google Scholar] [CrossRef]
- Torbica, A.; Belović, M.; Popović, L.; Čakarević, J.; Jovičić, M.; Pavličević, J. Comparative Study of Nutritional and Technological Quality Aspects of Minor Cereals. Food Sci. Technol. 2021, 58, 311–322. [Google Scholar] [CrossRef]
- Zhou, M.; Robards, K.; Glennie-Holmes, M.; Helliwell, S. Oat Lipids. J. Am. Oil Chem. Soc. 1999, 76, 169. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Soanes, D.M.; Kershaw, M.J.; Talbot, N.J. Functional Analysis of Lipid Metabolism in Magnaporthe Grisea Reveals a Requirement for Peroxisomal Fatty Acid Beta-Oxidation during Appressorium-Mediated Plant Infection. Mol. Plant-Microbe Interact. 2007, 20, 475–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal Activities of Four Fatty Acids against Plant Pathogenic Fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Jetter, R.; Kunst, L.; Samuels, A.L. Composition of Plant Cuticular Waxes. In Annual Plant Reviews Volume 23: Biology of the Plant Cuticle; Blackwell Publishing Ltd.: Oxford, UK, 2006; pp. 145–181. [Google Scholar] [CrossRef]
- Hamzehzarghani, H.; Kushalappa, A.C.; Dion, Y.; Rioux, S.; Comeau, A.; Yaylayan, V.; Marshall, W.D.; Mather, D.E. Metabolic Profiling and Factor Analysis to Discriminate Quantitative Resistance in Wheat Cultivars against Fusarium Head Blight. Physiol. Mol. Plant Pathol. 2005, 66, 119–133. [Google Scholar] [CrossRef]
- Gauthier, L.; Atanasova-Penichon, V.; Chéreau, S.; Richard-Forget, F. Metabolomics to Decipher the Chemical Defense of Cereals against Fusarium Graminearum and Deoxynivalenol Accumulation. Int. J. Mol. Sci. 2015, 16, 24839–24872. [Google Scholar] [CrossRef] [PubMed]
| Genotype | Starch (%) | TDF (%) | β-D-Glucans (%) | Total Lipids (%) | DON (µg·kg−1) | |
|---|---|---|---|---|---|---|
| Control | Ozon | 40.59 ± 0.08 a | 27.02 ± 0.66 a | 3.13 ± 0.05 a | 3.66 ± 0.17 a | 0.00 ± 0.00 a |
| Stoper | 43.65 ± 0.62 a | 23.47 ± 0.11 a | 4.17 ± 0.04 a | 4.02 ± 0.02 a | 187.50 ± 15.91 a | |
| Vok | 42.25 ± 0.25 a | 25.13 ± 0.66 a | 3.50 ± 0.00 a | 4.08 ± 0.20 a | 61.88 ± 2.65 a | |
| SW Betania | 39.51 ± 0.26 a | 26.31 ± 0.93 a | 3.16 ± 0.03 a | 4.50 ± 0.05 a | 378.76 ± 29.17 a | |
| Mean | 41.50 | 25.53 | 3.49 | 4.29 | 157.03 | |
| FC inoculation | Ozon | 30.82 ± 0.34 b | 27.87 ± 4.63 a | 2.66 ± 0.06 a | 2.93 ± 0.17 a | 1949.41 ± 189.40 b |
| Stoper | 37.76 ± 0.95 b | 27.06 ± 0.24 a | 3.89 ± 0.39 a | 4.35 ± 0.05 a | 1469.91 ± 9.82 b | |
| Vok | 30.99 ± 0.00 b | 32.73 ± 0.14 a | 1.67 ± 0.04 a | 3.39 ± 0.00 a | 2339.72 ± 3.80 b | |
| SW Betania | 31.48 ± 0.17 b | 29.54 ± 0.62 a | 3.42 ± 0.12 a | 3.72 ± 0.35 a | 2625.00 ± 7.58 b | |
| Mean | 32.76 | 29.30 | 2.91 | 3.60 | 2096.01 | |
| FG inoculation | Ozon | 34.90 ± 0.26 c | 29.88 ± 0.54 a | 6.87 ± 0.25 a | 3.11 ± 0.06 a | 1207.47 ± 91.53 c |
| Stoper | 39.43 ± 0.00 c | 26.31 ± 0.26 a | 2.16 ± 0.01 a | 4.30 ± 0.01 a | 990.19 ± 3.11 c | |
| Vok | 36.81 ± 0.35 c | 28.11 ± 4.23 a | 1.51 ± 0.25 a | 3.51 ± 0.39 a | 1609.28 ± 0.00 c | |
| SW Betania | 38.34 ± 0.17 c | 25.77 ± 0.07 a | 3.60 ± 0.21 a | 3.95 ± 0.45 a | 1461.79 ± 6.28 c | |
| Mean | 37.37 | 27.52 | 3.54 | 3.72 | 1317.18 | |
| Genotype | Linoleic Acid (%) | Oleic Acid (%) | Palmitic Acid (%) | A-linolenic Acid (%) | Stearic Acid (%) | Cis-vaccenic Acid (%) | |
|---|---|---|---|---|---|---|---|
| Control | Ozon | 40.97 ± 0.08 a | 36.72 ± 0.23 a | 16.95 ± 0.01 a | 2.22 ± 0.06 a | 1.76 ± 0.11 a | 1.40 ± 0.30 a |
| Stoper | 37.69 ± 0.20 a | 41.77 ± 0.21 a | 15.86 ± 0.16 a | 1.98 ± 0.01 a | 1.70 ± 0.18 a | 1.01 ± 0.06 a | |
| Vok | 40.41 ± 0.49 a | 36.94 ± 0.62 a | 17.72 ± 0.28 a | 1.84 ± 0.13 a | 2.18 ± 0.15 a | 0.93 ± 0.13 a | |
| SW Betania | 38.14 ± 0.10 a | 41.33 ± 0.21 a | 16.10 ± 0.32 a | 1.63 ± 0.02 a | 1.77 ± 0.26 a | 1.05 ± 0.19 a | |
| Mean | 39.30 | 39.19 | 16.66 | 1.92 | 1.85 | 1.09 | |
| FC inoculation | Ozon | 44.25 ± 0.17 b | 31.14 ± 0.64 b | 17.80 ± 0.23 a | 3.55 ± 0.22 b | 1.83 ± 0.42 a | 1.46 ± 0.04 a |
| Stoper | 39.58 ± 0.09 b | 39.85 ± 0.44 b | 15.57 ± 0.77 a | 2.24 ± 0.07 b | 1.82 ± 0.12 a | 0.51 ± 0.58 a | |
| Vok | 42.65 ± 1.34 b | 33.89 ± 1.61 b | 17.46 ± 0.24 a | 2.88 ± 0.08 b | 1.78 ± 0.01 a | 1.35 ± 0.11 a | |
| SW Betania | 39.86 ± 1.70 b | 38.33 ± 0.28 b | 17.57 ± 0.05 a | 2.22 ± 0.02 b | 2.04 ± 0.06 a | 1.08 ± 0.02 a | |
| Mean | 41.59 | 35.80 | 17.10 | 2.72 | 1.86 | 1.10 | |
| FG inoculation | Ozon | 41.27 ± 0.29 b | 35.25 ± 0.05 b | 17.12 ± 0.07 a | 2.60 ± 0.12 a | 2.21 ± 0.21 a | 1.58 ± 0.08 a |
| Stoper | 40.41 ± 0.35 b | 39.00 ± 0.15 b | 15.58 ± 0.65 a | 2.39 ± 0.13 a | 1.58 ± 0.28 a | 1.07 ± 0.25 a | |
| Vok | 42.93 ± 0.53 b | 34.19 ± 0.09 b | 17.35 ± 0.23 a | 1.99 ± 0.01 a | 2.25 ± 0.28 a | 1.32 ± 0.06 a | |
| SW Betania | 42.77 ± 1.69 b | 34.80 ± 0.84 b | 17.33 ± 0.68 a | 1.77 ± 0.09 a | 2.01 ± 0.12 a | 1.35 ± 0.04 a | |
| Mean | 41.85 | 35.81 | 16.85 | 2.18 | 2.01 | 1.33 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havrlentová, M.; Šliková, S.; Gregusová, V.; Kovácsová, B.; Lančaričová, A.; Nemeček, P.; Hendrichová, J.; Hozlár, P. The Influence of Artificial Fusarium Infection on Oat Grain Quality. Microorganisms 2021, 9, 2108. https://doi.org/10.3390/microorganisms9102108
Havrlentová M, Šliková S, Gregusová V, Kovácsová B, Lančaričová A, Nemeček P, Hendrichová J, Hozlár P. The Influence of Artificial Fusarium Infection on Oat Grain Quality. Microorganisms. 2021; 9(10):2108. https://doi.org/10.3390/microorganisms9102108
Chicago/Turabian StyleHavrlentová, Michaela, Svetlana Šliková, Veronika Gregusová, Bernadett Kovácsová, Andrea Lančaričová, Peter Nemeček, Jana Hendrichová, and Peter Hozlár. 2021. "The Influence of Artificial Fusarium Infection on Oat Grain Quality" Microorganisms 9, no. 10: 2108. https://doi.org/10.3390/microorganisms9102108
APA StyleHavrlentová, M., Šliková, S., Gregusová, V., Kovácsová, B., Lančaričová, A., Nemeček, P., Hendrichová, J., & Hozlár, P. (2021). The Influence of Artificial Fusarium Infection on Oat Grain Quality. Microorganisms, 9(10), 2108. https://doi.org/10.3390/microorganisms9102108






