Resistance to Boscalid, Fluopyram and Fluxapyroxad in Blumeriella jaapii from Michigan (U.S.A.): Molecular Characterization and Assessment of Practical Resistance in Commercial Cherry Orchards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Efficacy of Fungicides Containing Fluopyram or Fluxapyroxad in Controlling CLS
2.2. Orchard Sampling
2.3. In Vitro Sensitivity Determination of B. jaapii to SDHI Fungicides
2.4. Amplification and Identification of Mutations within SdhB, SdhC, and SdhD of B. jaapii
3. Results
3.1. Field Efficacy of Fungicides Containing Fluopyram or Fluxapyroxad in Controlling CLS
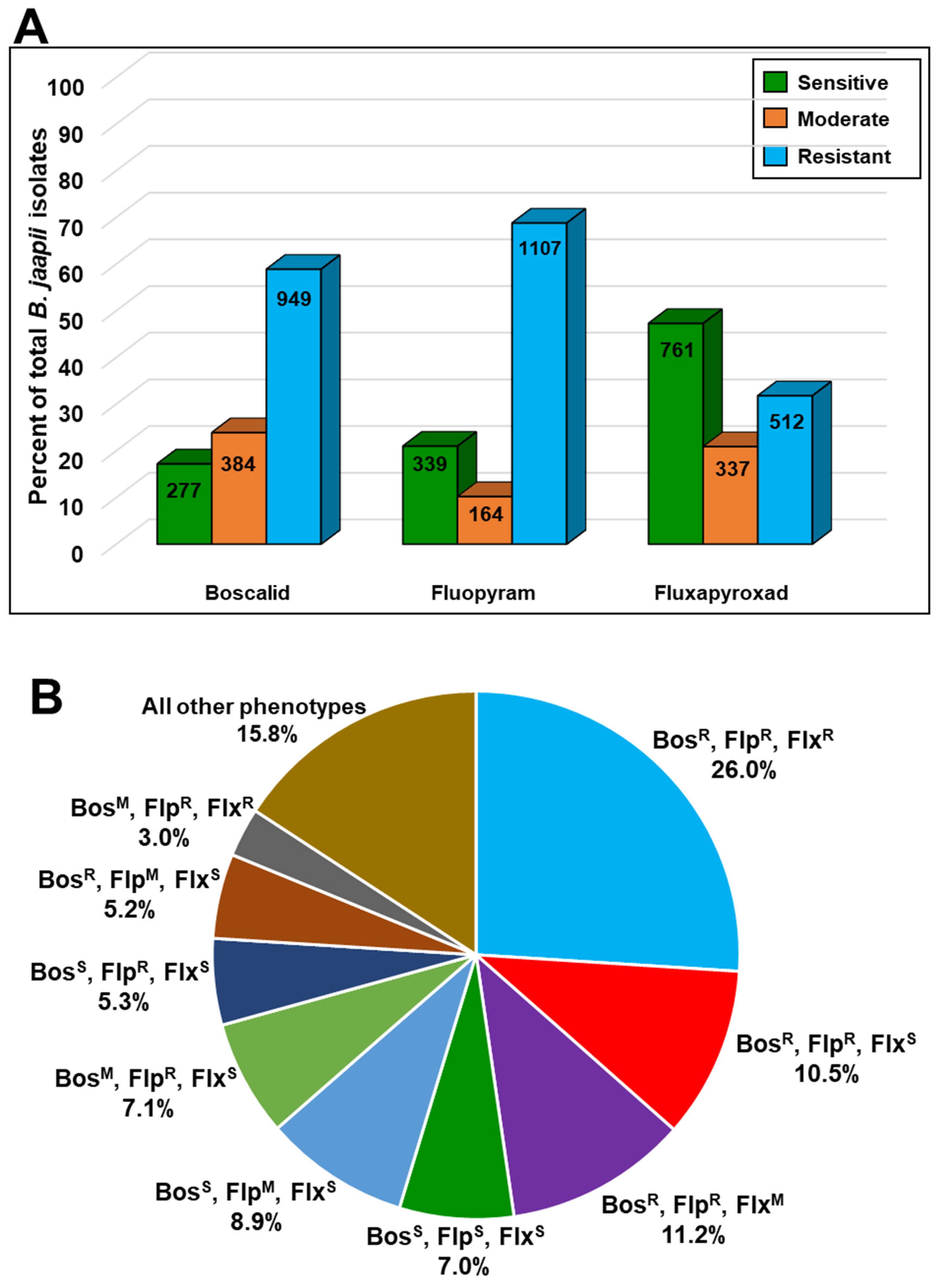
3.2. Orchard Sampling and In Vitro Fungicide Sensitivity Screening
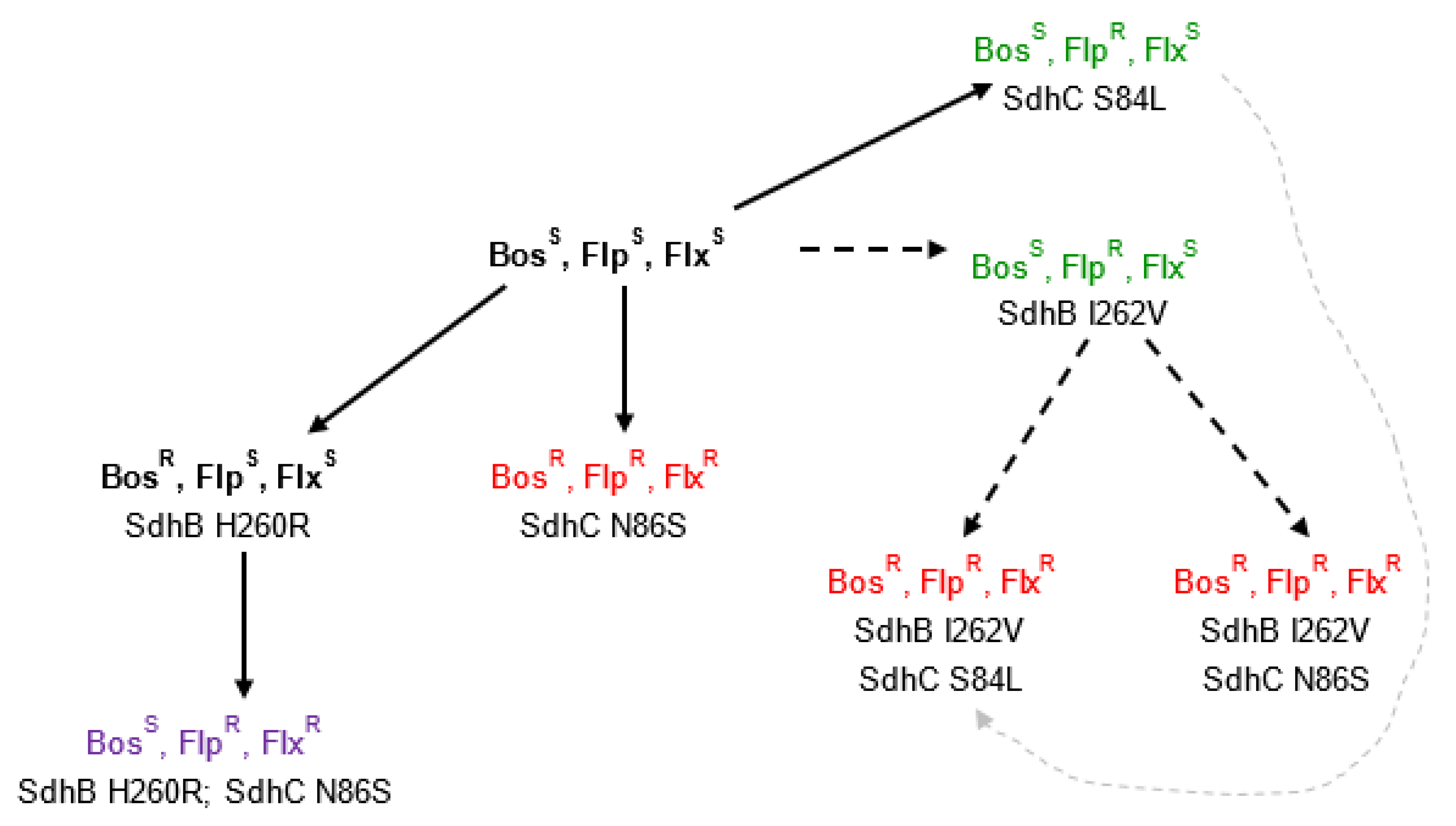
3.3. Identification of Mutations within SdhB, SdhC, and SdhD of B. jaapii
3.4. Assessment of Practical Fungicide Resistance in Michigan Tart Cherry Orchards
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andersen, K.L.; Sebolt, A.M.; Sundin, G.W.; Iezzoni, A.F. Assessment of the inheritance of resistance and tolerance in cherry (Prunus sp.) to Blumeriella jaapii, the causal agent of cherry leaf spot. Plant Pathol. 2018, 67, 682–691. [Google Scholar] [CrossRef]
- Proffer, T.J.; Berardi, R.; Ma, Z.; Nugent, J.E.; Ehret, G.R.; McManus, P.S.; Sundin, G.W. Occurrence, distribution, and PCR-based detection of resistance to sterol demethylation-inhibiting fungicides in populations of Blumeriella jaapii in Michigan. Phytopathology 2006, 96, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Proffer, T.J.; Jacobs, J.L.; Sundin, G.W. Overexpression of the 14α-demethylase target gene (CYP51) mediates fungicide resistance in Blumeriella jaapii. Appl. Environ. Microbiol. 2006, 72, 2581–2585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Outwater, C.A.; Proffer, T.J.; Rothwell, N.L.; Peng, J.; Sundin, G.W. Boscalid resistance in Blumeriella jaapii: Distribution, effect on field efficacy, and molecular characterization. Plant Dis. 2019, 103, 1112–1118. [Google Scholar] [CrossRef]
- Avenot, H.F.; Michailides, T.J. progress in understanding molecular mechanisms of resistance to succinate dehydrogenase inhibiting (SDHI) fungicides in phytopathogenic fungi. Crop Prot. 2010, 29, 643–651. [Google Scholar] [CrossRef]
- Sierotzki, H.; Scalliet, G. A review of current knowledge of resistance aspects for the next-generation succinate dehydrogenase inhibitor fungicides. Phytopathology 2013, 103, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Mallik, I.; Arabiat, S.; Pasche, J.S.; Bolton, M.D.; Patel, J.S.; Gudmestad, N.C. Molecular characterization and detection of mutations associated with resistance to succinate dehydrogenase-inhibiting fungicides in Alternaria solani. Phytopathology 2014, 104, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Cecchini, G. Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 2003, 72, 77–109. [Google Scholar] [CrossRef] [Green Version]
- Avenot, H.F.; Thomas, A.; Gitaitis, R.D.; Langston, D.B.; Stevenson, K.L. Molecular characterization of boscalid- and penthiopyrad-resistant isolates of Didymella bryoniae and assessment of their sensitivity to fluopyram. Pest Manag. Sci. 2011, 68, 645–651. [Google Scholar] [CrossRef]
- Veloukas, T.; Markoglou, A.N.; Karaoglandis, G.S. Differential effect of SdhB gene mutations on the sensitivity to SDHI fungicides in Botrytis cinerea. Plant Dis. 2013, 97, 118–122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amiri, A.; Heath, S.M.; Peres, N.A. Resistance to fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from strawberry. Plant Dis. 2014, 98, 532–539. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Brannen, P.M.; Schnabel, G. Resistance in Alternaria alternata to SDHI fungicides causes rare disease outbreak in peach orchards. Plant Dis. 2015, 99, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.-J.; Fernández-Ortuño, D.; Schnabel, G. Monitoring resistance to SDHI fungicides in Botrytis cinerea from strawberry fields. Plant Dis. 2016, 100, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Ortuño, D.; Pérez-García, A.; Chamorro, A.; de la Peña, E.; de Vicente, A.; Torés, J.A. Resistance to the SDHI fungicides boscalid, fluopyram, fluxapyroxad, and penthiopyrad in Botrytis cinerea from commercial strawberry fields in Spain. Plant Dis. 2017, 101, 1306–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popko, J.T.; Sang, H.; Lee, J.; Yamada, T.; Hoshino, Y.; Jung, G. Resistance of Sclerotinia homoeocarpa field isolates to succinate dehydrogenase inhibitor fungicides. Plant Dis. 2018, 102, 2625–2631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Elliott, M.R.; Yamada, T.; Jung, G. Field assessment of six point mutations in SDH subunit genes conferring varying resistance levels to SDHIs in Clarireedia spp. Plant Dis. 2021, 105, 1685–1691. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Nuckols, T.A.; Harris, D.; Stevenson, K.L.; Brewer, M.T. Differences in fungicide resistance profiles and multiple resistance to a quinone-outside inhibitor (QoI), two succinate dehydrogenase inhibitors (SDHI), and a demethylation inhibitor (DMI) for two Stagonosporopsis species causing gummy stem blight of cucurbits. Pest Manag. Sci. 2019, 75, 3093–3101. [Google Scholar] [PubMed]
- Shi, Y.; Zhu, F.; Sun, B.; Xie, X.; Chai, A.; Li, B. Two adjacent mutations in the conserved domain of SdhB confer various resistance phenotypes to fluopyram in Corynespora cassiicola. Pest Manag. Sci. 2021, 77, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Köller, W. Fungicide resistance in plant pathogens. In CRC Handbook of Pest Management in Agriculture, 2nd ed.; Pimentel, D., Ed.; CRC Press: Boca Raton, FL, USA, 1991; Volume 2, pp. 679–720. [Google Scholar]
- Holb, I.J.; Lakatos, P.; Abonyi, F. Some aspects of disease management of cherry leaf spot (Blumeriella jaapii) with special reference to pesticide use. Int. J. Hortic. Sci. 2010, 16, 45–49. [Google Scholar] [CrossRef]
- Villani, S.M.; Ayer, K.; Cox, K.D. Molecular characterization of the sdhB gene and baseline sensitivity to penthiopyrad, fluopyram, and benzovindiflupyr in Venturia inaequalis. Plant Dis. 2016, 100, 1709–1716. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Rojas, J.A.; Sang, H.; Proffer, T.J.; Outwater, C.A.; Vilgalys, R.; Sundin, G.W. Draft genome resource for Blumeriella jaapii, the cherry leaf spot pathogen. Phytopathology 2020, 110, 1507–1510. [Google Scholar] [CrossRef]
- Peng, J.; Sang, H.; Proffer, T.J.; Gleason, J.; Outwater, C.A.; Jung, G.; Sundin, G.W. A method for the examination of SDHI fungicide resistance mechanisms in phytopathogenic fungi using a heterologous expression system in Sclerotinia sclerotiorum. Phytopathology 2021, 111, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Kiiker, R.; Juurik, M.; Heick, T.M.; Mäe, A. Changes in DMI, SDHI, and QoI fungicide sensitivity in the Estonian Zymoseptoria tritici population between 2019 and 2020. Microorganisms 2021, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Rehfus, A.; Miessner, S.; Achenbach, J.; Strobel, D.; Bryson, R.; Stammler, G. Emergence of succinate dehydrogenase inhibitor resistance of Pyrenophora teres in Europe. Pest. Manag. Sci. 2016, 72, 1977–1988. [Google Scholar] [CrossRef] [PubMed]
- Succinate Dehydrogenase Inhibitor (SDHI) Working Group of the Fungicide Resistance Action Committee (FRAC). Available online: https://www.frac.info/docs/default-source/working-groups/sdhi-fungicides/sdhi-meeting-minutes/minutes-of-the-2020-sdhi-meeting-jan-june-september-2020-with-recommendations-for-2020.pdf?sfvrsn=d7b8499a_2 (accessed on 28 August 2021).
- van den Bosch, F.; Paveley, N.; Shaw, M.; Hobbelen, P.; Oliver, R. The dose rate debate: Does the risk of fungicide resistance increase or decrease with dose? Plant Pathol. 2011, 60, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Ayer, K.M.; Choi, M.-W.; Smart, S.T.; Moffett, A.E.; Cox, K.D. The effects of succinate dehydrogenase inhibitor fungicide dose and mixture on development of resistance in Venturia inaequalis. Appl. Environ. Microbiol. 2020, 86, e01196-20. [Google Scholar] [CrossRef]
- Sutton, T.B.; Aldwinckle, H.S.; Agnello, A.M.; Walgenbach, J.F. Compendium of Apple and Pear Diseases and Pests, 2nd ed.; American Phytopathological Society Press: St. Paul, MN, USA, 2014. [Google Scholar]
- Ogawa, J.M.; Zehr, E.I.; Bird, G.W.; Ritchie, D.F.; Uriu, K.; Uyemoto, J.K. Compendium of Stone Fruit Diseases; American Phytopathological Society Press: St. Paul, MN, USA, 1995. [Google Scholar]
- MacHardy, W.E. Apple Scab: Biology, Epidemiology, and Management; American Phytopathological Society Press: St. Paul, MN, USA, 1996. [Google Scholar]
- Chapman, K.S.; Sundin, G.W.; Beckerman, J.L. Identification of resistance to multiple fungicides in field populations of Venturia inaequalis. Plant Dis. 2011, 95, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Lesniak, K.S.; Proffer, T.J.; Beckerman, J.L.; Sundin, G.W. Occurrence of QoI resistance and detection of the G143A mutation in Michigan populations of Venturia inaequalis. Plant Dis. 2011, 95, 927–934. [Google Scholar] [CrossRef] [Green Version]
- Beckerman, J.L.; Sundin, G.W.; Rosenberger, D.A. Do some IPM concepts contribute to the development of fungicide resistance? Lessons learned from the apple scab pathosystem in the United States. Pest Manag. Sci. 2015, 71, 331–342. [Google Scholar] [CrossRef]
| Phenological Stages and Fungicides (g ha−1) Applied x | |||||||
|---|---|---|---|---|---|---|---|
| Treatment Description y | Years | Petal Fall | Shuck Split | 1st Cover | 2nd Cover | 3rd Cover | 4th Cover |
| Control | --- z | --- | --- | --- | --- | --- | |
| Fx + Py (P) | 2017–2019 | Ch 2780 | Ch 2780 | Fx 100 + Py 100 | Fx 100 + Py 100 | Fx 100 + Py 100 | Fx 100 + Py 100 |
| Fp + Tr (P) | 2017–2019 | Ch 2780 | Ch 2780 | Fp 92 + Tr 92 | Fp 92 + Tr 92 | Fp 92 + Tr 92 | Fp 92 + Tr 92 |
| Fx (P) | 2018–2019 | Ch 2780 | Ch 2780 | Fx 100 | Fx 100 | Fx 100 | Fx 100 |
| Fp (P) | 2018–2019 | Ch 2780 | Ch 2780 | Fp 92 | Fp 92 | Fp 92 | Fp 92 |
| Purpose | Primer Name | Sequence (5′-3′) | Amplicon Size |
|---|---|---|---|
| Amplification and sequencing of SdhB | SdhB_amp_F | CCCAATAAGACACCTCAACTC | 1094 bp |
| SdhB_amp_R | ATAACACTCTCGCATCCCTA | ||
| SdhB_seq_F | GGTTGATCCGACGTTGAC | Not applicable | |
| SdhB_seq_R | GTACGGCTTGATGGACTTG | ||
| Amplification and sequencing of SdhC | SdhC_amp_F | ATGTTGGCTCAACGAGCTG | 1007 bp |
| SdhC_amp_R | CTAATACGCCAATGCCAAGTAC | ||
| SdhC_seq_F | CTGCTGCCTTTGAGGATAG | Not applicable | |
| SdhC_seq_R | GCTTGTAGATGGAGAGGTTG | ||
| Amplification and sequencing of SdhD | SdhD_amp_F | ATGGCATCAATTGTGCGAC | 690 bp |
| SdhD_amp_R | CTATGCCGCCCAGATCCT | ||
| SdhD_seq_F | TTCCCGGTTCCTTGAGAGT | Not applicable | |
| SdhD_seq_R | CCGTGGGAATGTAGTCGATTATG |
| Cherry Leaf Spot Rating z | |||||
|---|---|---|---|---|---|
| Infection (%) | Defoliation (%) | ||||
| Year | Treatment Description y | Harvest | Post-Harvest | Harvest | Post-Harvest |
| 2017 | 2 August | 2 August | |||
| Fluxapyroxad + pyraclostrobin | -- | 37.3 c x | -- | 6.7 b | |
| Fluopyram + trifloxystrobin | -- | 63.3 b | -- | 12.2 b | |
| Untreated control | -- | 88.6 a | -- | 89.6 a | |
| 2018 | 20 July | 30 August | 20 July | 30 August | |
| Fluxapyroxad + pyraclostrobin | 14.3 b | 56.1 c | 1.9 b | 26.7 d | |
| Fluopyram + trifloxystrobin | 21.5 ab | 84.4 b | 2.9 b | 45.6 cd | |
| Fluxapyroxad | 11.9 b | 83.4 b | 1.9 b | 56.0 bc | |
| Fluopyram | 25.2 ab | 90.9 ab | 5.7 b | 72.4 b | |
| Untreated control | 38.4 a | 100.0 a | 19.5 a | 91.1 a | |
| 2019 | 11 July | 8 August | 11 July | 8 August | |
| Fluxapyroxad + pyraclostrobin | 55.7 bc | 99.4 a | 34.2 bc | 59.2 b | |
| Fluopyram + trifloxystrobin | 52.4 c | 97.8 a | 26.7 c | 65.1 b | |
| Fluxapyroxad | 70.5 ab | 100.0 a | 39.1 b | 90.4 a | |
| Fluopyram | 75.6 a | 100.0 a | 42.7 b | 91.1 a | |
| Untreated control | 74.6 a | 100.0 a | 57.2 a | 96.9 a | |
| Fungicide Phenotype | Number of Isolates | Percentage of Isolates | ||
|---|---|---|---|---|
| Boscalid | Fluopyram | Fluxapyroxad | ||
| S | S | S | 112 | 7.0 |
| R | R | R | 418 | 26.0 |
| S | R | S | 85 | 5.3 |
| S | S | R | 0 | 0.0 |
| R | S | S | 22 | 1.4 |
| R | R | S | 169 | 10.5 |
| R | S | R | 5 | 0.4 |
| S | R | R | 15 | 0.9 |
| M | M | M | 15 | 0.9 |
| S | M | S | 144 | 8.9 |
| S | S | M | 2 | 0.1 |
| M | S | S | 9 | 0.6 |
| S | M | M | 6 | 0.4 |
| M | M | S | 23 | 1.4 |
| R | M | S | 83 | 5.2 |
| R | S | M | 14 | 0.9 |
| R | M | M | 42 | 2.6 |
| M | R | M | 63 | 3.9 |
| M | M | R | 5 | 0.3 |
| S | M | R | 5 | 0.3 |
| S | R | M | 15 | 0.9 |
| M | R | S | 114 | 7.1 |
| M | S | R | 0 | 0.0 |
| M | R | R | 48 | 3.0 |
| R | M | R | 16 | 1.0 |
| R | R | M | 180 | 11.2 |
| Resistance/Sensitivity Phenotype | Presence/Absence of Mutation | |||||||
|---|---|---|---|---|---|---|---|---|
| SdhB | SdhC | |||||||
| Isolate Name | Location | Bosc | Fluo | Fluxa | H260R | I262V | S84L | N86S |
| R0RB-17 | Oceana | R | S | S | + | |||
| VNSB-8 | Oceana | R | S | S | + | |||
| V2OB-12 | Oceana | R | S | S | + | |||
| ACBB-3 | Leelanau | S | R | S | + | |||
| LSTB-3 | Mason | S | R | S | + | |||
| ARPB-4 | Leelanau | S | R | S | + | |||
| WYOB-4 | Oceana | S | R | S | + | |||
| BMTB-1 | Oceana | S | R | S | + | |||
| JGFB-5 | Mason | S | R | S | + | |||
| NWSBC-2 | Leelanau | S | R | S | + | |||
| BMTB-5 | Oceana | S | R | S | + | |||
| GOOB-24 | Leelanau | S | R | R | + | |||
| GSOB-6 | Leelanau | S | R | R | + | |||
| WYOB-21 | Oceana | S | R | R | + | + | ||
| ARPB-16 | Leelanau | R | R | R | + | |||
| JGFB-3 | Benzie | R | R | R | + | |||
| DRTB-4 | Oceana | R | R | R | + | |||
| ACBB-21 | Leelanau | R | R | R | + | |||
| DNFB-12 | Leelanau | R | R | R | + | + | ||
| DCOB-26 | Grand Trav. | R | R | R | + | + | ||
| JWNB-18 | Oceana | R | R | R | + | + | ||
| LSTB-1 | Mason | R | R | R | + | + | ||
| Fluopyram Number (and Percentage) of Isolates | Fluxapyroxad Number (and Percentage) of Isolates | |||||
|---|---|---|---|---|---|---|
| Year | Sensitive | Moderate | Resistant | Sensitive | Moderate | Resistant |
| 2017 | 0 (0.0) | 21 (75.0) | 7 (25.0) | 13 (46.4) | 10 (35.7) | 5 (17.9) |
| 2018 | 1 (2.5) | 16 (39.5) | 23 (57.5) | 7 (17.5) | 33 (82.5) | 0 (0.0) |
| 2019 | 0 (0.0) | 9 (36.0) | 16 (64.0) | 4 (16.0) | 18 (72.0) | 3 (12.0) |
| Fluopyram | Fluxapyroxad | ||||
|---|---|---|---|---|---|
| Orchard b | n | % Moderate | % Resistant | % Moderate | % Resistant |
| WC1 | 21 | 28.6 | 23.8 | 52.3 | 4.8 |
| WC2 | 17 | 100 | 0.0 | 76.5 | 0.0 |
| WC3 | 30 | 66.7 | 33.3 | 90.0 | 0.0 |
| WC4 | 29 | 41.4 | 58.6 | 96.6 | 3.4 |
| WC5 | 27 | 74.1 | 25.9 | 81.5 | 7.4 |
| WC6 | 33 | 50.0 | 43.3 | 73.3 | 0.0 |
| WC7 | 26 | 26.9 | 73.1 | 92.3 | 7.7 |
| WC8 | 27 | 51.9 | 48.1 | 77.8 | 3.7 |
| WC9 | 28 | 7.1 | 92.9 | 89.3 | 10.7 |
| WC10 | 30 | 26.7 | 73.3 | 40.0 | 46.7 |
| NW1 | 27 | 63.0 | 37.0 | 51.9 | 0.0 |
| NW2 | 27 | 66.7 | 33.3 | 55.6 | 0.0 |
| NW3 | 24 | 75.0 | 0.0 | 16.7 | 0.0 |
| NW4 | 30 | 40.0 | 60.0 | 70.0 | 0.0 |
| NW5 | 24 | 12.5 | 87.5 | 83.3 | 0.0 |
| NW6 | 29 | 41.4 | 58.6 | 86.3 | 10.3 |
| NW7 | 26 | 61.5 | 38.5 | 69.2 | 0.0 |
| NW8 | 26 | 23.1 | 76.9 | 84.7 | 11.5 |
| NW9 | 28 | 32.1 | 67.9 | 78.6 | 10.7 |
| NW10 | 27 | 25.9 | 74.1 | 81.5 | 7.4 |
| NW11 | 26 | 34.6 | 0.0 | 23.1 | 3.8 |
| NW12 | 27 | 37.1 | 37.1 | 55.6 | 11.1 |
| NW13 | 27 | 63.0 | 37.0 | 48.2 | 3.7 |
| NW14 | 27 | 77.8 | 18.5 | 29.6 | 3.7 |
| NW15 | 24 | 45.8 | 54.2 | 75.0 | 0.0 |
| NW16 | 27 | 59.3 | 25.9 | 48.2 | 7.4 |
| NW17 | 27 | 48.2 | 18.5 | 51.9 | 7.4 |
| NW18 | 21 | 71.4 | 28.6 | 28.6 | 0.0 |
| NW19 | 27 | 48.1 | 51.9 | 66.7 | 11.1 |
| NW20 | 27 | 63.0 | 37.0 | 74.1 | 0.0 |
| NW21 | 26 | 23.1 | 57.7 | 50.0 | 0.0 |
| NW22 | 26 | 38.5 | 61.5 | 76.9 | 23.1 |
| NW23 | 27 | 55.6 | 40.7 | 85.2 | 0.0 |
| NW24 | 27 | 48.1 | 48.1 | 66.7 | 0.0 |
| NW25 | 23 | 56.5 | 39.1 | 95.7 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gleason, J.; Peng, J.; Proffer, T.J.; Slack, S.M.; Outwater, C.A.; Rothwell, N.L.; Sundin, G.W. Resistance to Boscalid, Fluopyram and Fluxapyroxad in Blumeriella jaapii from Michigan (U.S.A.): Molecular Characterization and Assessment of Practical Resistance in Commercial Cherry Orchards. Microorganisms 2021, 9, 2198. https://doi.org/10.3390/microorganisms9112198
Gleason J, Peng J, Proffer TJ, Slack SM, Outwater CA, Rothwell NL, Sundin GW. Resistance to Boscalid, Fluopyram and Fluxapyroxad in Blumeriella jaapii from Michigan (U.S.A.): Molecular Characterization and Assessment of Practical Resistance in Commercial Cherry Orchards. Microorganisms. 2021; 9(11):2198. https://doi.org/10.3390/microorganisms9112198
Chicago/Turabian StyleGleason, Jacqueline, Jingyu Peng, Tyre J. Proffer, Suzanne M. Slack, Cory A. Outwater, Nikki L. Rothwell, and George W. Sundin. 2021. "Resistance to Boscalid, Fluopyram and Fluxapyroxad in Blumeriella jaapii from Michigan (U.S.A.): Molecular Characterization and Assessment of Practical Resistance in Commercial Cherry Orchards" Microorganisms 9, no. 11: 2198. https://doi.org/10.3390/microorganisms9112198
APA StyleGleason, J., Peng, J., Proffer, T. J., Slack, S. M., Outwater, C. A., Rothwell, N. L., & Sundin, G. W. (2021). Resistance to Boscalid, Fluopyram and Fluxapyroxad in Blumeriella jaapii from Michigan (U.S.A.): Molecular Characterization and Assessment of Practical Resistance in Commercial Cherry Orchards. Microorganisms, 9(11), 2198. https://doi.org/10.3390/microorganisms9112198






