Comparative Evaluation of Four Potent Neospora caninum Diagnostic Antigens Using Immunochromatographic Assay for Detection of Specific Antibody in Cattle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Experimental Mice and Rabbits
2.3. Parasites and Cell Cultures
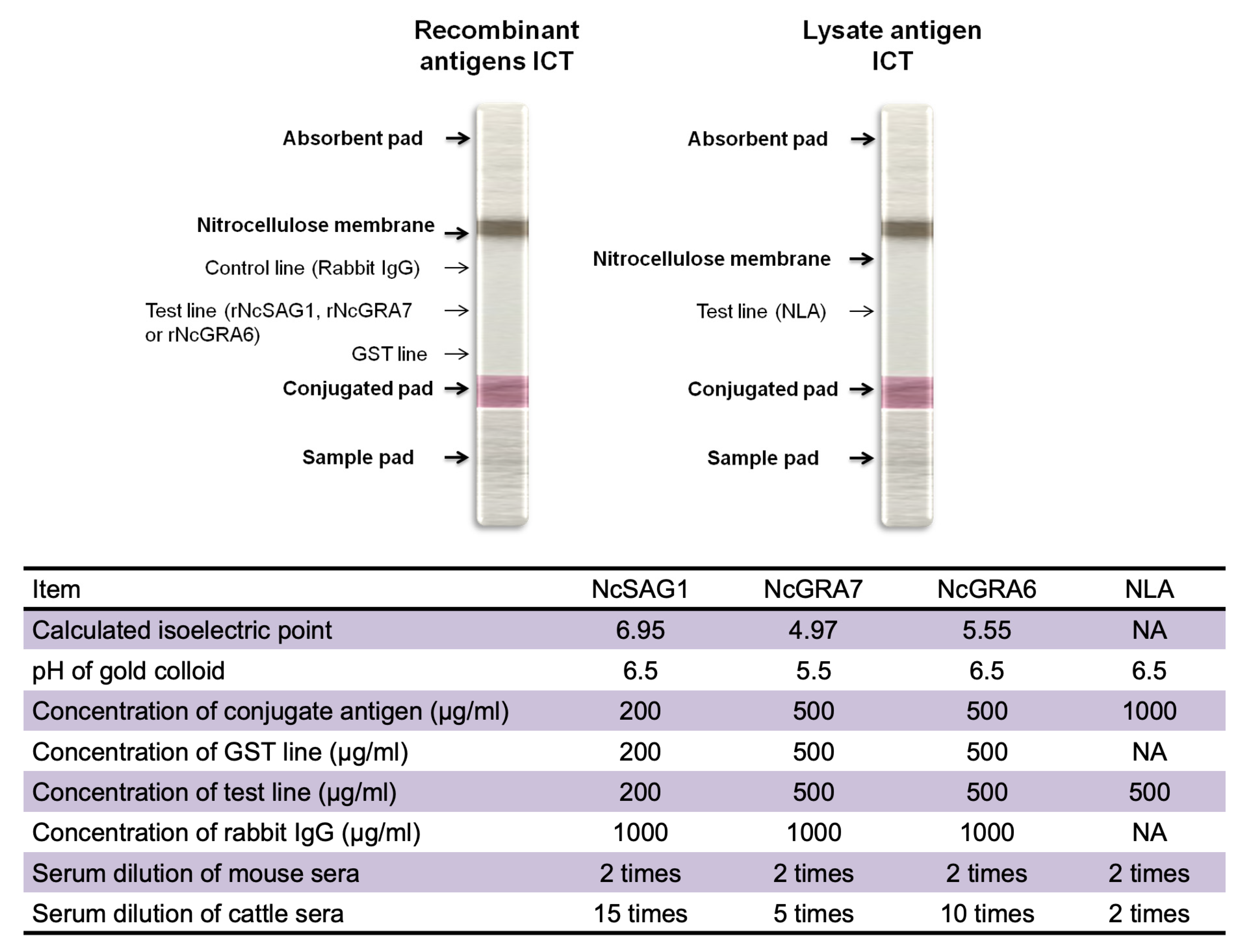
2.4. Recombinant and Lysate Antigens Preparation
2.5. Experimental Sample from Mice
2.6. Cattle Experimental and Field Samples
2.7. Preparation of Polyclonal Antibodies in Rabbits
2.8. Indirect ELISA
2.9. ICT
2.10. Statistical Analyses
3. Results and Discussion
3.1. First Assessment of Various Antigen-Based ICTs Using Experimental Mouse Sera
3.2. Evaluation of Various Antigen-Based ICTs against Experimental and Control Cattle Sera
3.3. Evaluation and Analyses of the NcSAG1-Based ICT against Field Sera from Cattle
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fereig, R.M.; Nishikawa, Y. From signaling pathways to distinct immune responses: Key factors for establishing or combating Neospora caninum infection in different susceptible hosts. Pathogens 2020, 9, 384. [Google Scholar] [CrossRef]
- Dubey, J.P.; Carpenter, J.L.; Speer, C.A.; Topper, M.J.; Uggla, A. Newly recognized fatal protozoan disease of dogs. J. Am. Vet. Med. Assoc. 1988, 192, 1269–1285. [Google Scholar] [PubMed]
- Dubey, J.P.; Lindsay, D.S. A review of Neospora caninum and neosporosis. Vet. Parasitol. 1996, 67, 1–59. [Google Scholar] [CrossRef]
- Ortega-Mora, L.M.; Gottstein, B.; Conraths, F.J.; Buxton, D. Protozoal Abortion in Farm Ruminants: Guidelines for Diagnosis and Control; CAB International: Wallingford, UK, 2007. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G. Neosporosis in animals—The last five years. Vet. Parasitol. 2011, 180, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Uzeda, R.S.; Schares, G.; Ortega-Mora, L.M.; Madruga, C.R.; Aguado-Martinez, A.; Corbellini, L.G.; Corbellini, L.G.; Driemeier, D.; Gondim, L.F. Combination of monoclonal antibodies improves immunohistochemical diagnosis of Neospora caninum. Vet. Parasitol. 2013, 197, 477–486. [Google Scholar] [CrossRef]
- Baszler, T.V.; Gay, L.J.; Long, M.T.; Mathison, B.A. Detection by PCR of Neospora caninum in fetal tissues from spontaneous bovine abortions. J. Clin. Microbiol. 1999, 37, 4059–4064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdelbaky, H.H.; Nishimura, M.; Shimoda, N.; Hiasa, J.; Fereig, R.M.; Tokimitsu, H.; Inokuma, H.; Nishikawa, Y. Evaluation of Neospora caninum serodiagnostic antigens for bovine neosporosis. Parasitol. Int. 2019, 75, 102045. [Google Scholar] [CrossRef]
- Sinnott, F.A.; Monte, L.G.; Collares, T.F.; Silveira, R.M.; Borsuk, S. Review on the immunological and molecular diagnosis of neosporosis (years 2011–2016). Vet. Parasitol. 2017, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Aguado-Martínez, A.; Alvarez-García, G.; Fernández-García, A.; Risco-Castillo, V.; Arnaiz-Seco, I.; Rebordosa-Trigueros, X.; Navarro-Lozano, V.; Ortega-Mora, L.M. Usefulness of rNcGRA7- and rNcSAG4-based ELISA tests for distinguishing primo-infection, recrudescence, and chronic bovine neosporosis. Vet. Parasitol. 2008, 157, 182–195. [Google Scholar] [CrossRef]
- Teixeira, L.; Marques, A.; Meireles, C.S.; Seabra, A.R.; Rodrigues, D.; Madureira, P.; Faustino, A.M.; Silva, C.; Ribeiro, A.; Ferreira, P.; et al. Characterization of the B-cell immune response elicited in BALB/c mice challenged with Neospora caninum tachyzoites. Immunology 2005, 116, 38–52. [Google Scholar] [CrossRef]
- Dubey, J.P.; Schares, G. Diagnosis of bovine neosporosis. Vet. Parasitol. 2006, 140, 1–534. [Google Scholar] [CrossRef]
- Hiasa, J.; Kohara, J.; Nishimura, M.; Xuan, X.; Tokimitsu, H.; Nishikawa, Y. ELISAs based on rNcGRA7 and rNcSAG1 antigens as an indicator of Neospora caninum activation. Vet. Parasitol. 2012, 187, 379–385. [Google Scholar] [CrossRef]
- Hiasa, J.; Nishimura, M.; Itamoto, K.; Xuan, X.; Inokuma, H.; Nishikawa, Y. Enzyme-linked immunosorbent assays based on Neospora caninum dense granule protein 7 and profilin for estimating the stage of neosporosis. Clin. Vacc. Immunol. 2012, 19, 411–417. [Google Scholar] [CrossRef]
- Takashima, Y.; Takasu, M.; Yanagimoto, I.; Hattori, N.; Batanova, T.; Nishikawa, Y.; Kitoh, K. Prevalence and dynamics of antibodies against NcSAG1 and NcGRA7 antigens of Neospora caninum in cattle during the gestation period. J. Vet. Med. Sci. 2013, 75, 1413–1418. [Google Scholar] [CrossRef] [Green Version]
- Lally, N.C.; Jenkins, M.C.; Dubey, J.P. Evaluation of two Neospora caninum recombinant antigens for use in an enzyme-linked immunosorbent assay for the diagnosis of bovine neosporosis. Clin. Diag. Lab. Immunol. 1996, 3, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Fereig, R.M.; Shimoda, N.; Abdelbaky, H.H.; Kuroda, Y.; Nishikawa, Y. Neospora GRA6 possesses immune-stimulating activity and confers efficient protection against Neospora caninum infection in mice. Vet. Parasitol. 2019, 267, 61–68. [Google Scholar] [CrossRef]
- Liao, M.; Zhang, S.; Xuan, X.; Zhang, G.; Huang, X.; Igarashi, I.; Fujisaki, K. Development of rapid immunochromatographic test with recombinant NcSAG1 for detection of antibodies to Neospora caninum in cattle. Clin. Diagn. Lab. Immunol. 2005, 12, 885–887. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, A.M.; Costa, M.F.; Paule, B.; Vale, V.; Ribeiro, M.; Nascimento, I.; Schaer, R.E.; Almeida, M.A.; Meyer, R.; Freire, S.M. Serologic immunoreactivity to Neospora caninum antigens in dogs determined by indirect immunofluorescence, western blotting and dot-ELISA. Vet. Parasitol. 2005, 130, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Chahan, B.; Gaturaga, I.; Huang, X.; Liao, M.; Fukumoto, S.; Hirata, S.; Nishikawa, Y.; Suzuki, H.; Sugimoto, C.; Nagasawa, H.; et al. Serodiagnosis of Neospora caninum infection in cattle by enzyme-linked immunosorbent assay with recombinant truncated NcSAG1. Vet. Parasitol. 2003, 118, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Abdelbaky, H.H.; Fereig, R.M.; Nishikawa, Y. Identification of the antigenic region of Neospora caninum dense granule protein 7 using ELISA. Parasitol. Int. 2018, 67, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Bjerkas, I.; Jenkins, M.C.; Dubey, J.P. Identification and characterization of Neospora caninum tachyzoite antigens useful for diagnosis of neosporosis. Clin. Diagn. Lab. Immunol. 1994, 1, 214–221. [Google Scholar] [CrossRef]
- Fereig, R.M.; Kuroda, Y.; Terkawi, M.A.; Mahmoud, M.E.; Nishikawa, Y. Immunization with Toxoplasma gondii peroxiredoxin 1 induces protective immunity against toxoplasmosis in mice. PLoS ONE 2017, 12, e0176324. [Google Scholar] [CrossRef]
- Nishimura, M.; Kohara, J.; Kuroda, Y.; Hiasa, J.; Tanaka, S.; Muroi, Y.; Kojima, N.; Furuoka, H.; Nishikawa, Y. Oligomannose-coated liposome-entrapped dense granule protein 7 induces protective immune response to Neospora caninum in cattle. Vaccine 2013, 31, 3528–3535. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Shimoda, N.; Fereig, R.M.; Moritaka, T.; Umeda, K.; Nishimura, M.; Ihara, F.; Kobayashi, K.; Himori, Y.; Suzuki, Y.; et al. Neospora caninum dense granule protein 7 regulates the pathogenesis of neosporosis by modulating host immune response. Appl. Environ. Microbiol. 2018, 84, e01350-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, H.S.; E Almeida, L.T.; Fernandes, V.; Senra, R.L.; Fontes, P.P.; Bittar, E.R.; Mendes, T.A.O. Chimeric protein designed by genome scale immunoinformatic enhances serodiagnosis of bovine neosporosis. J. Clin. Microbiol. 2020, 13, e01343-19. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.; Nishikawa, Y. Development of an immunochromatographic assay based on dense granule protein 7 for serological detection of Toxoplasma gondii infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fereig, R.M.; Abdelbaky, H.H.; Ihara, F.; Nishikawa, Y. Development and evaluation of the first immunochromatographic test that can detect specific antibodies against Cryptosporidium parvum. Acta Trop. 2018, 185, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Kelley, J.; Walter, L.; Trowsdale, J. Comparative genomics of major histocompatibility complexes. Immunogenetics 2005, 56, 683–695. [Google Scholar] [CrossRef] [PubMed]
- Haley, P.J. The lymphoid system: A review of species differences. J. Toxicol. Pathol. 2017, 30, 111–113. [Google Scholar] [CrossRef] [Green Version]






| Animal | Serum Samples (Sample Number) | Number of Positive Samples | |||||||
|---|---|---|---|---|---|---|---|---|---|
| NcSAG1 | NcGRA7 | NcGRA6 | NLA | ||||||
| ELISA (IgG and/or IgM) | ICT | ELISA (IgG and/or IgM) | ICT | ELISA (IgG and/or IgM) | ICT | ELISA (IgG and/or IgM) | ICT | ||
| Mouse | 2 WPI # (n = 5) | 5 | 5 | 5 | 5 | 5 | 4 | 5 | 4 |
| 4 WPI (n = 5) | 5 | 5 | 5 | 0 | 5 | 0 | 5 | 3 | |
| 8 WPI (n = 5) | 5 | 5 | 5 | 0 | 5 | 0 | 5 | 1 | |
| Total positive (n = 15) | 15 | 15 | 15 | 5 | 15 | 4 | 15 | 8 | |
| Non-infected (n = 5) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| T. gondii (n = 3) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Cattle | 4 WPI (n = 4) | 4 | 4 | 4 | 3 | 2 | 3 | 4 | 4 |
| 8 WPI (n = 5) | 5 | 5 | 3 | 0 | 0 | 0 | 5 | 5 | |
| Neosporosis (n = 8) * | 8 | 8 | 6 | 5 | 5 | 0 | 8 | 8 | |
| Total positive (n = 17) | 17 | 17 | 13 | 8 | 7 | 3 | 17 | 17 | |
| Non-infected (n = 6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Item | NcSAG1 (n = 53) | ||
|---|---|---|---|
| Positive (%) | Negative (%) | CI (95%) | |
| IgG-ELISA | 19 (35.8) | 34 (64.2) | 23.5–50.25 |
| IgM-ELISA | 5 (9.4) | 48 (90.6) | 3.52–21.42 |
| IgG or IgM-ELISA * | 19 (35.8) | 34 (64.2) | 23.5–50.25 |
| IgG and IgM ELISA # | 5 (9.4) | 48 (90.6) | 3.52–21.42 |
| ICT | 21 (39.6) | 32 (60.4) | 26.76–53.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fereig, R.M.; Abdelbaky, H.H.; Nishikawa, Y. Comparative Evaluation of Four Potent Neospora caninum Diagnostic Antigens Using Immunochromatographic Assay for Detection of Specific Antibody in Cattle. Microorganisms 2021, 9, 2133. https://doi.org/10.3390/microorganisms9102133
Fereig RM, Abdelbaky HH, Nishikawa Y. Comparative Evaluation of Four Potent Neospora caninum Diagnostic Antigens Using Immunochromatographic Assay for Detection of Specific Antibody in Cattle. Microorganisms. 2021; 9(10):2133. https://doi.org/10.3390/microorganisms9102133
Chicago/Turabian StyleFereig, Ragab M., Hanan H. Abdelbaky, and Yoshifumi Nishikawa. 2021. "Comparative Evaluation of Four Potent Neospora caninum Diagnostic Antigens Using Immunochromatographic Assay for Detection of Specific Antibody in Cattle" Microorganisms 9, no. 10: 2133. https://doi.org/10.3390/microorganisms9102133







