Abstract
Background: Aspergillus section Fumigati is one of the Aspergillus sections more frequently related to respiratory symptoms and by other health outcomes. This study aimed to characterize Aspergillus section Fumigati distribution in eleven firefighter headquarters (FFHs) to obtain an accurate occupational exposure assessment. Methods: A sampling approach protocol was performed using active (impaction method) and passive sampling methods (floor surfaces swabs, electrostatic dust collectors (EDCs), and settled dust). All samples were analysed by culture-based methods and passive sampling was used for molecular detection of Aspergillus section Fumigati. Results: Of all the matrices, the highest counts of Aspergillus sp. were obtained on settled dust filters (3.37% malt extract agar—MEA, 19.09% dichloran glycerol—DG18) followed by cleaning cloths (1.67% MEA; 7.07% DG18). Among the Aspergillus genus, the Fumigati section was predominant in Millipore and EDC samples in MEA (79.77% and 28.57%, respectively), and in swabs and settled dust filters in DG18 (44.76% and 30%, respectively). The Fumigati section was detected more frequently in DG18 (33.01%) compared to MEA (0.33%). The Fumigati section was observed in azole supplemented media (itraconazole and voriconazole) in several passive sampling methods employed and detected by qPCR in almost all passive samples, with EDCs being the matrix with the highest prevalence (n = 61; 67.8%). Conclusion: This study confirms that Aspergillus sp. is widespread and the Fumigati section is present in all FFHs. The presence of fungi potentially resistant to azoles in the FFHs was also observed. Further studies are needed to identify the best corrective and preventive measures to avoid this section contamination in this specific occupational environment.
1. Introduction
Aspergillus species are filamentous fungi commonly observed in different environmental compartments such as soil, water and air, with an emphasis on decaying vegetation, seeds and grains, where they prosper as saprophytes. Aspergillus species are also found in different indoor environments, and some species are considered opportunistic pathogens for humans [1,2]. Aspergillus conidia can be abundant in outdoor and indoor environments and are easily dispersed in the air depending on the developed activities. Since the conidia are very small, they are easily inhaled and may colonize the upper and lower respiratory tract of exposed individuals [2,3,4]. Aspergillus section Fumigati is one of the Aspergillus sections more frequently related to respiratory symptoms due to the small size of the conidia, thermotolerance, its nutritional versatility, and several other virulence factors [2,5,6,7]. Additionally, the development of resistance to antifungal drugs, mainly in this Aspergillus section, is a phenomenon with growing prevalence in Europe, being associated with therapeutic failure and high mortality rates [8].
An approach to ensure an accurate Aspergillus section Fumigati exposure assessment, considering its clinical and toxicological relevance, was recently suggested for occupational environments [4]. Among the recommendations, the combination of active and passive sampling methods, the use of culture-dependent and -independent methods, and the screening of azole resistance were emphasized and extensively justified by the results of previous assessments [4,7,9,10,11,12,13,14,15,16].
Several occupational environments in Portugal have already been characterized regarding the Aspergillus genus prevalence, demonstrating its critical dissemination indoors [4,7,9]. More recently, an assessment of Portuguese firefighters’ ambulances identified hazardous levels of Aspergillus section Fumigati in ambulance air, which would be able to reach the alveoli [17,18]; additionally, there are other relevant findings, such as toxigenic fungi with clinical relevance found in ambulance air, contamination of surfaces increased after cleaning at some sites and mycotoxins detected in mops and electrostatic dust cloths [18]. Thus, a concern regarding microbiological contamination in the headquarters was raised and the requirement for a profound characterization was identified. This study aimed to characterize Aspergillus section Fumigati distribution in firefighter headquarters following previous recommendations [4] to obtain an accurate occupational exposure assessment.
2. Material and Methods
2.1. Firefighter Headquarters Characterization
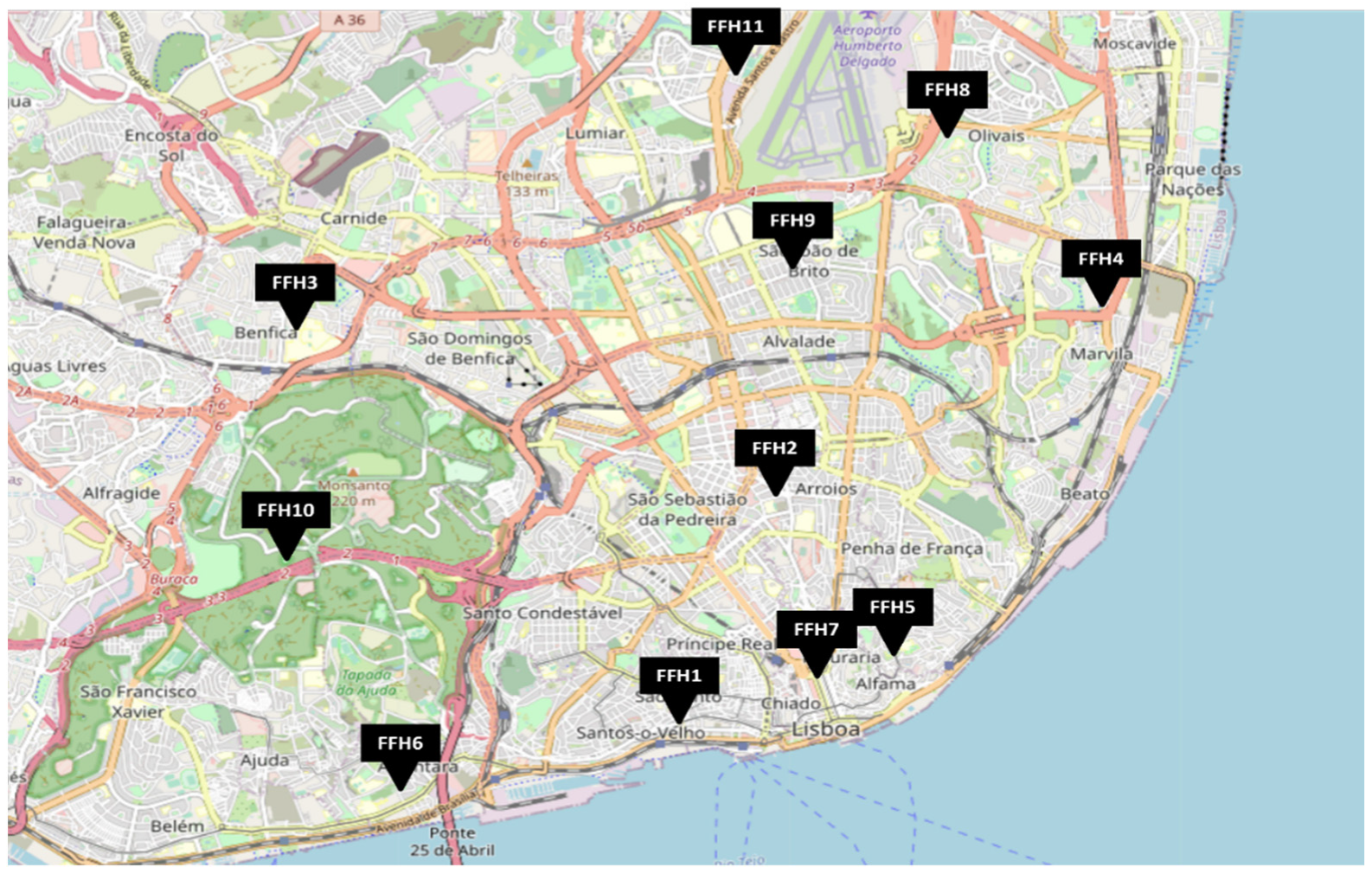
This study is part of an enlarged exploratory study aiming to assess microbial contamination in firefighter headquarters (FFHs). Eleven FFHs located in the Lisbon area were assessed between September 2020 and May of 2021 (Figure 1).
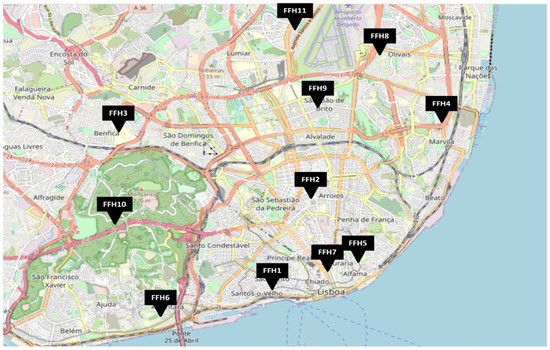
Figure 1.
Geographical distribution of the FFH (Firefighter headquarter) assessed.
In general, all FFHs were composed by dormitory, balneary, kitchen, canteen, bar, living room, administrative room, reception and gym, these being the chosen places for the sampling campaign. A local characterization of each FFH and the corresponding sampling sites was performed before all the procedures. The sampling campaign covered a range of facilities types, from centenary buildings to more recent ones, the latter being until two years old. The number of occupants per turn ranged from seven to fifty. Visible problems in wall conditions such as cracks, infiltrations, air leaks and mould growth were also registered, detected in older FFHs. Regarding the cleaning routine, all areas of the headquarters were cleaned and disinfected once a day (Table 1).

Table 1.
Characterization of the 11 FFHs sampled.
2.2. Sampling Approaches
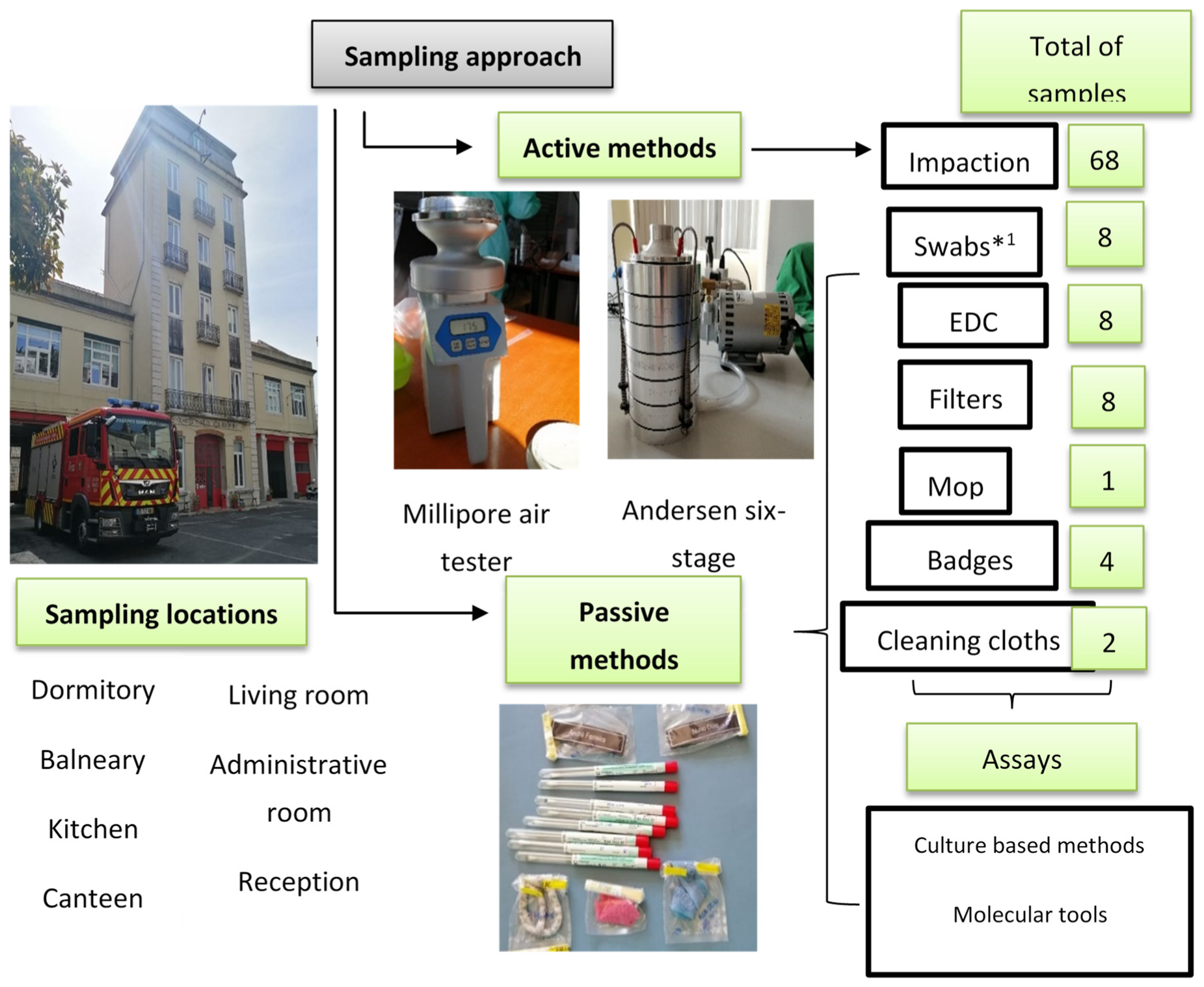
At each chosen site, a sampling approach protocol was performed using active and passive sampling methods (Figure 2). Indoor air was collected from selected areas by the impaction method on each plate using two devices (Millipore air tester and Andersen six-stage air sampler), according to the manufacturers’ guidelines. Different culture media were used to promote selectivity of bacteria and fungi [18,19].
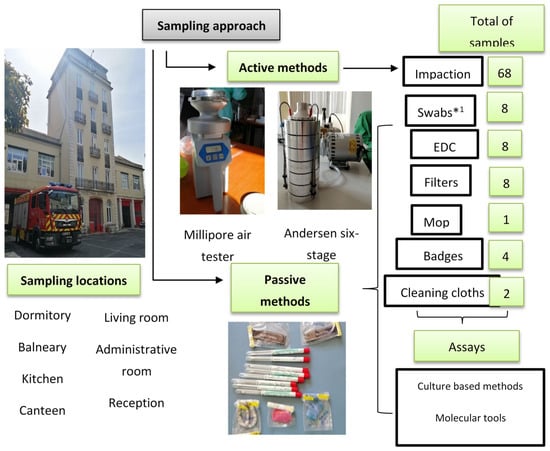
Figure 2.
Sampling approach and assays applied in FFH2 assessment; EDC: Electrostatic dust collector. *1 swabs were analysed only through culture-based methods.
The following passive sampling methods were used: floor surfaces swabs, electrostatic dust collectors (EDCs), and settled dust. For the collection of settled dust, a vacuum cleaner equipped with a filter (for further analysis) was used and a composite sample [20] of the settled dust from each FFH was obtained. Cloths and mops used in cleaning routines were collected, as well as the identification badges from firefighters’ uniforms.
A total of 760 indoor air samples were collected by the impactors, 190 samples from each culture medium. Regarding passive sampling methods, 82 EDCs, 90 swabs, 90 filters, 11 settled dust composite samples, 67 firefighter uniform badges, 25 cleaning cloths and 14 mops were obtained. Table S1 presented the sample distribution per sampling method in each FFH.
Indoor air was collected through active sampling methods in each selected area. About 250 L were collected at a flow rate of 140 L/min by Millipore air tester (Millipore, Billerica, MA, USA). A six-stage Andersen air sampler was also used, with a flow rate of 28.3 L/min, for 9 min in each culture medium, according to manufacturer’s recommendations [18]. An outdoor air sample form each FFH was taken for reference.
Concerning passive sampling methods, the floor surfaces were swabbed using a 10 × 10 cm square stencil, which was disinfected between each sample with a 70% alcohol solution [11]. The EDCs were placed in each sampling area 1.5 m above the ground for 30 days [21]. All samples (filters, settled dust, mops, cleaning cloths and identification badges) were kept in sterilized bags and transported under refrigeration (0–4 °C) to the laboratory [18].
Swabs were washed with 1 mL of 0.1% Tween 80 saline (0.9% NaCl) for 30 min on the orbital shaker (250 rpm, 30 min). The identification badges and a piece (2 cm2) of each settled dust filter, cleaning cloth and mop were processed similarly with 10 mL of the same solution [12]. EDCs were weighted and processed with 20 mL of the same washing solution. A composite sample with the settled dust obtained from each FFH was washed in a ratio of 1 g per 9.1 mL of 0.1% Tween 80 saline (0.9% NaCl) for 30 min at 250 rpm [18].
2.3. Aspergillus Section Fumigati Prevalence
All samples were analysed by culture-based methods. The impacted air and 150 µL of the suspensions resulting from washing the passive samples were inoculated in two different culture media: malt extract agar (MEA) supplemented with chloramphenicol (0.05%) (Frilabo, Maia, Portugal) and dichloran glycerol (DG18) agar supplemented with chloramphenicol (0.01%) (Frilabo, Maia, Portugal). The same extracts were used to screen for azole resistance by inoculating 150 µL of the samples onto Sabouraud dextrose agar (SDA) supplemented with 4 mg/L itraconazole (ITR), 2 mg/L voriconazole (VOR), 0.5 mg/L Posaconazole (POS), or SDA alone (as control) adapted from the [22]. The reference strain A. fumigatus ATCC 204305 was used as negative control and a pan-azole-resistant strain was used as positive control (both kindly provided by Reference Unit for Parasitic and Fungal Infections, Department of Infectious Diseases of the National Institute of Health, from Dr. Ricardo Jorge).
All plates, including those of the air impaction devices used and those where the extracts of the passive samples were inoculated, were incubated at 27 °C and examined for Aspergillus sp. densities (colony-forming units, CFU·m−3, CFU·m−2, CFU·g−1) after 3 (azole-resistance screening) and 5–7 days (MEA and DG18). This temperature was selected to enable the identification of all fungi in samples, for a broader characterization of fungal contamination, besides Aspergillus section Fumigati.
Fungal densities were determined depending on the passive sampling method used and applying appropriate formulas (Table S2) as previously described [9,10] Fungal isolates were identified by macroscopic and microscopic morphology using tease mount or Scotch tape mount and lactophenol cotton blue mount procedures [23]. Whenever colony overgrowth was observed due to fungi with fast growing rates (Chrysonilia sitophila, Trichoderma sp. and Mucorales order), a quantitative cut off of 500 isolates (CFU) was applied for air sampling and the mean of the results obtained from each environmental matrix was used for passive sampling [9,10,12,13].
2.4. Molecular Detection of Aspergillus Section Fumigati
Samples extracts (8.8 mL) from passive sampling (excluding surface swabs) were used for molecular detection of Aspergillus section Fumigati (Table 2). Fungal DNA was extracted using the ZR Fungal/Bacterial DNA MiniPrep Kit (Zymo Research, Irvine, USA) and molecular identification was performed by real-time PCR (qPCR) using the CFX-Connect PCR System (Bio-Rad). Reactions included 1× iQ Supermix (Bio-Rad, Portugal), 0.5 μM of each primer, and 0.375 μM of TaqMan probe in a total volume of 20 μL. Amplification followed a three-step PCR: 40 cycles with denaturation at 95 °C for 30 s, annealing at 52 °C for 30 s, and extension at 72 °C for 30 s.

Table 2.
Sequence of primers and TaqMan probes used for real-time PCR of Aspergillus section Fumigati.
A non-template control and a positive control consisting of DNA obtained from a reference that belonged to the culture collection of the Reference Unit for Parasitic and Fungal Infections, Department of Infectious Diseases of the National Institute of Health, from Dr. Ricardo Jorge. These strains have been sequenced for ITS, B-tubulin, and Calmodulin.
2.5. Statistical Analysis
Data were analysed using SPSS statistical software, V26.0, for Windows (Microsoft, USA). The results were considered significant at the 5% significance level. To test the normality of the data, the Shapiro–Wilk test (n’s < 50) or the Kolmogorov–Smirnov test (n’s > 50) was used. To compare Fungal contamination, Aspergillus sp. and Aspergillus section Fumigati between FFHs, media, sampling method and sections, the Kruskal–Wallis test was used, since the assumption of normality was not verified.
To study the relationship between fungal contamination, Aspergillus sp. and Aspergillus section Fumigati, Spearman’s correlation coefficient was used, since the normality assumption was not verified. To study the relationship between sections, FFHs, media and sampling method, the Chi-Square test by Monte Carlo simulation was used, since the assumptions of applicability of the Chi-Square test were not verified. To identify the association trend, multiple correspondence analysis was used, having been discretized the variables fungal contamination (<53.76, [53.76; 294.2[, [294.2; 2052.05[, ≥2052.05), Aspergillus sp. (<3.93, [3.93; 100[, ≥100) and Aspergillus section Fumigati (<3.93, [3.93; 11.78[, [11.78; 212.31[, ≥212.31), considering the quartiles.
3. Results
3.1. Aspergillus Section Fumigati Distribution
Among all the FFHs, and concerning Aspergillus genera, the highest value obtained by the Andersen six-stage air samples was observed on FFH5 (3.81%), and the same trend was obtained from the cleaning cloths and filters from the same FFH (0.51% and 7.33%). Concerning Millipore air samples, the FFH10 presented the highest counts (1.87%). Fumigati was the predominant section in Andersen six-stage FFH7 (88.37%), while for Millipore air samples, the highest counts were observed in FFH9 (100%).
In FFH4, the Aspergillus genera was predominant in EDCs (0.55%) and in settled dust (0.65%) samples, while in FFH2, the section Fumigati was the most frequent in EDCs (100%). Aspergillus sp. was identified in mops from FFH1 (0.44%), Fumigati being the predominant section (100%). Similar results were obtained in swabs samples from FFH3, Aspergillus being the most frequent genera (0.08%) and Fumigati being the prevalent section (100%).
Regarding samples collected from all FFHs, the genus Aspergillus was present in almost all matrices, with a prevalence of 1.52% in MEA (Millipore; six-stage Andersen; EDCs; cleaning cloths; mops; settled dust filters; swabs) and 2.20% in DG18 (Millipore; six-stage Andersen; EDCs; cleaning cloths; settled dust filters; swabs), being absent in mops, identification badges and settled dust samples in MEA and identification badges in DG18 (Table 3).

Table 3.
Aspergillus sp. distribution in all matrices in MEA and DG18 from all FFHs.
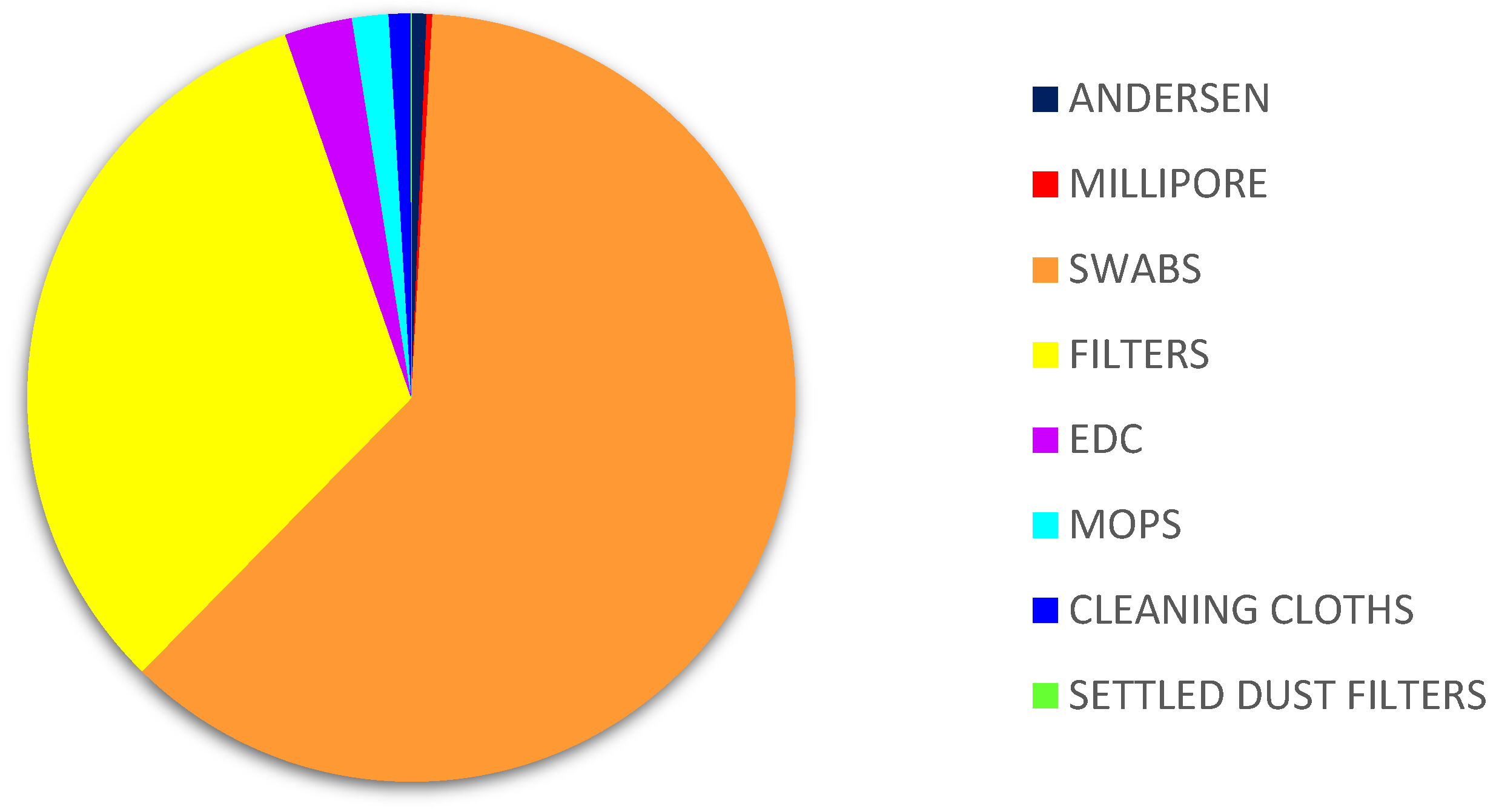
Of all the matrices, the highest counts of Aspergillus sp. were obtained on filters (3.37% MEA, 19.09% DG18) followed by cleaning cloths (1.67% MEA; 7.07% DG18). The matrices where the lowest prevalence was reported were air samples from Millipore (0.10% MEA, 0.77% DG18), six-stage Andersen (0.11% MEA) and settled dust (0.89% DG18) (Figure 3).
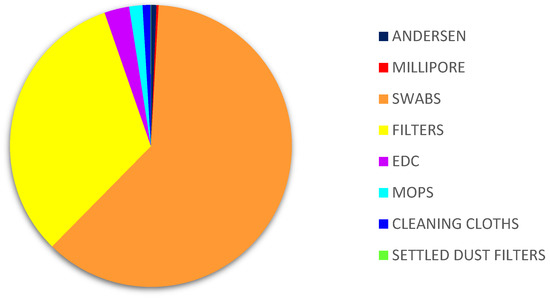
Figure 3.
Aspergillus sp. distribution in samples on DG18. DG18: Dichloran–Glycerol Agar; EDC: Electrostatic dust collector.
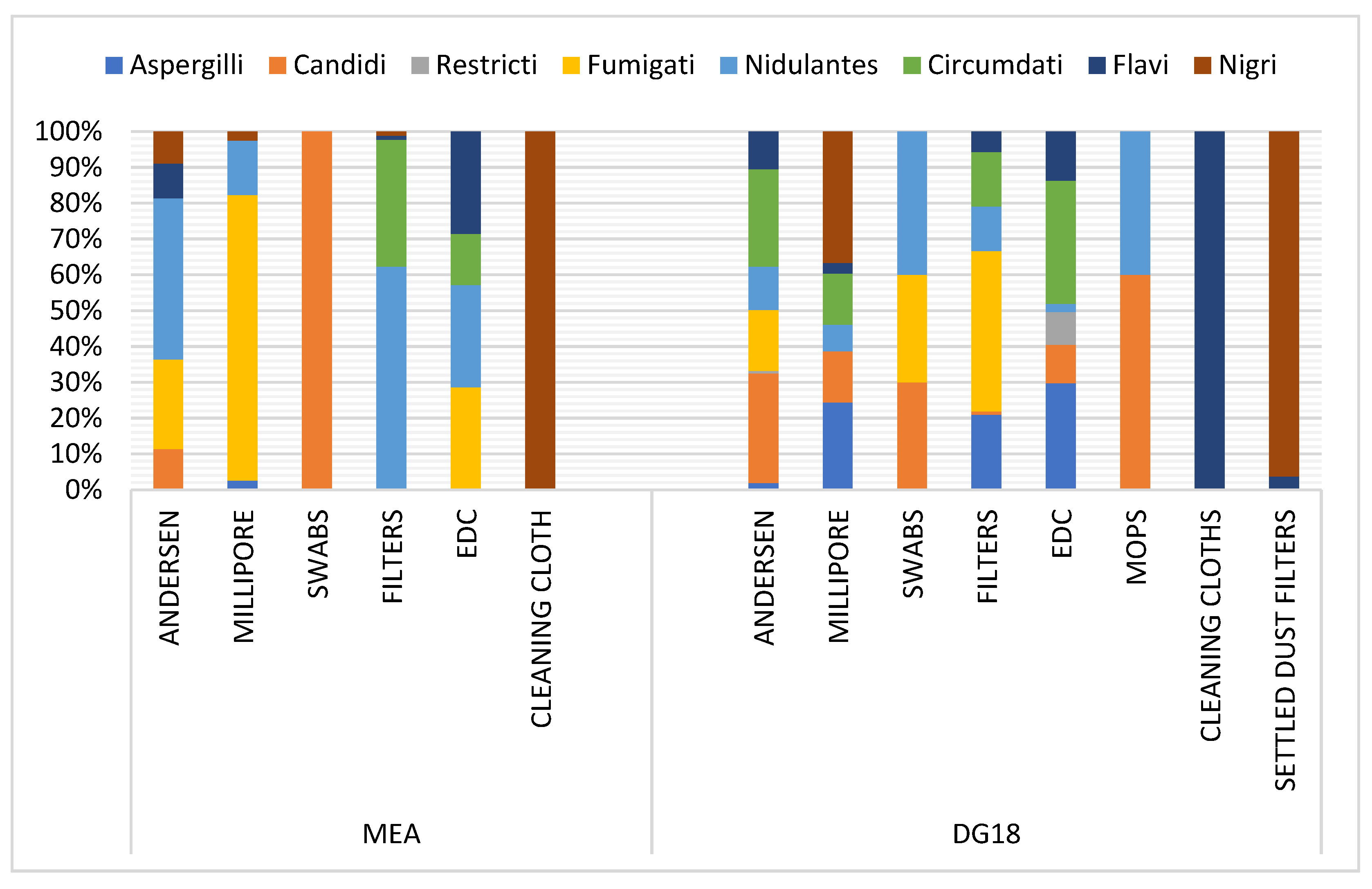
Among the Aspergillus genus, the Fumigati section was predominant in Millipore and EDC samples in MEA (79.77% and 28.57%, respectively), and in swabs and settled dust filters in DG18 (44.76% and 30%, respectively). This section was also observed in six-stage Andersen samples (25.04% MEA; 16.99% DG18) (Figure 4). Among Aspergillus sp., section Fumigati was identified in the Andersen air sampler through all six stages. In FFH8, the section was the only one identified (100% on DG18) in stage 1 (7 µm). In FFH2, despite the lower frequency (4% MEA), the section was reported on the 2nd stage (4.7 µm). The section was the only one found on DG18 in stage 3 (3.1 µm) in FFH7. The same trend was obtained in FFH2 (100% MEA) in stage 4 (2.1 µm) and in FFH6 (100% MEA) in stage 5 (1.1 µm). In addition, FFH5, FFH6 and FFH10 had similar results (100% MEA) in stage 6 (0.65 µm). In stage 6, section Fumigati was the only found in the two culture media from FFH8 (100% MEA and DG18). Concerning the Fumigati section, positive samples (n = 23), the section was more frequently detected in samples performed by the Andersen sampling device (Table S3) and was more frequently detected in DG18 (33.01%) compared to MEA (0.33%) (Table S4).
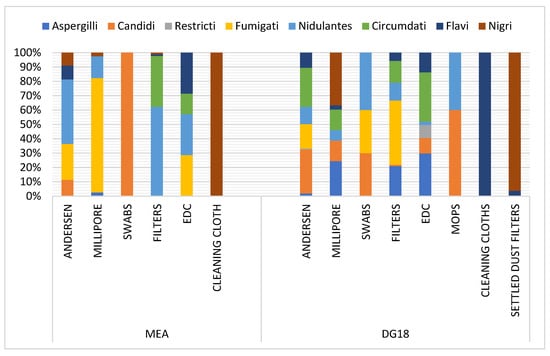
Figure 4.
Distribution of Aspergillus sections per matrice in MEA (Malt Extract Agar) and DG18 (Dichloran–Glycerol Agar).
3.2. Screening of Azole Resistance
Passive matrices (82 EDC, 102 swabs, 89 filters, 11 settled dust, 67 uniform name tags, 25 cleaning cloths and 14 mops) were extracted as described and screened for antifungal resistance to three commonly used medical azoles. Growth of Aspergillus sp. was observed in Sabouraud (SDA: 1.11%) and in two azole-supplemented SDA media (ITR: 0.11%; VOR: 0.11%), with no growth observed in the POS media. The Fumigati section was the only among Aspergillus sp. observed in three culture media (SDA: 8.9%; ITR: 100%; VOR: 85.3%) (Table 4).

Table 4.
Prevalence of Aspergillus section Fumigati among Aspergillus sp. in azole-supplemented SDA media.
The relative frequency of azole-resistant Aspergillus section Fumigati isolates among all Aspergillus sp. isolates in the azole resistance screening was 17.8%. Isolates able to grow in 4 mg/L itraconazole and/or 2 mg/L voriconazole (all of them from Aspergillus genus) were identified in three different headquarters, from the following samples: 2 filters in FFH1; 1 EDC in FFH3; and 4 EDCs in FFH6.
Of all screened matrices, Aspergillus sp. was identified only in mops, EDCs, settled dust filters and settled dust. Concerning the Aspergillus sections, the Fumigati section was prevalent in the SDA of 100% in mops, 4.4% in EDCs and 3.7% in filters. Fumigati was the only section found in ITR (filters and EDCs: 100%) and the most predominant in VOR (EDCs: 97.1%) (Table 4).
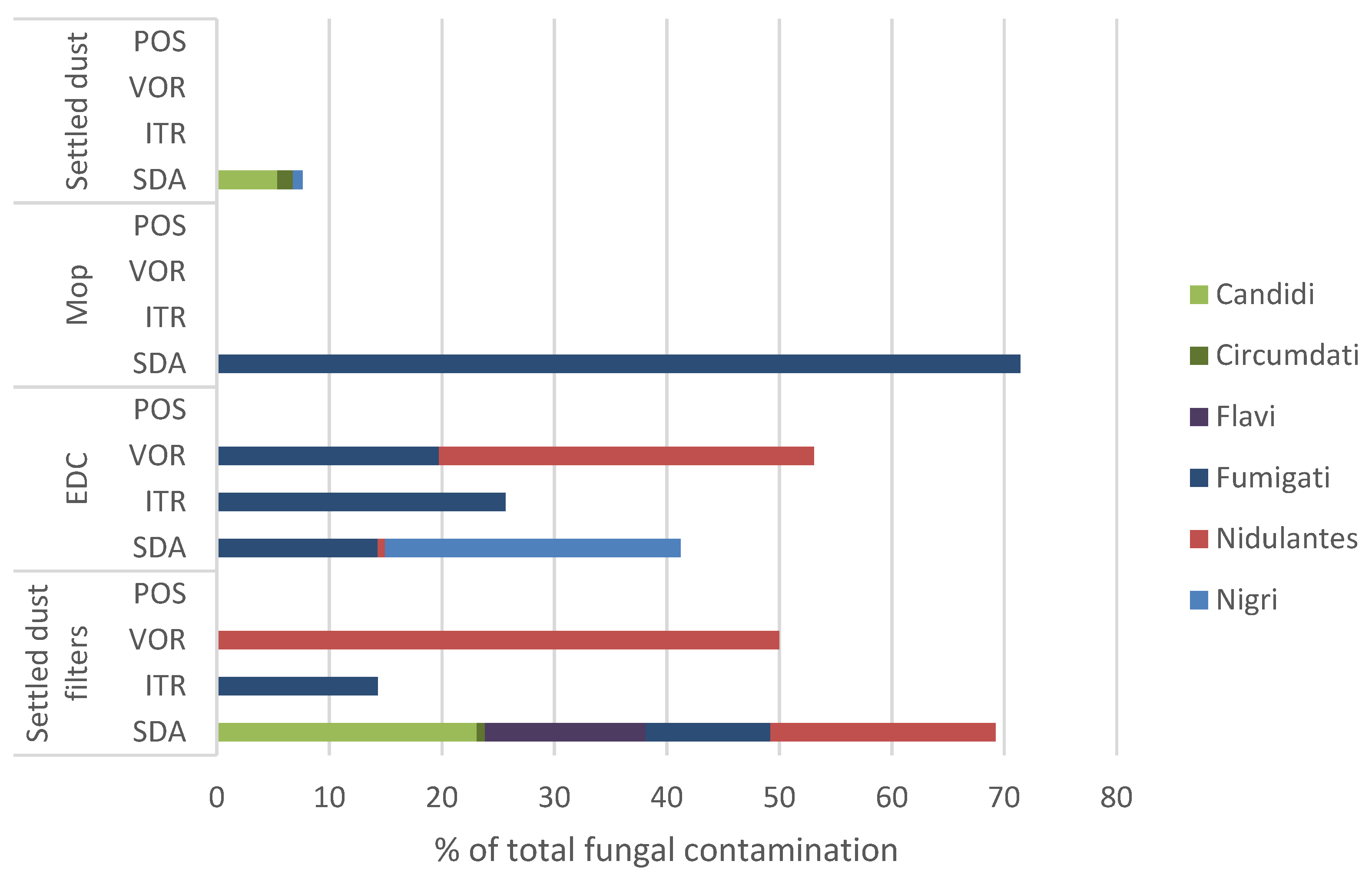
The results of Aspergillus sp. relative distribution per sample type and culture media are depicted in Figure 5. The Fumigati section was predominant among Aspergillus sp. in mop samples in SDA media, abundant in EDCs and settled dust filter samples, including in ITR and VOR, and absent in settled dust.
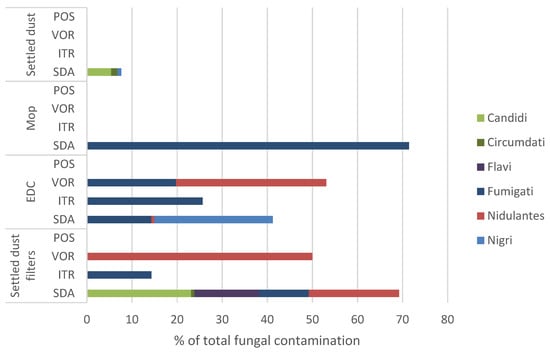
Figure 5.
Relative distribution of Aspergillus sections per matrix type in SDA and azole-supplemented SDA media (ITR, VOR, POS) regarding total fungal contamination. SDA (Sabouraud Dextrose Agar); ITR (Itraconazole); VOR (Voriconazole); POS (Posaconazole); EDC (Electrostatic dust collector).
3.3. Molecular Detection
The Aspergillus section Fumigati was detected by qPCR in almost all passive samples, with EDCs being the matrix with the highest prevalence (n = 61; 67.8%) followed by settled dust filters (n = 60; 66.6%). This section was also detected in seven mops (50%), in 15 cleaning cloths (60%), and, to a lesser extent, in identification badges (n = 4; 5.9%) (Table S5).
3.4. Correlation Analysis
Regarding the comparison of fungal contamination, Aspergillus sp. and Aspergillus section Fumigati between sampling methods, statistically significant differences were detected in (i) fungal contamination (, p = 0.000); (ii) Aspergillus sp. (, p = 0.000). In both cases, it was found that settled dust filters, EDCs and the group of others (mops, swabs and cleaning cloths) were the sampling methods that presented the highest values (Table 5).

Table 5.
Comparison of fungal contamination, Aspergillus sp. and Aspergillus section Fumigati between sampling methods. Kruskal–Wallis test.
Among the media used (MEA, DG18, SDA, ITR, VOR, and POS), statistically significant differences were detected for fungal contamination (, p = 0.000), Aspergillus sp. (, p = 0.000) and Aspergillus section Fumigati (, p = 0.000). It was found that the SDA and ITR+VOR were the ones with the highest values (Table 6).

Table 6.
Comparison of Fungal contamination, Aspergillus sp. and Aspergillus section Fumigati between media. Kruskal–Wallis test.
Regarding FFHs, statistically significant differences were detected for fungal contamination (, p = 0.014), Aspergillus sp. (, p = 0.014) and Aspergillus section Fumigati (, p = 0.013). Regarding fungal contamination, FFHs 3, 7, 8, 9 and 11, were the ones with the highest values, while for Aspergillus sp., FFHs 3, 6, 7 and 9 were the ones presenting the highest values. Concerning to the Aspergillus section Fumigati, FFHs 6, 7 and 8, were the ones where the highest values were observed (Table 7).

Table 7.
Comparison of fungal contamination, Aspergillus sp. and Aspergillus section Fumigati between FFHs. Kruskal–Wallis test.
Significant correlations were detected between Aspergillus sp. and Aspergillus section Fumigati (rS = 0.137, p = 0.036) and with fungal contamination (rS = 0.705, p = 0.000) and between Aspergillus section Fumigati and fungal contamination (rS = 0.130), p = 0.047). These results reveal that higher values for Aspergillus sp. are related to higher counts for Aspergillus section Fumigati and fungal contamination, as well as between Aspergillus section Fumigati and fungal contamination (Table 8).

Table 8.
Relationship between fungal contamination, Aspergillus sp. and Aspergillus section Fumigati. Spearman’s correlation coefficient.
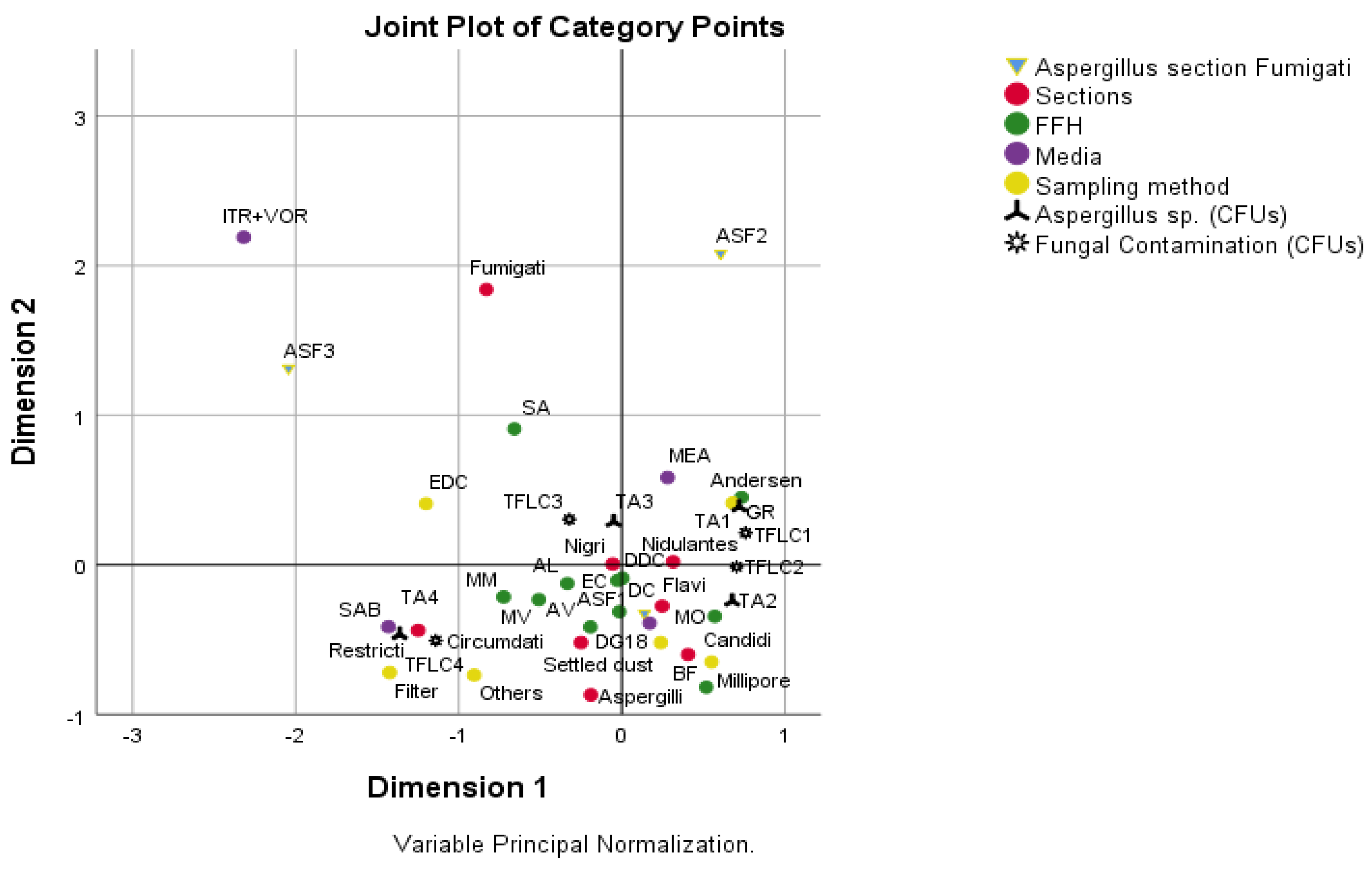
Significant associations were detected between sections, FFHs, media and sampling method (p’s < 0.05, Qui-Square test by Monte Carlo Simulation). From an analysis of Figure 6, the following associations were identified: (i) FFHs 1, 3, 7, 8 and 9 with SAB, settled dust filters and other sampling methods, Aspergillus sp. with values ≥21.31, fungal contamination with values ≥2052.05, and sections Restricti, Circumdati and Aspergilli; (ii) FFHs 1, 2, 4, 8 and 10 with DG18, section Fumigati with values [0; 3.93[, Nigri, Nidulantes, Flavi and Candidi sections, Settled dust and Millipore sampling method, Aspergillus sp. with values [3.93; 11.78[ and fungal contamination with values [53.76; 294.2[; (iii) FFH5 with Aspergillus sp. with values <3.93, fungal contamination with values <53.76, MEA, Nidulantes section, Andersen sampling method; iv) FFH6 with EDCs, Fungal contamination with values [294.2; 2052.05[, Aspergillus sp. with values [11.78; 21.31[ and Nigri section.
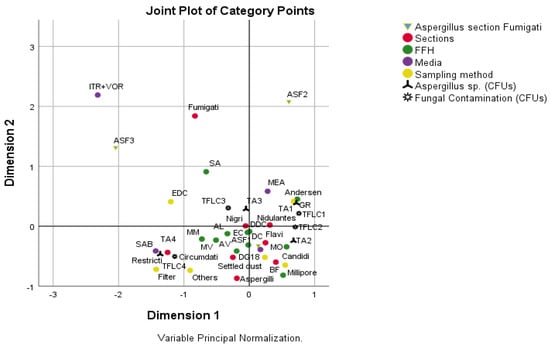
Figure 6.
Results of multiple correspondence analysis. Study of the association between Aspergillus section Fumigati, sections, FFHs, media, sampling method, Aspergillus sp. and fungal contamination. Aspergillus section Fumigati: ASF1—[0; 3.93[, ASF2—[3.93; 100[, ASF3—≥100; Aspergillus sp. (CFUs—Colony forming units;): TA1—<3.93, TA2—[3.93; 11.78[, TA3—[11.78; 21.31[, TA4—≥21.31; Fungal contamination (CFUs): TFLC1—<53.76, TFLC2—[53.76; 294.2[, TFLC3—[294.2; 2052.05[, TFLC4—≥2052.05; FFH (firefighter headquarter).
4. Discussion
The presence of fungi in an indoor environment is influenced by a wide range of variables, such as human occupancy and their activities, humidity levels, ventilation, environmental characteristics, water infiltrations, building and decoration materials and outdoor air [25,26]. Furthermore, there is strong scientific evidence corroborating the relationship between the building dampness, visible mould, and moisture damage with adverse respiratory health effects [4,27,28,29,30,31,32]. Moisture, nutrients and temperature are proved to be the most important variables that influence the growth and dissemination of fungi on building materials [33]. Thus, the results regarding Aspergillus sp. and Fumigati section contamination in the assessed FFHs were the expected considering the observed FFH conditions (in 8 from the 11 FFHs): leakages, visible mould growth and cracks on the walls/floor.
The identification of the Aspergillus section Fumigati through passive and active sampling has already been reported [4,34,35,36,37,38]. However, other studies developed in Portuguese occupational environments presented a lower prevalence of Aspergillus sp. and, more specifically, section Fumigati [9,10,12,13,35]. Indeed, in primary health care centres the Fumigati section presence was 33.3% on surface swabs and 1.3% in EDCs [9,10]. In one central hospital, Aspergillus sp. presented an overall prevalence of 17.25%, with the Fumigati section only being observed in the vacuum bag [12]; in Portuguese bakeries, the Fumigati section was found on air samples (3.2% on DG18) and in EDCs (8.3% on MEA and 50% on DG18) [13]; in three fitness centres, only four isolates of this section were found in one air sample [35].
In this study, a multiple approach protocol was performed comprising the two sampling methods for a better characterization of contamination in FFHs. Aspergillus counts were revealed to be higher in settled dust filters and cleaning cloths. Previous studies also identified passive sampling as suitable to determine Aspergillus section Fumigati by culture-based methods through sampling of filtering respiratory protective devices and other environmental matrices in settings such as waste-sorting plants, veterinary clinics, dairies, ambulances, and many other indoor and occupational environments [4,10,16,18,32]. In fact, passive sampling is able to characterize contamination levels over a wider period of time, compared to air sampling [21,36]. Thus, higher fungal counts and greater fungal diversity are expected by passive sampling.
The Aspergillus section Fumigati was predominant in swabs and settled dust filters in DG18, and in EDCs in MEA, suggesting that reliable matrices for Aspergillus section Fumigati exposure assessment were chosen [4]. The significant differences in fungal counts between passive and active sampling highlight the advantages associated with a multi-approach protocol that comprises active and passive sampling simultaneously, overcoming the limitations associated with each sampling method [4,7,9,10,11,12,13,14,15,16,39].
Regardless of the lower prevalence of Aspergillus sp. in air samples performed by Millipore and six-stage Andersen, the Fumigati section was predominant in Millipore air samples in MEA. Furthermore, the underestimation of microbial contamination collected by impaction devices (Andersen six-stage and Millipore sampling devices), due to cell damage during sampling process, has already been stated [18,40,41]. However, the six-stage Andersen sampler allows the hazardous range where the Fumigati section has lung penetrability to be identified [17,18,41]. Indeed, in four of the assessed FFHs, section Fumigati at stage 6 (0.65 µm) of reaching alveoli was observed. This has the potential to cause respiratory diseases (inflammation activation) by activating macrophages, B cells and T cells [42]. The same concern was raised in a study held in Portuguese ambulances used in emergency clinical services [18].
Aspergillus section Fumigati was more frequent in DG18 compared to MEA counts. Despite the recommendation of using MEA for aerobiological studies in a Portuguese regulatory framework dedicated to the assessment of indoor air quality (IAQ) (25APA 2010), DG18 is an efficient option due to its restrictive character, inhibiting the development of fastidious fungal species [21]. The results of some fungal overgrowth on the MEA plates may have influenced the development of Aspergillus sp. and, more specifically, of the Fumigati section, due to chemical competition [43], highlighting the use of both culture media for a wider fungal characterization [4,7,9,10,11,12,13,14,15,16,39]. Sample dilution prior to inoculation (to avoid the excessive development of fast-growing fungi on media plates) was not performed due to the limitations of this option. Indeed, the removal of rare types of organisms leads to differences in species richness and diversity, decreasing competition among microorganisms, causing a probable overgrowth in some species that were not as prevalent in the original community [44].
The higher mean rank values obtained for total fungal contamination, Aspergillus sp. counts, and Aspergillus section Fumigati with SDA and ITR+VOR media, compared to MEA or DG18, suggest Sabouraud is a more suitable media for the recovery of fungi and Aspergillus sp. The use of Sabouraud as a standard medium to assess outdoor airborne fungi by air sampling was generally supported in a recent study on media comparison [45], and has also been supported in clinical applications [46]. However, Saboraud enhanced Chrysonilia sitophila in other performed assessments [9,18].
The ability to use protection against respiratory devices or filters as a sampling approach depends on the features of the assessed setting, activities developed and duration of use, e.g., mop sampling depends on cleaning procedures. The EDC device, on the other hand, allows for the recovery of fungal contamination in a consistent and standardized manner (regarding retention material and collection period), and it is a low-cost, low-maintenance sampling strategy that has been increasingly used in the assessment of occupational exposure to fungal burden [19] and in indoor air quality studies [21,32].
The fact that, among Aspergillus sections, only the Fumigati section was found in azole-supplemented media, confirms the presence of fungi potentially resistant to azoles in FFHs. If this azole-resistance phenotype is further confirmed by molecular analysis or antifungal susceptibility testing, it might represent a health risk for workers in this setting, especially in the FFHs where contamination by Aspergillus section Fumigati was higher. This health risk arises from the fact that azole-resistant fungi might cause invasive infections, especially in immunocompromised individuals, which are of difficult control due to the limited treatment options [2,4,8,19]. In addition to being the etiological agent of invasive aspergillosis, the Fumigati section is also responsible for more common respiratory symptoms such as asthma, allergic sinusitis, cough and bronchial hyperresponsiveness [47].
Culture-based methods allowed the Aspergillus section Fumigati to be identified in various matrices (settled dust filters, swabs, EDCs and air samples from Millipore and Andersen), confirming the results of molecular detection in EDCs and filters. The use of qPCR further enabled the Fumigati section to be detected in additional matrices (identification badges, mops and cleaning cloths) where it was undetectable by culture. This may be associated with the absence of fungal viability due to an impediment to grow in culture (e.g., due to competition for nutrients), while the molecular tools enable even non-viable microorganisms to be identified [48]. Failure to detect the Aspergillus section Fumigati by qPCR in swabs and air samples (in contrast to the results obtained by culture) may be associated with ineffective DNA extraction in sample processing, or the presence of inhibitors (such as particles from air samples), misleading the results [4,49,50]. Without diminishing the advantages of molecular analysis, classical culture-based methods are still necessary to assess the viability of pathogenic microorganisms related to their infectivity potential. Indeed, a microorganism’s viability is associated to the potential of inflammatory and cytotoxic responses and, consequently, the infection potential. Therefore, molecular tools must be used in parallel with classic methods [4,51].
Correlation was found in this study between total fungal counts, Aspergillus sp. counts and Fumigati section counts not following the trend previously found in health care environments [9]. This means that the measures used to avoid fungal contamination in this setting are also effective concerning Aspergillus contamination. Nevertheless, Aspergillus genera assessment should always be performed, as specific Aspergillus sections (Flavi, Fumigati, Circumdati and Nidulantes) are indicators of harmful fungal contamination when found on air samples and require intervention, as referred to by the American Industrial Hygiene Association and Portuguese regulatory framework concerning IAQ [25,52].
Thus, considering the lack of scientific information in this specific environment, further studies are needed to characterize the overall exposure to fungal contamination and other microbiological agents, as well as regarding the most suitable corrective and preventive measures used to avoid exposure. Additionally, further research on azole-resistance profile must be conducted to better estimate the risk of exposure to resistant Aspergillus section Fumigati in this setting, namely, screening azole resistance at selective conditions for Aspergillus section Fumigati, molecular analysis of resistance mutations, and antifungal susceptibility testing.
5. Conclusions
Overall, this study confirms the widespread nature of Aspergillus sp. and the presence of section Fumigati in all FFHs. The presence of fungi potentially resistant to azoles in FFHs was also reported. Further studies are needed to identify the best corrective and preventive measures to avoid fungal contamination in this specific occupational environment.
This study corroborates the importance of applying a wide sampling approach when assessing occupational exposure to section Fumigati in FFHs. The same tendency of complementarity was found between culture-based methods and molecular methods, since qPCR added the detection of the Fumigati section in additional matrices, while in some samples, the section was only possible to be identified and not detected by qPCR.
DG18 should be considered in future legal and technical recommendations focusing on the assessment of occupational exposure to Aspergillus genera.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9102112/s1, Table S1. Samples distribution per sampling method in each FFH. Table S2. Formulas applied for the calculation of CFU. M−3/m−2 * CFU.m−2.day−1. Table S3. Aspergillus section Fumigati distribution in each FFH per sampling method. Table S4. Aspergillus section Fumigati prevalence in positive samples from FFH in MEA and DG18. Table S5. Identification and detection of the Aspergillus section Fumigati in the assessed FFH.
Author Contributions
Conceptualization, C.V.; methodology, C.V.; formal analysis, B.G., M.D., E.C. and L.A.C.; investigation, C.V.; resources, C.V.; writing—original draft preparation, C.V., B.G., M.D., E.C. and L.A.C.; writing—review and editing, C.V. and L.A.C.; supervision, C.V.; project administration, C.V.; funding acquisition, C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Instituto Politécnico de Lisboa, for funding the Project “Occupational exposure of ambulance drivers to bioburden” (IPL/2020/BIO-AmbuDrivers_ESTeSL)”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Through the UIDB/05608/2020 and UIDP/05608/2020.
Acknowledgments
H&TRC authors gratefully acknowledge the FCT/MCTES national support through the UIDB/05608/2020 and UIDP/05608/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seyedmousavi, S.; Guillot, J.; Arné, P.; de Hoog, G.S.; Mouton, J.W.; Melchers, W.J.G.; Verweij, P.E. Aspergillus and aspergilloses in wild and domestic animals: A global health concern with parallels to human disease. Med Mycol. 2015, 53, 765–797. [Google Scholar] [CrossRef] [PubMed]
- Sabino, R. Exposure to Fungi in Health Care Facilities. Ref. Modul. Life Sci. 2020, 1–10. [Google Scholar] [CrossRef]
- Walsh, T.J.; Anaissie, E.J.; Denning, D.W.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Segal, B.H.; Steinbach, W.J.; Stevens, D.A.; et al. Treatment of Aspergillosis: Clinical Practice Guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 327–360. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Aranha Caetano, L.; Viegas, S. Occupational exposure to Aspergillus section Fumigati: Tackling the knowledge gap in Portugal. Environ. Res. 2021, 194. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.C. Aspergillus fumigatus: Growth and virulence. Med Mycol. 2006, 44, S77–S81. [Google Scholar] [CrossRef]
- Varga, J.; Baranyi, N.; Chandrasekaran, M.; Vágvölgyi, C.; Kocsubé, S. Mycotoxin producers in the Aspergillus genus: An update. Acta Biol. Szeged. 2015, 59, 151–167. [Google Scholar]
- Viegas, C.; Faria, T.; Aranha Caetano, L.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. prevalence in different occupational settings. J. Occup. Environ. Hyg. 2017, 14, 771–785. [Google Scholar] [CrossRef]
- Stop neglecting fungi. Nat. Microbiol. 2017, 2. [CrossRef]
- Viegas, C.; Almeida, B.; Gomes, A.Q.; Carolino, E.; Caetano, L.A. Aspergillus spp. prevalence in Primary Health Care Centres: Assessment by a novel multi-approach sampling protocol. Environ Res. 2019, 175, 133–141. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; Viegas, S. Bioburden in healthcare centers: Is the compliance with Portuguese legislation enough to prevent and control infection? Build. Environ. 2019, 160. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Cavaleiro, J.R.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Marchand, G.; et al. Settled dust assessment in clinical environment: Useful for the evaluation of a wider bioburden spectrum. Int. J. Environ. Health Res. 2019, 26, 1–19. [Google Scholar] [CrossRef]
- Viegas, C.; Almeida, B.; Monteiro, A.; Paciência, I.; Rufo, J.; Aguiar, L.; Lage, B.; Gonçalves, L.M.D.; Aranha Caetano, L.; Carolino, E.; et al. Exposure assessment in one central Hospital: A multi-approach protocol to achieve an accurate risk characterization. Environ. Res. 2019, 181, 108947. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; Caetano, L.A.; Carolino, E.; Quintal-Gomes, A.; Twaruzek, M.; Kosicki, R.; Viegas, S. Characterization of Occupational Exposure to Fungal Burden in Portuguese Bakeries. Microorganisms 2019, 7, 234. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Almeida, B.; Caetano, L.A.; Afanou, A.; Straumfors, A.; Veríssimo, C.; Gonçalves, P.; Sabino, R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Viegas, C.; Caetano, L.A.; Cox, J.; Korkalainen, M.; Haines, S.R.; Dannemiller, K.C.; Viegas, S.; Reponen, T. The effects of waste sorting in environmental microbiome, THP-1 cell viability and inflammatory responses. Environ. Res. 2020, 185. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Almeida, B.; Carolino, E.; Quintal Gomes, A.; Viegas, S. Aspergillus spp. burden on filtering respiratory protective devices. Is there an occupational health concern? Air Qual. Atmos. Health 2020, 13. [Google Scholar] [CrossRef]
- Andersen, A. New sampler for the collection, sizing and enumeration of viable airborne particles. J. Bacteriol. 1958, 76, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Sousa, P.; Dias, M.; Caetano, L.A.; Ribeiro, E.; Carolino, E.; Twarużek, M.; Kosicki, R.; Viegas, S. Bioburden contamination and Staphylococcus aureus colonization associated with firefighter’s ambulances. Environ. Res. 2021, 197. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Dias, M.; Carolino, E.; Sabino, R. Culture Media and Sampling Collection Method for Aspergillus spp. Assessment: Tackling the Gap between Recommendations and the Scientific Evidence. Atmosphere 2021, 12, 23. [Google Scholar] [CrossRef]
- EPA. United States Environmental Protection Agency EPA Observational Economy Series Volume1: Composite Sampling. Available online: https://www.epa.gov/environmentaljustice/epa-environmental-justice-strategy-1995 (accessed on 1 September 2021).
- Viegas, C.; Dias, M.; Almeida, B.; Vicente, E.; Candeias, C.; Aranha Caetano, L.; Carolino, E.; Alves, C. Loading Rates of Dust and Bioburden in Dwellings in an Inland City of Southern Europe. Atmosphere 2021, 12, 378. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Routine and Extended Internal Quality Control for MIC Determination and Agar Dilution for Yeasts, Moulds and Dermatophytes as Recommended by EUCAST. Available online: http://www.eucast.org (accessed on 1 September 2021).
- De Hoog, D.; Guarro, J.; Gene, G.; Figueras, M. Atlas of Clinical Fungi—The Ultimate Benchtool for Diagnosis; Utr Centraalbureau voor Schimmelcultures: Utrecht, The Netherlands, 2016; Volume 4, Issue 1. [Google Scholar]
- Cruz-Perez, P.; Buttner, M.P.; Stetzenbach, L.D. Detection and quantitation of Aspergillus fumigatus in pure culture using polymerase chain reaction. Mol. Cell. Probes 2001, 15, 81–88. [Google Scholar] [CrossRef]
- WHO. World Health Organisation Guidelines for Indoor Air Quality: Dampness and Mould. Available online: https://www.who.int/publications/i/item/9789289041683 (accessed on 1 September 2021).
- APA. Qualidade do Ar em Espaços Interiores Um Guia Técnico Agência Portuguesa do Ambiente. Available online: https://www.voltimum.pt/artigos/biblioteca-de-flipbooks/qualidade-do-ar-em (accessed on 1 September 2021).
- Strachan, D.P. Damp housing and childhood asthma: Validation of reporting of symptoms. BMJ 1988, 297, 1223–1226. [Google Scholar] [CrossRef]
- Platt, S.D.; Martin, C.J.; Hunt, S.M.; Lewis, C.W. Damp housing, mould growth, and symptomatic health state. BMJ 1989, 298, 1673–1678. [Google Scholar] [CrossRef]
- Dales, R.E.; Burnett, R.; Zwanenburg, H. Adverse health effects among adults exposed to home dampness and molds. Am. Rev. Respir. Dis. 1991, 143, 505–509. [Google Scholar] [CrossRef]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health. Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef]
- Kanchongkittiphon, W.; Mendell, M.J.; Gaffin, J.M.; Wang, G.; Phipatanakul, W. Indoor environmental exposures and exacerbation of asthma: An update to the 2000 review by the Institute of Medicine. Environ. Health Perspect. 2015, 123, 6–20. [Google Scholar] [CrossRef]
- Adams, R.; Leppänen, H.; Karvonen, A.; Jacobs, J.; Borràs-Santos, A.; Valkonen, M.; Krop, E.; Haverinen-Shaughnessy, U.; Huttunen, K.; Zock, J.-P.; et al. Microbial exposures in moisture-damaged schools and associations with respiratory symptoms in students: A multi-country environmental exposure study. Indoor Air 2021. [Google Scholar] [CrossRef] [PubMed]
- Rajasekar, A.; Balasubramanian, R. Assessment of airborne bacteria and fungi in food courts. Build. Environ. 2011, 46, 2081–2087. [Google Scholar] [CrossRef]
- Alberti, C.; Bouakline, A.; Ribaud, P.; Lacroix, C.; Rousselot, P.; Leblanc, T.; Derouin, F.; Aspergillus group. Relationship between environmental fungal contamination and the incidence of invasive aspergillosis in haematology patients. J. Hosp. Infect. 2001, 48, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Viegas, C.; Verde, S.C.; Wolterbeek, H.T.; Almeida, S.M. Characterizing the fungal and bacterial microflora and concentrations in fitness centres. Indoor Built Environ. 2016, 25, 872–882. [Google Scholar] [CrossRef]
- Reponen, T. Sampling for Microbial Determinations. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 85–96. [Google Scholar]
- Cox, J.; Mbareche, H.; Lindsley, W.G.; Duchaine, C. Field sampling of indoor bioaerosols. Aerosol Sci. Technol. 2020, 54, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Melo, A.; Dias, M.; Almeida, B.; Caetano, L.A.; Veríssimo, C.; Viegas, C.; Sabino, R. Azole-resistant Aspergillus fumigatus harboring the tr34/l98h mutation: First report in Portugal in environmental samples. Microorganisms 2021, 9, 57. [Google Scholar] [CrossRef] [PubMed]
- Viegas, C.; Faria, T.; Cebola de Oliveira, A.; Aranha Caetano, L.; Carolino, E.; Quintal-Gomes, A.; Twarużek, M.; Kosicki, R.; Soszczyńska, E.; Viegas, S. A new approach to assess fungal contamination and mycotoxins occupational exposure in forklifts drivers from waste sorting. Mycotoxin Res. 2017, 33, 285–295. [Google Scholar] [CrossRef]
- Chowdhary, A.; Kathuria, S.; Xu, J.; Meis, J.F. Emergence of Azole-Resistant Aspergillus fumigatus Strains due to Agricultural Azole Use Creates an Increasing Threat to Human Health. PLoS Pathog. 2013, 9. [Google Scholar] [CrossRef]
- Mao, Y.; Ding, P.; Wang, Y.; Ding, C.; Wu, L.; Zheng, P.; Zhang, X.; Lia, X.; Wang, L.; Suna, Z. Comparison of culturable antibiotic-resistant bacteria in polluted and non-polluted air in Beijing, China. Environ. Int. 2019, 131, 104936. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Bowyer, P.; Sabino, R. The human lung and Aspergillus: You are what you breathe in? Med. Mycol. 2019, 57, S145–S154. [Google Scholar] [CrossRef]
- Viegas, C.; Faria, T.; dos Santos, M.; Carolino, E.; Gomes, A.Q.; Sabino, R.; Viegas, S. Fungal burden in waste industry: An occupational risk to be solved. Environ. Monit. Assess. 2015, 187. [Google Scholar] [CrossRef] [PubMed]
- Franklin, R.B.; Garland, J.L.; Bolster, C.H.; Mills, A.L. Impact of dilution on microbial community structure and functional potential: Comparison of numerical simulations and batch culture experiments. Appl. Environ. Microbiol. 2001, 67, 702–712. [Google Scholar] [CrossRef]
- Black, W.D. A comparison of several media types and basic techniques used to assess outdoor airborne fungi in Melbourne. Australia. PLoS ONE 2020, 15. [Google Scholar] [CrossRef]
- Nagano, Y.; Millar, B.C.; Goldsmith, C.E.; Walker, J.M.; Elborn, J.S.; Rendall, J.; Moore, J.E. Development of selective media for the isolation of yeasts and filamentous fungi from the sputum of adult patients with cystic fibrosis (CF). J. Cyst Fibros. 2008, 7, 566–572. [Google Scholar] [CrossRef][Green Version]
- Bush, R.K.; Portnoy, J.M.; Saxon, A.; Terr, A.I.; Wood, R.A. The medical effects of mold exposure. J. Allergy Clin. Immunol. 2006, 117, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Franchitti, E.; Pascale, E.; Fea, E.; Anedda, E.; Traversi, D. Methods for bioaerosol characterization: Limits and perspectives for human health risk assessment in organicwaste treatment. Atmosphere 2020, 11, 452. [Google Scholar] [CrossRef]
- McDevitt, J.J.; Lees, P.S.J.; Merz, W.G.; Schwab, K.J. Inhibition of quantitative PCR analysis of fungal conidia associated with indoor air particulate matter. Aerobiologia 2007, 23, 35–45. [Google Scholar] [CrossRef]
- Mensah-Attipoe, J.; Taubel, M. Analysis Approaches for Fungi in Indoor Environmental Assessments. In Exposure to Microbiological Agents in Indoor and Occupational Environments, 1st ed.; Viegas, C., Viegas, S., Gomes, A.Q., Täubel, M., Sabino, R., Eds.; Springer: Dordrecht, The Netherlands, 2017; pp. 109–126. [Google Scholar]
- Yoo, K.; Lee, T.K.; Choi, E.J.; Yang, J.; Shukla, S.K.; Hwang, S.; Park, J. Molecular approaches for the detection and monitoring of microbial communities in bioaerosols: A review. J. Environ. Sci. 2016, 51, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Hung, L.L.; Miller, J.D.; Dillon, K.H. Field Guide for the Determination of Biological Contaminants in Environmental Samples, 2nd ed.; AIHA: Fairfax, VA, USA, 2005. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).