Denitrification of Permeable Sand Sediment in a Headwater River Is Mainly Influenced by Water Chemistry, Rather Than Sediment Particle Size and Heterogeneity
Abstract
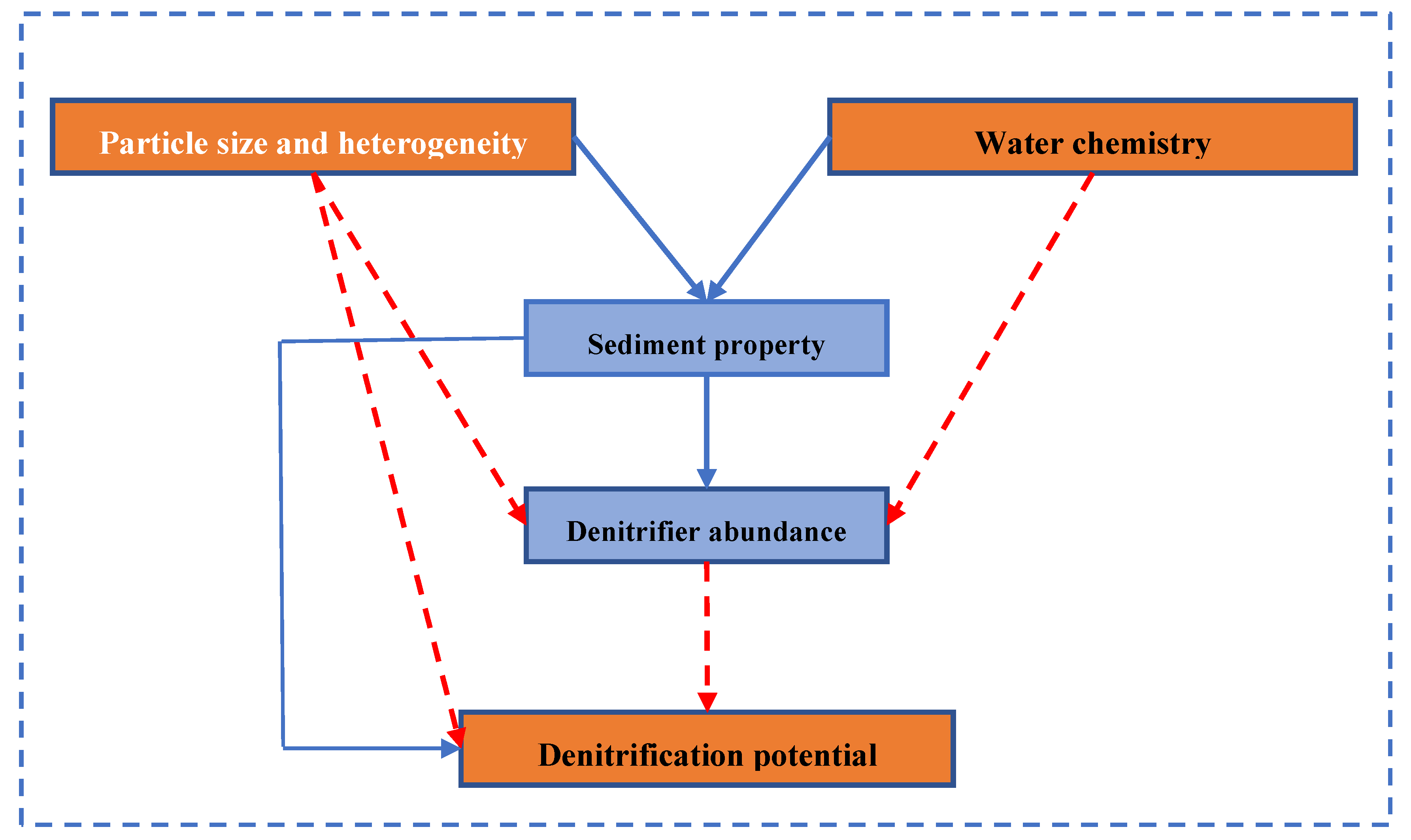
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Sediment Variables and Denitrification Rate Measurement
2.4. Denitrifier Community Analysis and Denitrification Enzyme Activity Prediction
2.5. Denitrifier Abundance Measurement
2.6. Statistical Analysis
3. Results
3.1. Changes of Abiotic Environmental Factors
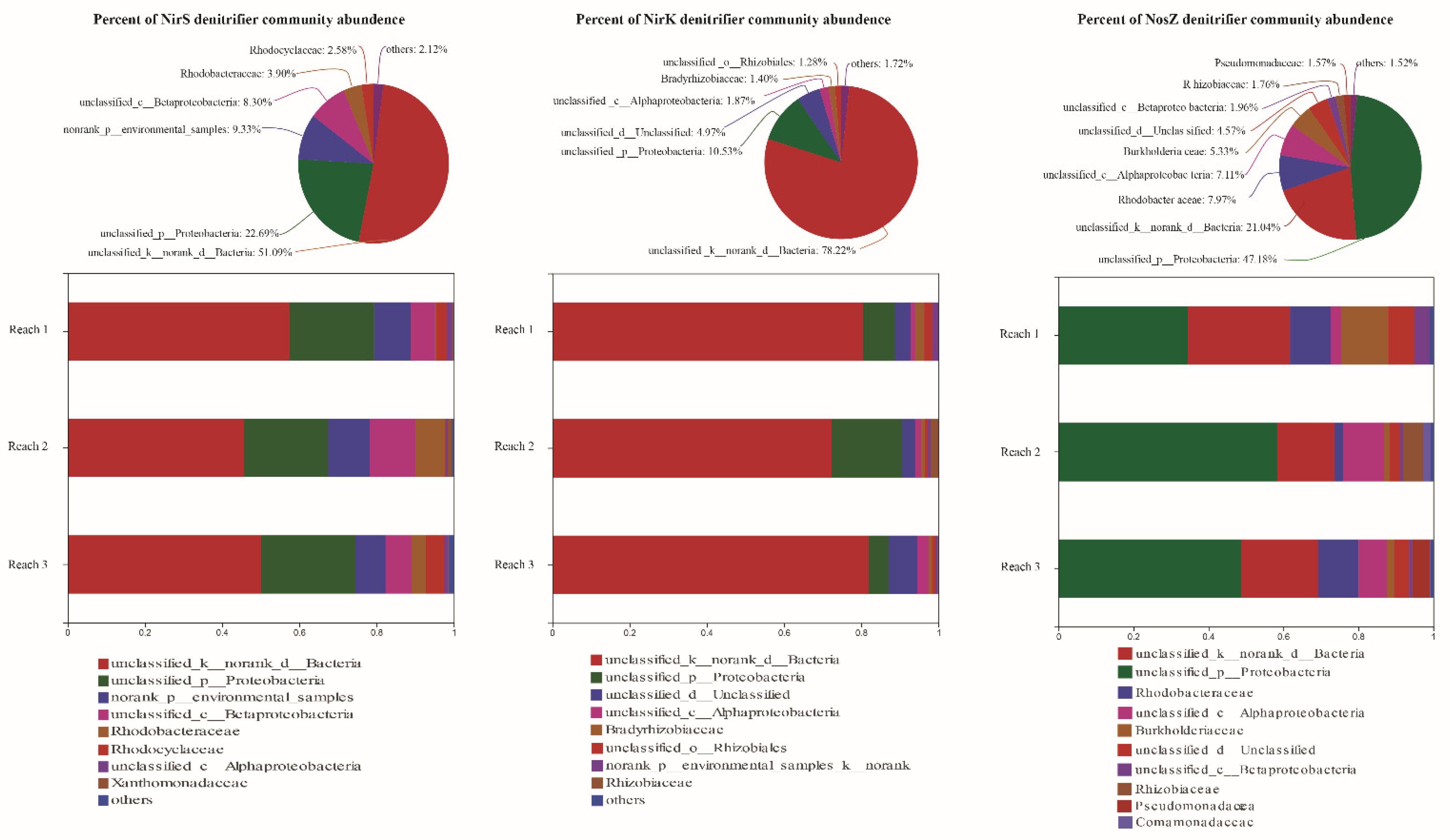
3.2. Three Denitrifier Community Compositions and Their Relationships with Abiotic Environmental Facotors
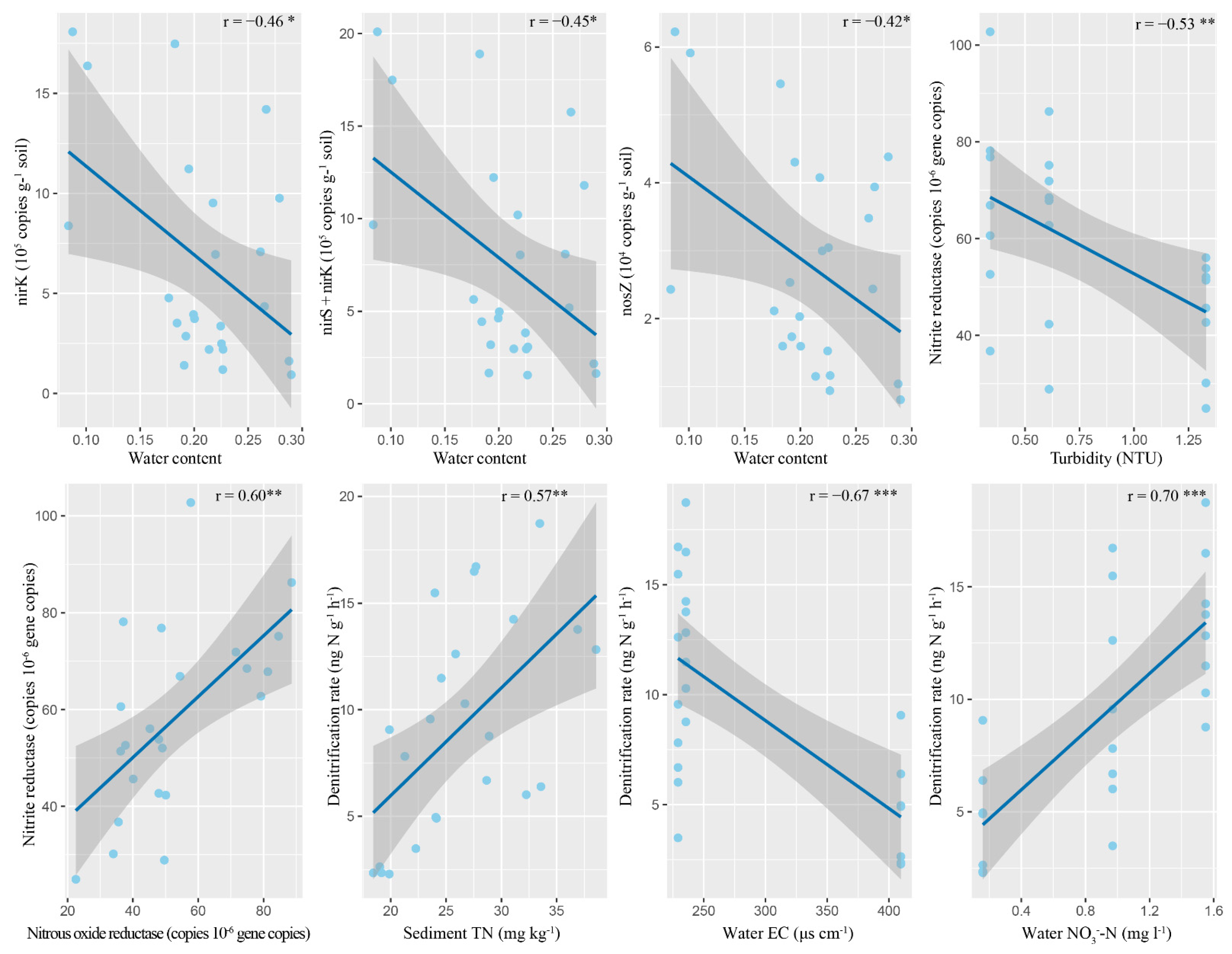
3.3. Denitrification Gene Abundances and Their Relationships with Abiotic Environmental Factors
3.4. Denitrification Enzyme Activities and Their Relationships with Abiotic Environmental Factors
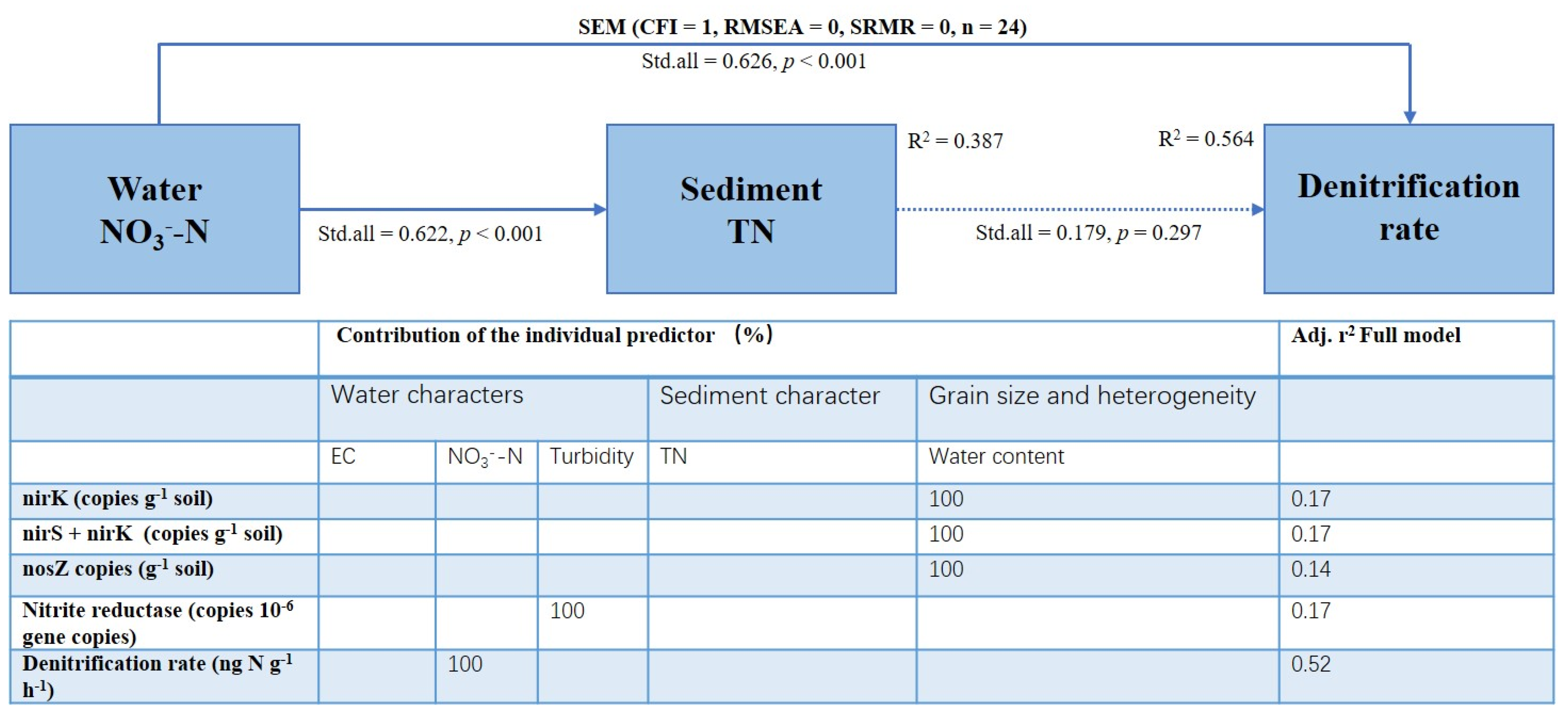
3.5. Denitrification Rates and Their Relationships with Abiotic and Biotic Environmental Factors
4. Discussion
4.1. Characteristics of Denitrifier Communities and Denitrification Rates in Permeable Sandy Sediments
4.2. Key Factors Controlling Denitrification of Permeable Sandy Sediments
4.3. Impacts of Particle Size and Heterogeneity on the Denitrification Process of Permeable Sandy Sediments
4.4. Problems and Prospects of the Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Krause, S.; Lewandowski, J.; Grimm, N.B.; Hannah, D.M.; Pinay, G.; McDonald, K.; Martí, E.; Argerich, A.; Pfister, L.; Klaus, J.; et al. Ecohydrological interfaces as hot spots of ecosystem processes. Water Resour. Res. 2017, 53, 6359–6376. [Google Scholar] [CrossRef] [Green Version]
- Earle, S. Physical Geology; BCcampus: Victoria, BC, Canada, 2014. [Google Scholar]
- Huettel, M.; Røy, H.; Precht, E.; Ehrenhauss, S. Hydrodynamical impact on biogeochemical processes in aquatic sediments. Hydrobiologia 2003, 494, 231–236. [Google Scholar] [CrossRef]
- Gangi, A.F. Permeability of unconsolidated sands and porous rocks. J. Geophys. Res. Space Phys. 1985, 90, 3099. [Google Scholar] [CrossRef]
- Probandt, D.; Knittel, K.; Tegetmeyer, H.E.; Ahmerkamp, S.; Holtappels, M.; Amann, R. Permeability shapes bacterial communities in sublittoral surface sediments. Environ. Microbiol. 2017, 19, 1584–1599. [Google Scholar] [CrossRef] [PubMed]
- Keil, R.G.; Montluçon, D.B.; Prahl, F.G.; Hedges, J.I. Sorptive preservation of labile organic matter in marine sediments. Nat. Cell Biol. 1994, 370, 549–552. [Google Scholar] [CrossRef]
- Shum, K.; Sundby, B. Organic matter processing in continental shelf sediments—the subtidal pump revisited. Mar. Chem. 1996, 53, 81–87. [Google Scholar] [CrossRef]
- Rao, A.M.; McCarthy, M.J.; Gardner, W.S.; Jahnke, R.A. Respiration and denitrification in permeable continental shelf deposits on the South Atlantic Bight: N2:Ar and isotope pairing measurements in sediment column experiments. Cont. Shelf Res. 2008, 28, 602–613. [Google Scholar] [CrossRef]
- Kessler, A.; Glud, R.N.; Cardenas, M.B.; Larsen, M.; Bourke, M.F.; Cook, P. Quantifying denitrification in rippled permeable sands through combined flume experiments and modeling. Limnol. Oceanogr. 2012, 57, 1217–1232. [Google Scholar] [CrossRef]
- Gao, H.; Schreiber, F.; Collins, G.; Jensen, M.M.; Kostka, J.E.; Lavik, G.; de Beer, D.; Zhou, H.-Y.; Kuypers, M.M.M. Aerobic denitrification in permeable Wadden Sea sediments. ISME J. 2009, 4, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Huettel, M.; Berg, P.; Kostka, J.E. Benthic Exchange and Biogeochemical Cycling in Permeable Sediments. Annu. Rev. Mar. Sci. 2014, 6, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Hellemann, D.; Tallberg, P.; Bartl, I.; Voss, M.; Hietanen, S. Denitrification in an oligotrophic estuary: A delayed sink for riverine nitrate. Mar. Ecol. Prog. Ser. 2017, 583, 63–80. [Google Scholar] [CrossRef] [Green Version]
- Bartl, I.; Hellemann, D.; Rabouille, C.; Schulz, K.; Tallberg, P.; Hietanen, S.; Voss, M. Particulate organic matter controls benthic microbial N retention and N removal in contrasting estuaries of the Baltic Sea. Biogeosciences 2019, 16, 3543–3564. [Google Scholar] [CrossRef] [Green Version]
- Rocha, C. Sandy sediments as active biogeochemical reactors: Compound cycling in the fast lane. Aquat. Microb. Ecol. 2008, 53, 119–127. [Google Scholar] [CrossRef]
- Wang, W.; Hu, M.; Shu, X.; Li, H.; Qi, W.; Yang, Y.; Zhang, Q. Microbiome of permeable sandy substrate in headwater river is shaped by water chemistry rather than grain size and heterogeneity. Sci. Total. Environ. 2021, 780, 146552. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Palmer, M.A.; Swan, C.M.; Brooks, S.; Poff, N.L. The influence of substrate heterogeneity on biofilm metabolism in a stream ecosystem. Ecology 2002, 83, 412–422. [Google Scholar] [CrossRef]
- Wilson, K.C.; Dodds, W. Centimeter-scale stream substratum heterogeneity and metabolic rates. Hydrobiologia 2008, 623, 53–62. [Google Scholar] [CrossRef]
- Parker, S.P.; Bowden, W.B.; Flinn, M.B.; Giles, C.D.; Arndt, K.A.; Beneš, J.P.; Jent, D.G. Effect of particle size and heterogeneity on sediment biofilm metabolism and nutrient uptake scaled using two approaches. Ecosphere 2018, 9, e02137. [Google Scholar] [CrossRef]
- Xin, X.-K.; Yin, W.; Li, K.-F. Estimation of non-point source pollution loads with flux method in Danjiangkou Reservoir area, China. Water Sci. Eng. 2017, 10, 134–142. [Google Scholar] [CrossRef]
- Smith, M.; Tiedje, J.M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 1979, 11, 261–267. [Google Scholar] [CrossRef]
- Xiong, Z.; Guo, L.; Zhang, Q.; Liu, G.; Liu, W. Edaphic Conditions Regulate Denitrification Directly and Indirectly by Altering Denitrifier Abundance in Wetlands along the Han River, China. Environ. Sci. Technol. 2017, 51, 5483–5491. [Google Scholar] [CrossRef]
- Bruesewitz, D.A.; Tank, J.L.; Hamilton, S.K. Incorporating spatial variation of nitrification and denitrification rates into whole-lake nitrogen dynamics. J. Geophys. Res. Space Phys. 2012, 117, 7. [Google Scholar] [CrossRef]
- Langille, M.G.I.; Zaneveld, J.; Caporaso, J.G.; McDonald, D.; Knights, D.; Reyes, J.A.; Clemente, J.C.; Burkepile, D.E.; Thurber, R.L.V.; Knight, R.; et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat. Biotechnol. 2013, 31, 814–821. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2012; ISBN 3-900051-07-0. [Google Scholar]
- Oksanen, J.; Blanchet, F.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. Vegan: Community Ecology Package, R package Version. 2, 5–7. 2020. Available online: https://github.com/vegandevs/vegan (accessed on 1 May 2021).
- Desnues, C.; Michotey, V.D.; Wieland, A.; Zhizang, C.; Fourçans, A.; Duran, R.; Bonin, P.C. Seasonal and diel distributions of denitrifying and bacterial communities in a hypersaline microbial mat (Camargue, France). Water Res. 2007, 41, 3407–3419. [Google Scholar] [CrossRef]
- Knapp, C.; Dodds, W.; Wilson, K.C.; O’Brien, J.M.; Graham, D.W. Spatial Heterogeneity of Denitrification Genes in a Highly Homogenous Urban Stream. Environ. Sci. Technol. 2009, 43, 4273–4279. [Google Scholar] [CrossRef]
- Garcia-Ruiz, R.; Pattinson, S.N.; Whitton, B.A. Denitrification in river sediments: Relationship between process rate and properties of water and sediment. Freshw. Biol. 1998, 39, 467–476. [Google Scholar] [CrossRef]
- Sirivedhin, T.; Gray, K.A. Factors affecting denitrification rates in experimental wetlands: Field and laboratory studies. Ecol. Eng. 2006, 26, 167–181. [Google Scholar] [CrossRef]
- Chen, Q.-L.; Ding, J.; Li, C.-Y.; Yan, Z.-Z.; He, J.-Z.; Hu, H.-W. Microbial functional attributes, rather than taxonomic attributes, drive top soil respiration, nitrification and denitrification processes. Sci. Total. Environ. 2020, 734, 139479. [Google Scholar] [CrossRef]
- Ma, L.; Xiong, Z.; Yao, L.; Liu, G.; Zhang, Q.; Liu, W. Soil properties alter plant and microbial communities to modulate denitrification rates in subtropical riparian wetlands. Land Degrad. Dev. 2020, 31, 1792–1802. [Google Scholar] [CrossRef]
- Robert, T. Denitrification. In Soil Microbiology; Robert, T., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2021; pp. 447–467. [Google Scholar]
- Grebliunas, B.D.; Perry, W.L. Carbon limitation of sediment bacterial production and denitrification in high nitrate low carbon systems. Environ. Earth Sci. 2016, 75, 1–9. [Google Scholar] [CrossRef]
- Chen, S.; Wang, F.; Zhang, Y.; Qin, S.; Wei, S.; Wang, S.; Hu, C.; Liu, B. Organic carbon availability limiting microbial denitrification in the deep vadose zone. Environ. Microbiol. 2018, 20, 980–992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, L.; Thamdrup, B.; Trimmer, M. Coupled nitrification and N2 gas production as a cryptic process in oxic riverbeds. Nat. Commun. 2021, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Highton, M.P.; Roosa, S.; Crawshaw, J.; Schallenberg, M.; Morales, S.E. Physical Factors Correlate to Microbial Community Structure and Nitrogen Cycling Gene Abundance in a Nitrate Fed Eutrophic Lagoon. Front. Microbiol. 2016, 7, 1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.; Luo, X.; Hao, X.; Ouyang, Y.; Zeng, L.; Wang, L.; Wen, S.; Wang, B.; Van Nostrand, J.D.; Chen, W.; et al. Microscale heterogeneity of the soil nitrogen cycling microbial functional structure and potential metabolism. Environ. Microbiol. 2021, 23, 1199–1209. [Google Scholar] [CrossRef]
- Ahmerkamp, S.; Marchant, H.K.; Peng, C.; Probandt, D.; Littmann, S.; Kuypers, M.M.M.; Holtappels, M. The effect of sediment grain properties and porewater flow on microbial abundance and respiration in permeable sediments. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Miksch, S.; Meiners, M.; Meyerdierks, A.; Probandt, D.; Wegener, G.; Titschack, J.; Jensen, M.A.; Ellrott, A.; Amann, R.; Knittel, K. Bacterial communities in temperate and polar coastal sands are seasonally stable. ISME Commun. 2021, 1, 1–11. [Google Scholar] [CrossRef]
- Liu, W.; Yao, L.; Jiang, X.; Guo, L.; Cheng, X.; Liu, G. Sediment denitrification in Yangtze lakes is mainly influenced by environmental conditions but not biological communities. Sci. Total Environ. 2018, 616-617, 978–987. [Google Scholar] [CrossRef]
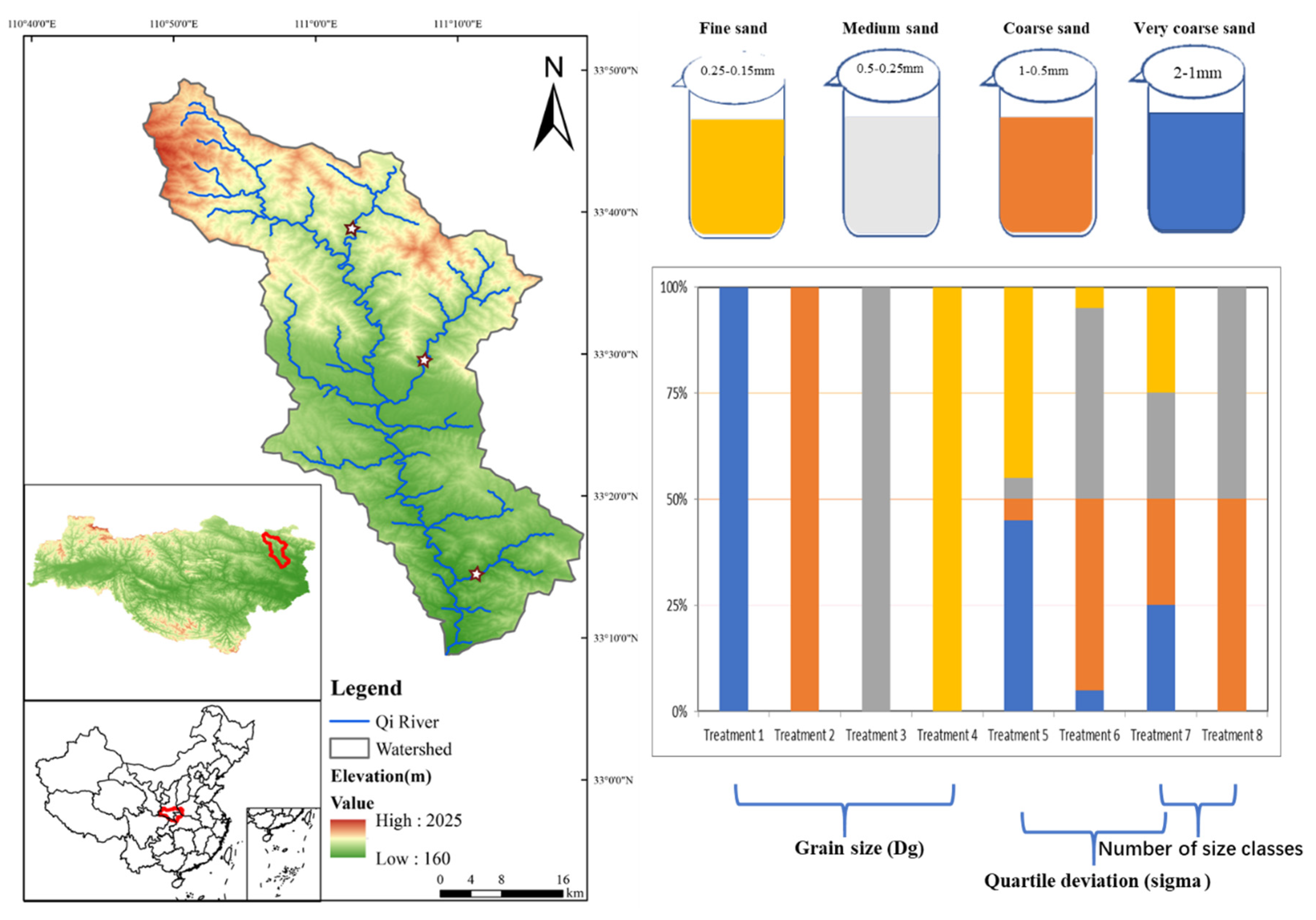

| Treatment Design | Particle Size | Particle Heterogeneity | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Quartile Deviation | Number of Size Classes | ||||||||
| Treatment | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 7 | 8 |
| Number of size classes (nsc) | 1 | 1 | 1 | 1 | 4 | 4 | 4 | 4 | 2 |
| Geometric mean grain size (Dg) (mm) | 1.41 | 0.71 | 0.35 | 0.19 | 0.52 | 0.50 | 0.51 | 0.51 | 0.50 |
| Geometric standard deviation (sigma) | 1.00 | 1.00 | 1.00 | 1.00 | 2.59 | 1.58 | 2.11 | 2.11 | 1.41 |
| Permeability (10−11 m2) | 144.21 | 36.57 | 8.89 | 2.62 | 19.61 | 18.13 | 18.87 | 18.87 | 18.13 |
| Sediment Characteristics | Reach | Particle Size | Particle Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Quartile Deviation | Number of Size Classes | |||||||
| Kruskal–Wallis chi-squared | p-value | Kruskal–Wallis chi-squared | p-value | Kruskal–Wallis chi-squared | p-value | Kruskal–Wallis chi-squared | p-value | |
| TOC (g kg−1dw) | 1.47 | 0.48 | 13.61 | <0.01 | 0.42 | 0.81 | 0.01 | 0.93 |
| TN (mg kg−1dw) | 28.36 | <0.01 | 9.04 | 0.03 | 8.31 | 0.02 | 1.50 | 0.22 |
| TP (mg kg−1dw) | 7.36 | 0.03 | 23.58 | <0.01 | 5.24 | 0.07 | 0.53 | 0.47 |
| NH4−-N (mg kg−1fw) | 15.45 | <0.01 | 5.16 | 0.16 | 0.51 | 0.77 | 2.65 | 0.10 |
| NO3−-N (mg kg−1fw) | 6.72 | 0.03 | 2.65 | 0.45 | 0.01 | 0.99 | 0.14 | 0.70 |
| Microbial Abundance and Enzyme Activity | Reach | Particle Size | Particle Heterogeneity | |||||
|---|---|---|---|---|---|---|---|---|
| Quartile Deviation | Number of Size Classes | |||||||
| Kruskal–Wallis chi-squared | p | Kruskal–Wallis chi-squared | p | Kruskal–Wallis chi-squared | p | Kruskal–Wallis chi-squared | p | |
| nirS (copies ng−1 DNA) | 3.71 | 0.16 | 2.49 | 0.48 | 0.09 | 0.96 | 3.86 | 0.05 |
| nirS (copies g−1 soil) | 1.94 | 0.38 | 4.03 | 0.26 | 0.27 | 0.86 | 3.86 | 0.05 |
| nirK (copies ng−1 DNA) | 4.12 | 0.13 | 5.16 | 0.16 | 0.27 | 0.88 | 0.43 | 0.51 |
| nirK (copies g−1 soil) | 3.85 | 0.15 | 6.59 | 0.09 | 0.62 | 0.73 | 1.19 | 0.28 |
| nirS + nirK (copies ng−1 DNA) | 2.99 | 0.22 | 5.15 | 0.16 | 0.27 | 0.88 | 1.19 | 0.28 |
| nirS +nirK (copies g−1 soil) | 3.19 | 0.20 | 6.59 | 0.09 | 0.62 | 0.73 | 1.19 | 0.28 |
| nosZ (copies ng−1 DNA) | 1.99 | 0.37 | 4.59 | 0.20 | 0.80 | 0.67 | 0.05 | 0.83 |
| nosZ (copies g−1 soil) | 2.56 | 0.28 | 5.36 | 0.15 | 2.22 | 0.33 | 0.05 | 0.843 |
| Nitrite reductase (copies 10−6 gene copies) | 7.29 | 0.03 | 3.00 | 0.39 | 1.16 | 0.56 | 0.43 | 0.51 |
| Nitrous oxide reductase (copies 10−6 gene copies) | 11.42 | <0.01 | 1.67 | 0.64 | 1.42 | 0.49 | 0.05 | 0.83 |
| Water Characteristics | Sediment Characteristics | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EC | Turbidity | NO3-N | Water content | STOC | STN | STP | SNH4+-N | SNO3−-N | |
| nirS (copies ng−1 DNA) | −0.08 | 0.36 | −0.09 | −0.07 | 0.05 | −0.10 | 0.28 | −0.20 | 0.34 |
| nirS (copies g−1 soil) | −0.06 | 0.28 | −0.06 | −0.24 | 0.02 | −0.08 | 0.19 | −0.18 | 0.27 |
| nirK (copies ng−1 DNA) | −0.26 | 0.04 | 0.25 | −0.35 | −0.22 | 0.02 | 0.10 | 0.01 | 0.03 |
| nirK (copies g−1 soil) | −0.26 | −0.05 | 0.29 | −0.46 * | −0.22 | 0.09 | <0.01 | 0.09 | −0.04 |
| nirS + nirK (copies ng−1 DNA) | −0.26 | 0.07 | 0.23 | −0.33 | −0.20 | 0.01 | 0.12 | −0.01 | 0.06 |
| nirS +nirK (copies g−1 soil) | −0.25 | −0.03 | 0.27 | −0.45 * | −0.21 | 0.08 | 0.02 | 0.07 | −0.01 |
| nosZ (copies ng−1 DNA) | −0.24 | 0.07 | 0.21 | −0.25 | −0.20 | 0.02 | 0.10 | 0.05 | 0.05 |
| nosZ (copies g−1 soil) | −0.24 | −0.06 | 0.28 | −0.42 * | −0.21 | 0.12 | −0.02 | 0.14 | −0.03 |
| Nitrite reductase (relative gene copies) | 0.07 | −0.53 ** | 0.14 | 0.19 | 0.39 | 0.09 | −0.15 | −0.27 | −0.13 |
| Nitrous oxide reductase (relative gene copies) | 0.31 | −0.37 | −0.14 | 012 | 0.37 | −0.09 | −0.29 | −0.37 | −0.08 |
| Denitrification rate (ng N g−1 h−1) | −0.67 ** | −0.11 | 0.74 ** | 0.12 | 0.12 | 0.57 ** | 0.16 | 0.28 | −0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Wang, X.; Shu, X.; Wang, B.; Li, H.; Zhang, Q. Denitrification of Permeable Sand Sediment in a Headwater River Is Mainly Influenced by Water Chemistry, Rather Than Sediment Particle Size and Heterogeneity. Microorganisms 2021, 9, 2202. https://doi.org/10.3390/microorganisms9112202
Wang W, Wang X, Shu X, Wang B, Li H, Zhang Q. Denitrification of Permeable Sand Sediment in a Headwater River Is Mainly Influenced by Water Chemistry, Rather Than Sediment Particle Size and Heterogeneity. Microorganisms. 2021; 9(11):2202. https://doi.org/10.3390/microorganisms9112202
Chicago/Turabian StyleWang, Weibo, Xu Wang, Xiao Shu, Baoru Wang, Hongran Li, and Quanfa Zhang. 2021. "Denitrification of Permeable Sand Sediment in a Headwater River Is Mainly Influenced by Water Chemistry, Rather Than Sediment Particle Size and Heterogeneity" Microorganisms 9, no. 11: 2202. https://doi.org/10.3390/microorganisms9112202
APA StyleWang, W., Wang, X., Shu, X., Wang, B., Li, H., & Zhang, Q. (2021). Denitrification of Permeable Sand Sediment in a Headwater River Is Mainly Influenced by Water Chemistry, Rather Than Sediment Particle Size and Heterogeneity. Microorganisms, 9(11), 2202. https://doi.org/10.3390/microorganisms9112202







