Secreted Secondary Metabolites Reduce Bacterial Wilt Severity of Tomato in Bacterial–Fungal Co-Infections
Abstract
1. Introduction
2. Results
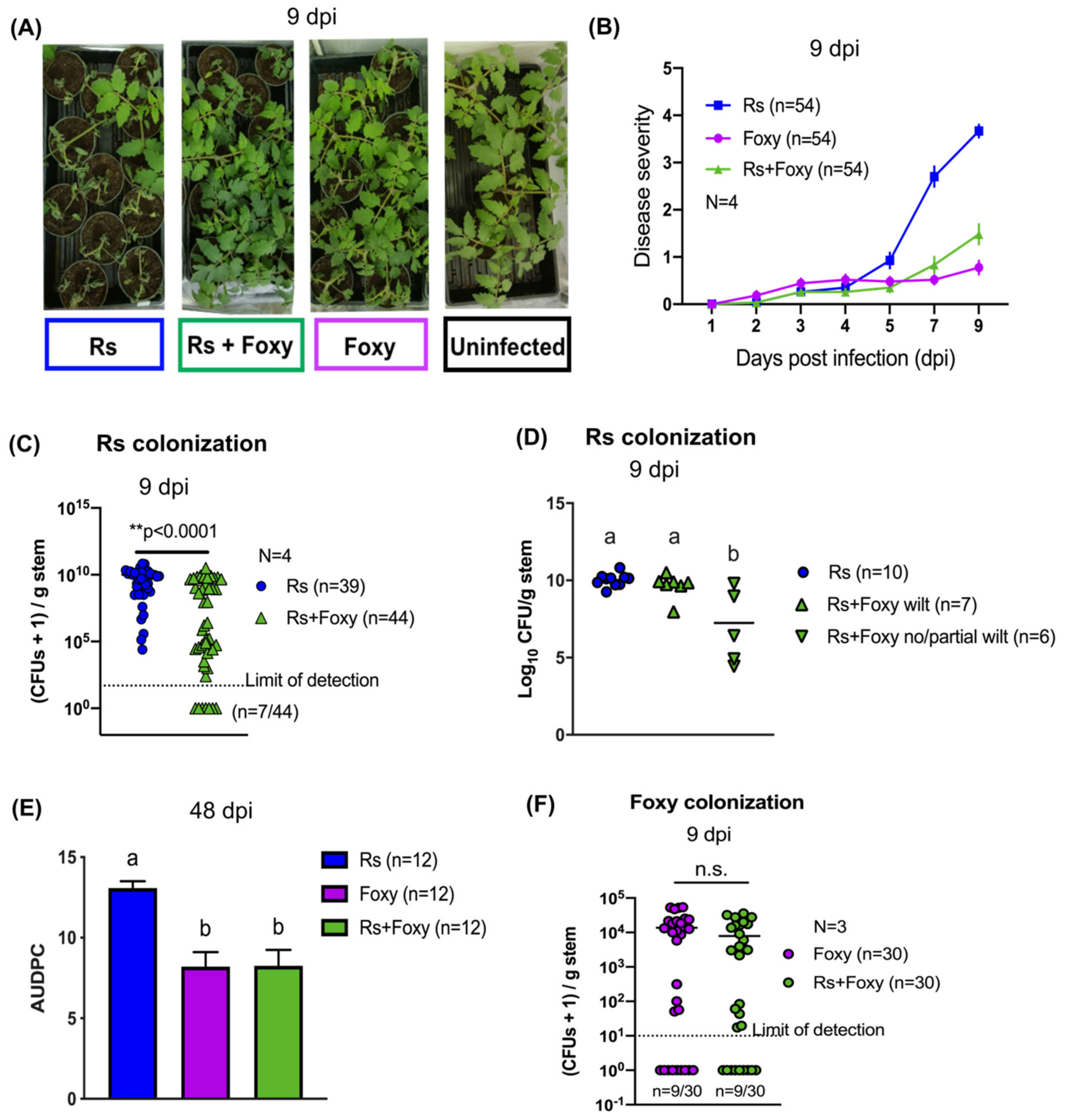
2.1. Bacterial Wilt Severity Is Significantly Reduced in Co-Infections
2.2. Co-Infection Disease Phenotype Requires Specific Interactions with Live F. oxysporum f. sp. lycopersici
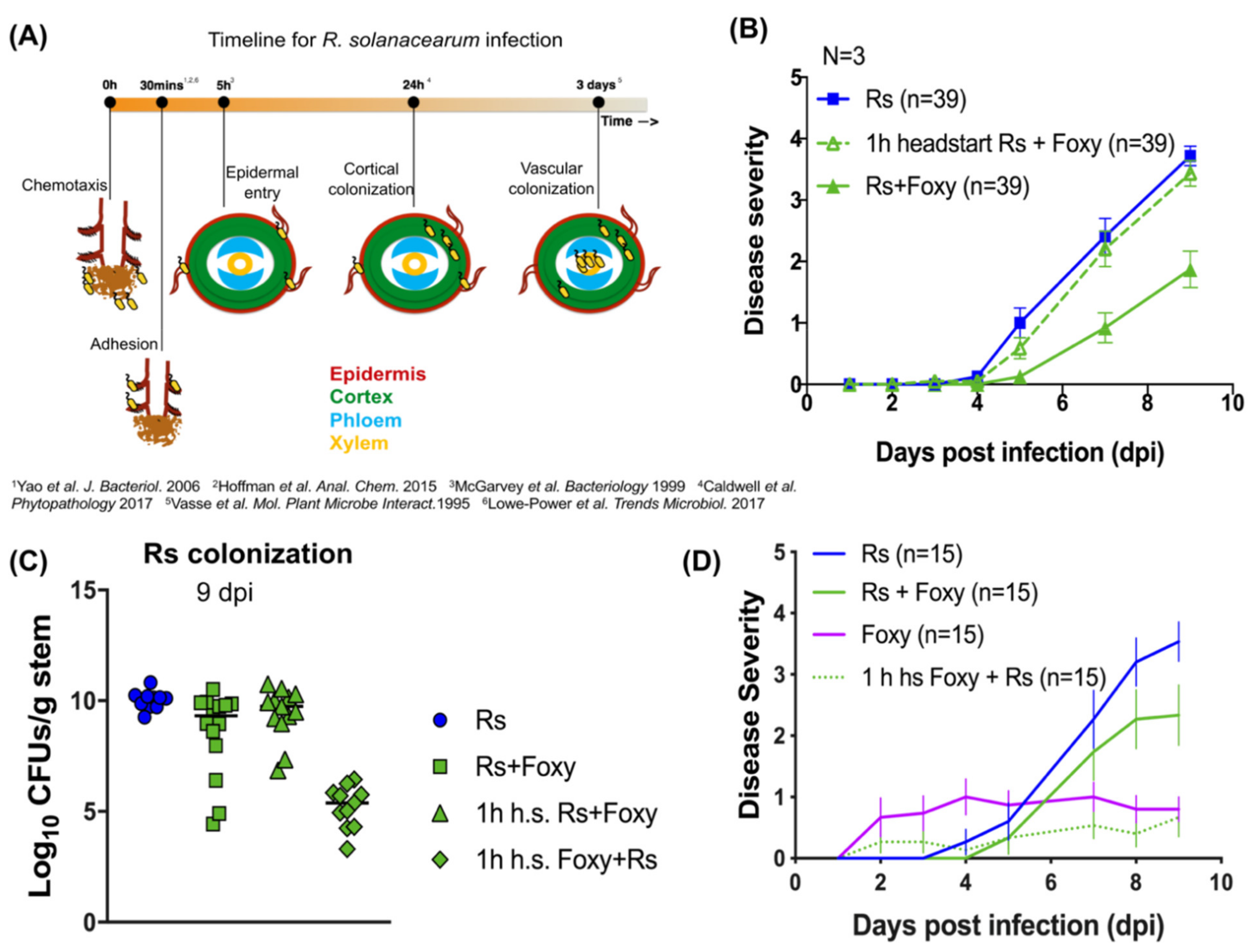
2.3. Early Interactions Play Vital Roles in Co-Infection Disease Outcome
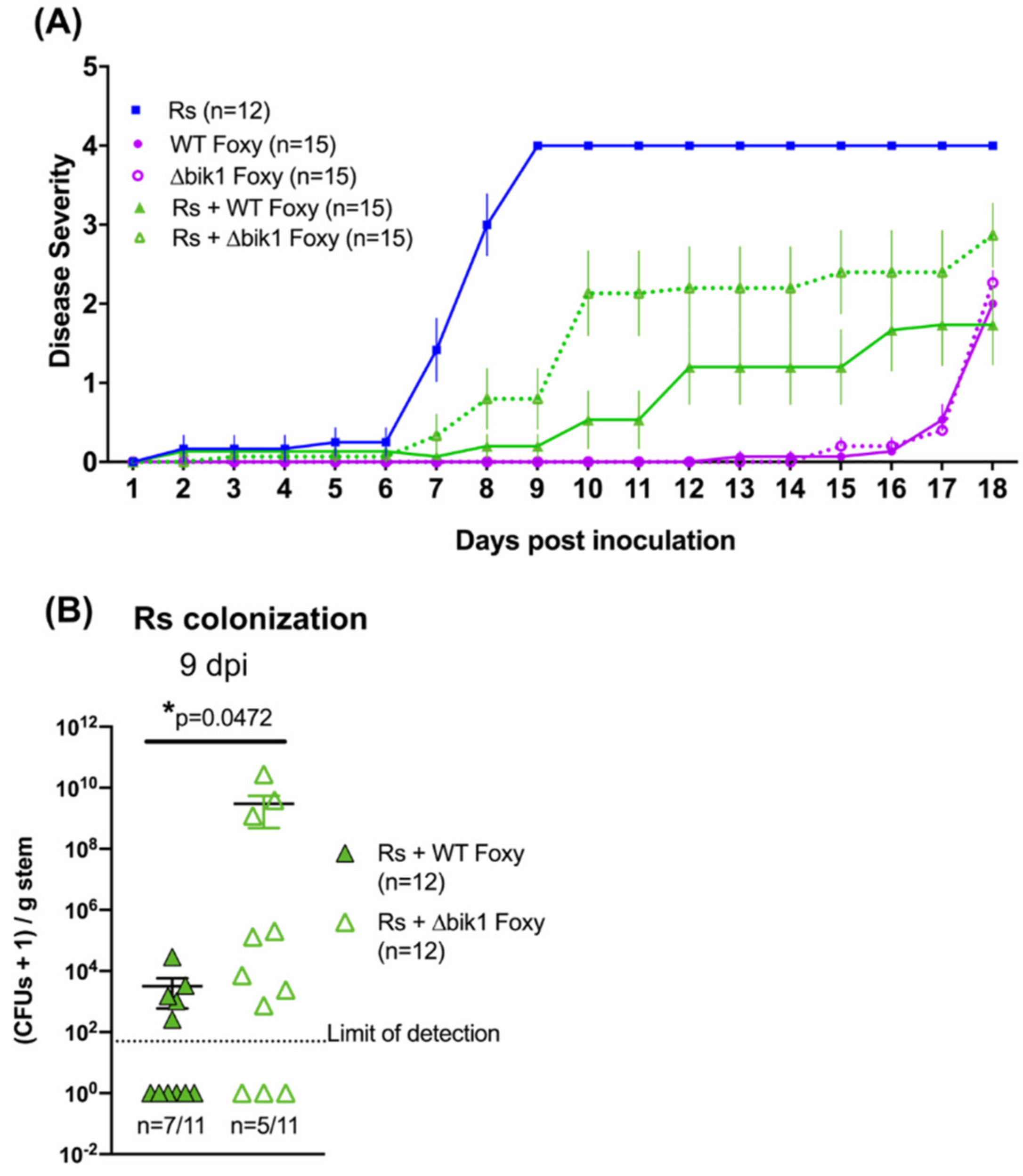
2.4. A Role for Bikaverin in Bacterial Wilt Suppression
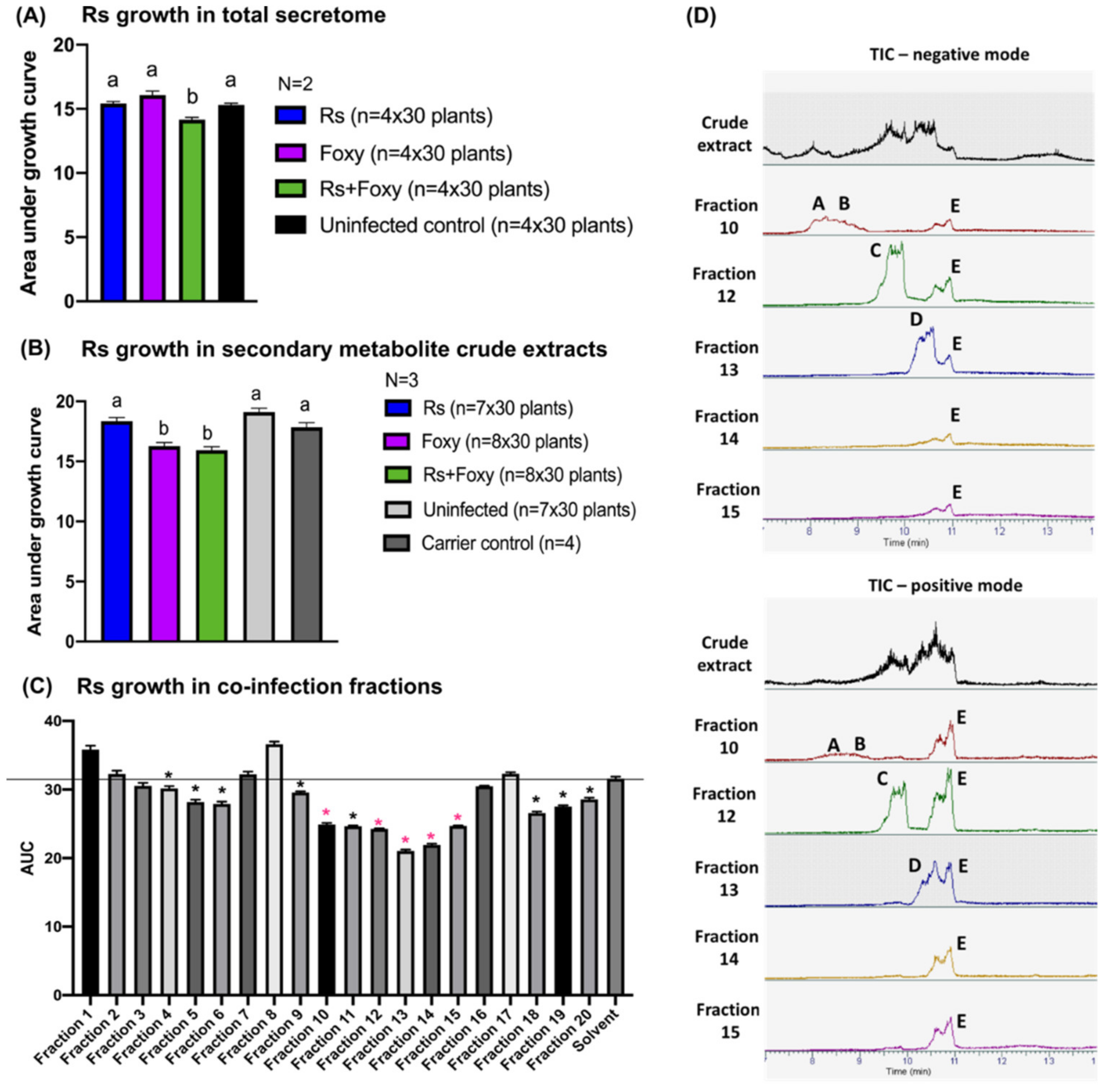
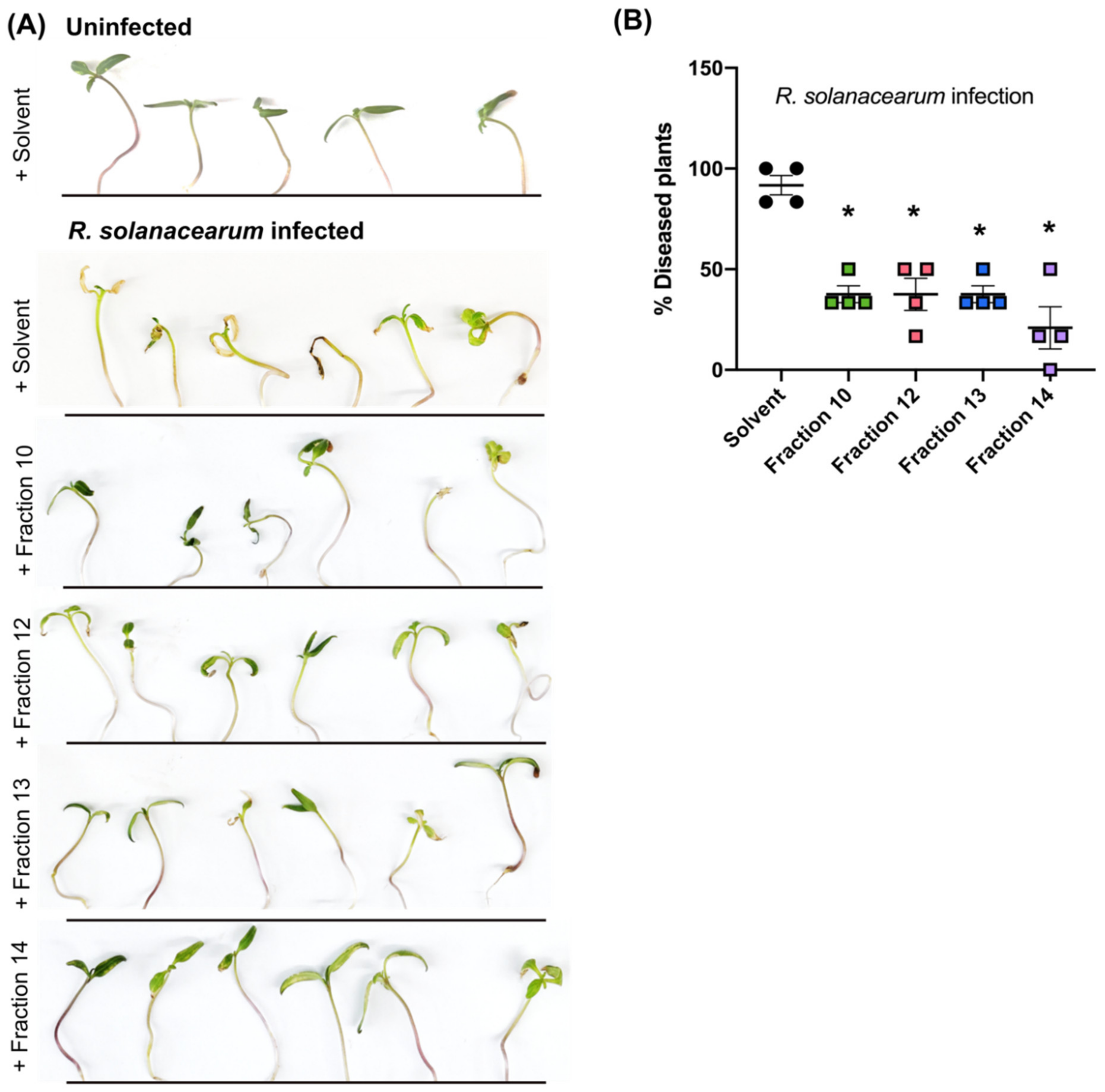
2.5. Secondary Metabolites in Early Co-Infection Secretome Reduce Bacterial Growth In Vitro
3. Discussion
4. Materials and Methods
4.1. Microbial Culture Maintenance and Growth Conditions
4.2. Media Recipes
4.3. Strain Construction and Transformation
4.4. Tomato Coinfection Procedures
4.5. Assessment of Bacterial Adhesion
4.6. Assessment of Microbial Load in Planta
4.7. Rhizosphere Secretome Growth Analyses
4.8. Metabolite Extraction and Fractionation
4.9. Analytical Chemistry
4.10. Evaluation of Extract Efficacy against Bacterial Disease
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wei, Z.; Gu, Y.; Friman, V.P.; Kowalchuk, G.A.; Xu, Y.; Shen, Q.; Jousset, A. Initial soil microbiome composition and functioning predetermine future plant health. Sci. Adv. 2019, 5, eaaw0759. [Google Scholar] [CrossRef] [PubMed]
- Steffan, B.N.; Venkatesh, N.; Keller, N.P. Let’s Get Physical: Bacterial-Fungal Interactions and Their Consequences in Agriculture and Health. J. Fungi 2020, 6, 243. [Google Scholar] [CrossRef]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host–Multi-Pathogen Warfare: Pathogen Interactions in Co-infected Plants. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Péréfarres, F.; Thébaud, G.; Lefeuvre, P.; Chiroleu, F.; Rimbaud, L.; Hoareau, M.; Reynaud, B.; Lett, J.M. Frequency-dependent assistance as a way out of competitive exclusion between two strains of an emerging virus. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133374. [Google Scholar] [CrossRef]
- Susi, H.; Barrès, B.; Vale, P.F.; Laine, A.L. Co-infection alters population dynamics of infectious disease. Nat. Commun. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tollenaere, C.; Susi, H.; Laine, A.L. Evolutionary and Epidemiological Implications of Multiple Infection in Plants. Trends Plant Sci. 2016, 21, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions in health and disease. Pathogens 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Bakaletz, L.O. Viral–bacterial co-infections in the respiratory tract. Curr. Opin. Microbiol. 2017, 35, 30–35. [Google Scholar] [CrossRef]
- Rawson, T.M.; Moore, L.S.P.; Zhu, N.; Ranganathan, N.; Skolimowska, K.; Gilchrist, M.; Satta, G.; Cooke, G.; Holmes, A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020, 71, 2459–2468. [Google Scholar] [CrossRef]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Singh, S.; Gautam, R.K.; Singh, D.R.; Sharma, T.V.R.S.; Sakthivel, K.; Roy, S.D. Genetic approaches for mitigating losses caused by bacterial wilt of tomato in tropical islands. Eur. J. Plant Pathol. 2015, 143, 205–221. [Google Scholar] [CrossRef]
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Billault, A.; Brottier, P.; Camus, J.C.; Cattolico, L.; et al. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef]
- Bosland, P.W. Fusarium Oxysporum, a Pathogen of Many Plant Species. Adv. Plant Pathol. 1988, 6, 281–289. [Google Scholar] [CrossRef]
- Spraker, J.E.; Wiemann, P.; Baccile, J.A.; Venkatesh, N.; Schumacher, J.; Schroeder, F.C.; Sanchez, L.M.; Keller, N.P. Conserved Responses in a War of Small Molecules between a Plant-Pathogenic Bacterium and Fungi. MBio 2018, 9, e00820-18. [Google Scholar] [CrossRef]
- CABI. Ralstonia solanacearum CABI Distribution Data. Available online: https://www.cabi.org/isc/datasheet/45009 (accessed on 1 October 2021).
- CABI. Fusarium oxysporum f. sp. lycoperisici CABI Distribution Data. Available online: https://www.cabi.org/isc/datasheet/24660 (accessed on 1 October 2021).
- Su, L.; Zhang, L.; Nie, D.; Kuramae, E.E.; Shen, B.; Shen, Q. Bacterial tomato pathogen Ralstonia solanacearum invasion modulates rhizosphere compounds and facilitates the cascade effect of fungal pathogen Fusarium solani. Microorganisms 2020, 8, 806. [Google Scholar] [CrossRef] [PubMed]
- Leclair, L.W.; Hogan, D.A. Mixed bacterial-fungal infections in the CF respiratory tract. Med. Mycol. 2010, 48, S125–S132. [Google Scholar] [CrossRef]
- Yang, S.; Hua, M.; Liu, X.; Du, C.; Pu, L.; Xiang, P.; Wang, L.; Liu, J. Bacterial and fungal co-infections among COVID-19 patients in intensive care unit. Microbes Infect. 2021, 23, 104806. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Allen, C. Chemotaxis is required for virulence and competitive fitness of the bacterial wilt pathogen Ralstonia solanacearum. J. Bacteriol. 2006, 188, 3697–3708. [Google Scholar] [CrossRef]
- Hoffman, M.D.; Zucker, L.I.; Brown, P.J.B.; Kysela, D.T.; Brun, Y.V.; Jacobson, S.C. Timescales and Frequencies of Reversible and Irreversible Adhesion Events of Single Bacterial Cells. Anal. Chem. 2015, 87, 12032–12039. [Google Scholar] [CrossRef]
- Mcgarvey, J.A.; Denny, T.P.; Schell, M.A. Spatial-Temporal and Quantitative Analysis of Growth and EPS I Production by Ralstonia solanacearum in Resistant and Susceptible Tomato Cultivars. Phytopathology 1999, 89, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, D.; Kim, B.-S.; Iyer-Pascuzzi, A.S. Ralstonia solanacearum Differentially Colonizes Roots of Resistant and Susceptible Tomato Plants. Phytopathology 2017, 107, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Vasse, J.; Frey, P.; Trigalet, A. Microscopic studies of intercellular infection and protoxylem invasion of tomato roots by Pseudomonas solanacearum. Mol. Plant-Microbe Interact. 1995, 8, 241–251. [Google Scholar] [CrossRef]
- Lowe-Power, T.M.; Khokhani, D.; Allen, C. How Ralstonia solanacearum Exploits and Thrives in the Flowing Plant Xylem Environment. Trends Microbiol. 2018, 26, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Olivain, C.; Humbert, C.; Nahalkova, J.; Fatehi, J.; L’Haridon, F.; Alabouvette, C. Colonization of tomato root by pathogenic and nonpathogenic Fusarium oxysporum strains inoculated together and separately into the soil. Appl. Environ. Microbiol. 2006, 72, 1523–1531. [Google Scholar] [CrossRef] [PubMed]
- Lagopodi, A.L.; Ram, A.F.J.; Lamers, G.E.M.; Punt, P.J.; Van den Hondel, C.A.M.J.J.; Lugtenberg, B.J.J.; Bloemberg, G.V. Novel aspects of tomato root colonization and infection by Fusarium oxysporum f. sp. radicis-lycopersici Revealed by confocal laser scanning microscopic analysis using the green fluorescent protein as a marker. Mol. Plant-Microbe Interact. 2002, 15, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Wen, T.; Zhao, M.; Liu, T.; Huang, Q.; Yuan, J.; Shen, Q. High abundance of Ralstonia solanacearum changed tomato rhizosphere microbiome and metabolome. BMC Plant Biol. 2020, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Kamilova, F.; Kravchenko, L.V.; Shaposhnikov, A.I.; Makarova, N.; Lugtenberg, B. Effects of the tomato pathogen Fusarium oxysporum f. sp. radicis-lycopersici and of the biocontrol bacterium Pseudomonas fluorescens WCS365 on the composition of organic acids and sugars in tomato root exudate. Mol. Plant-Microbe Interact. 2006, 19, 1121–1126. [Google Scholar] [CrossRef]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bhattarai, B. Variation of Soil Microbial Population in Different Soil Horizons. J. Microbiol. Exp. 2015, 2, 00044. [Google Scholar] [CrossRef]
- Cai, P.; Sun, X.; Wu, Y.; Gao, C.; Mortimer, M.; Holden, P.A.; Redmile-Gordon, M.; Huang, Q. Soil biofilms: Microbial interactions, challenges, and advanced techniques for ex-situ characterization. Soil Ecol. Lett. 2019. [Google Scholar] [CrossRef]
- Sharma, K.; Palatinszky, M.; Nikolov, G.; Berry, D.; Shank, E.A. Transparent soil microcosms for live-cell imaging and non-destructive stable isotope probing of soil microorganisms. eLife 2020, 9, e56275. [Google Scholar] [CrossRef]
- Abeysinghe, G.; Kuchira, M.; Kudo, G.; Masuo, S.; Ninomiya, A.; Takahashi, K.; Utad, A.S.; Hagiwara, D.; Nomura, N.; Takaya, N.; et al. Fungal mycelia and bacterial thiamine establish a mutualistic growth mechanism. Life Sci. Alliance 2020, 3. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Hogan, D.A.; Mylonakis, E. Medically important bacterialg-fungal interactions. Nat. Rev. Microbiol. 2010, 8, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Spraker, J.E.; Sanchez, L.M.; Lowe, T.M.; Dorrestein, P.C.; Keller, N.P. Ralstonia solanacearum lipopeptide induces chlamydospore development in fungi and facilitates bacterial entry into fungal tissues. ISME J. 2016, 10, 2317–2330. [Google Scholar] [CrossRef] [PubMed]
- Höfer, A.M.; Harting, R.; Aßmann, N.F.; Gerke, J.; Schmitt, K.; Starke, J.; Bayram, Ö.; Tran, V.T.; Valerius, O.; Braus-Stromeyer, S.A.; et al. The velvet protein Vel1 controls initial plant root colonization and conidia formation for xylem distribution in Verticillium wilt. PLoS Genet. 2021, 17, e1009434. [Google Scholar] [CrossRef] [PubMed]
- de Lamo, F.J.; Spijkers, S.B.; Takken, F.L.W. Protection to tomato wilt disease conferred by the nonpathogen Fusarium oxysporum Fo47 is more effective than that conferred by avirulent strains. Phytopathology 2021, 111, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Graham, J. Survival of Pseudomonas solanacearum Race 3 in Plant Debris and in Latently Infected Potato Tubers. Phytopathology 1979, 69, 1100. [Google Scholar] [CrossRef]
- Bacterial Wilt—Ralstonia solanacearum. Available online: https://extension.psu.edu/bacterial-wilt-ralstonia-solanacearum (accessed on 6 June 2021).
- Netzker, T.; Flak, M.; Krespach, M.K.; Stroe, M.C.; Weber, J.; Schroeckh, V.; Brakhage, A.A. Microbial interactions trigger the production of antibiotics. Curr. Opin. Microbiol. 2018, 45, 117–123. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatesh, N.; Koss, M.J.; Greco, C.; Nickles, G.; Wiemann, P.; Keller, N.P. Secreted Secondary Metabolites Reduce Bacterial Wilt Severity of Tomato in Bacterial–Fungal Co-Infections. Microorganisms 2021, 9, 2123. https://doi.org/10.3390/microorganisms9102123
Venkatesh N, Koss MJ, Greco C, Nickles G, Wiemann P, Keller NP. Secreted Secondary Metabolites Reduce Bacterial Wilt Severity of Tomato in Bacterial–Fungal Co-Infections. Microorganisms. 2021; 9(10):2123. https://doi.org/10.3390/microorganisms9102123
Chicago/Turabian StyleVenkatesh, Nandhitha, Max J. Koss, Claudio Greco, Grant Nickles, Philipp Wiemann, and Nancy P. Keller. 2021. "Secreted Secondary Metabolites Reduce Bacterial Wilt Severity of Tomato in Bacterial–Fungal Co-Infections" Microorganisms 9, no. 10: 2123. https://doi.org/10.3390/microorganisms9102123
APA StyleVenkatesh, N., Koss, M. J., Greco, C., Nickles, G., Wiemann, P., & Keller, N. P. (2021). Secreted Secondary Metabolites Reduce Bacterial Wilt Severity of Tomato in Bacterial–Fungal Co-Infections. Microorganisms, 9(10), 2123. https://doi.org/10.3390/microorganisms9102123






