Seroprevalence of Antibodies against Tick-Borne Pathogens in Czech Patients with Suspected Post-Treatment Lyme Disease Syndrome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Studied Patient Group
2.2. Laboratory Tests
3. Results
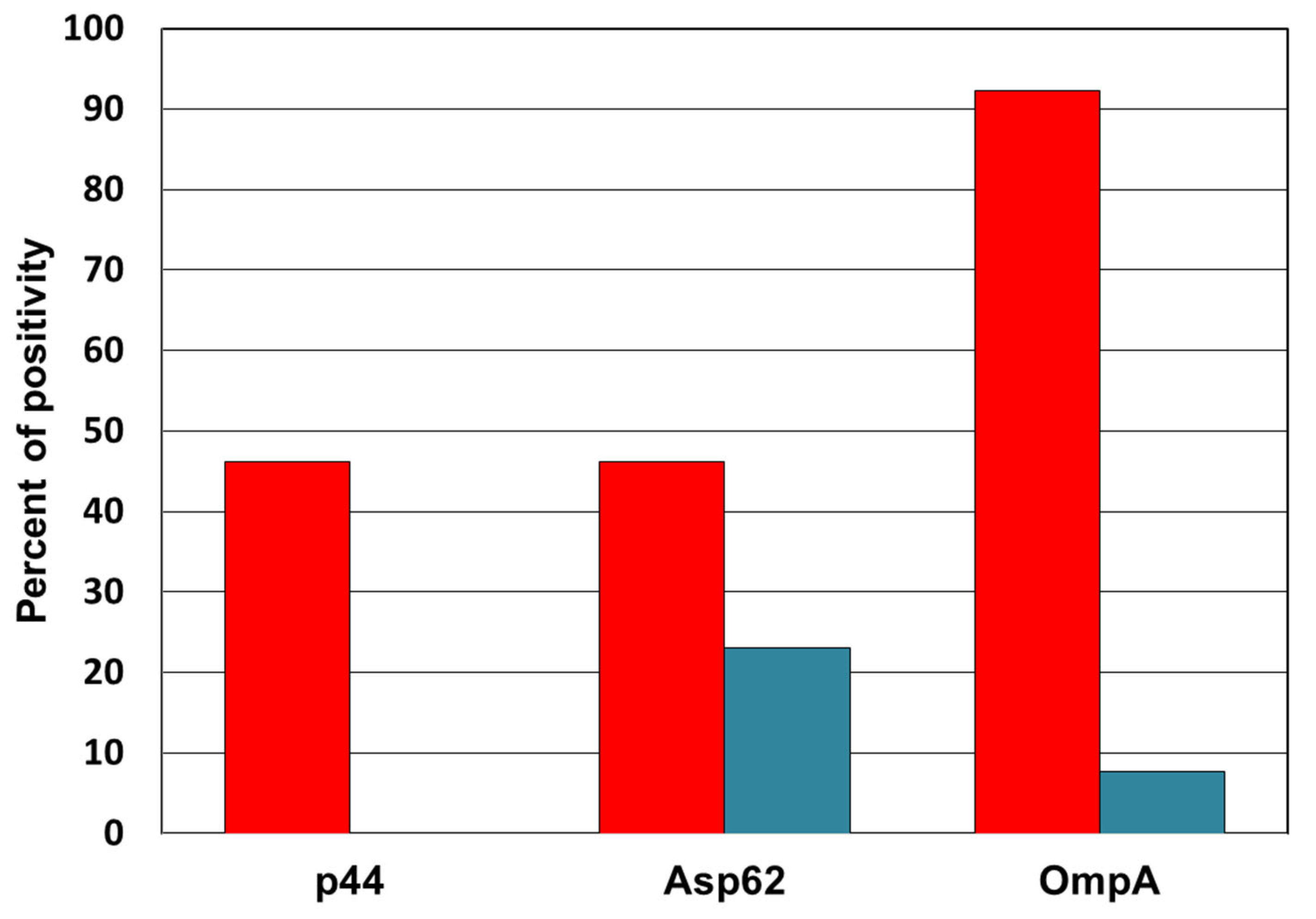
3.1. Detection of Anti-Anaplasma Antibodies
3.2. Detection of Anti-Bartonella Antibodies
3.3. Detection of Anti-Babesia Antibodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kugeler, K.J.; Schwartz, A.M.; Delorey, M.J.; Mead, P.S.; Hinckley, A.F. Estimating the Frequency of Lyme Disease Diagnoses, United States, 2010–2018. Emerg. Infect. Dis. 2021, 27, 616–619. [Google Scholar] [CrossRef] [PubMed]
- Rudenko, N.; Golovchenko, M. Sexual Transmission of Lyme Borreliosis? The Question That Calls for an Answer. Trop. Med. Infect. Dis. 2021, 6, 87. [Google Scholar] [CrossRef]
- European Parliament Resolution on Lyme Disease (Borreliosis) (2018/2774(RSP). Available online: https://www.europarl.europa.eu/doceo/document/B-8-2018-0514_EN.html (accessed on 15 September 2021).
- Steere, A.C.; Strle, F.; Wormser, G.P.; Hu, L.T.; Branda, J.A.; Hovius, J.W.R.; Li, X.; Mead, P.S. Lyme borreliosis. Nat. Rev. Dis. Prim. 2016, 2, 16090. [Google Scholar] [CrossRef]
- Krupka, M.; Raska, M.; Belakova, J.; Horynová, M.S.; Novotny, R.; Weigl, E. Biological aspects of lyme disease spirochetes: Unique bacteria of the Borrelia burgdorferi species group. Biomed. Pap. 2007, 151, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, P.S. Epidemiology of Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 187–210. [Google Scholar] [CrossRef]
- Rudenko, N.; Golovchenko, M.; Grubhoffer, L.; Oliver, J.H. Updates on Borrelia burgdorferi sensu lato complex with respect to public health. Ticks Tick-Borne Dis. 2011, 2, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingry, L.C.; Batra, D.; Replogle, A.; Rowe, L.A.; Pritt, B.S.; Petersen, J.M. Whole Genome Sequence and Comparative Genomics of the Novel Lyme Borreliosis Causing Pathogen, Borrelia mayonii. PLoS ONE 2016, 11, e0168994. [Google Scholar] [CrossRef] [PubMed]
- Gern, L. Borrelia burgdorferi sensu lato, the agent of Lyme borreliosis: Life in the wilds. Parasite 2008, 15, 244–247. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Signs and Symptoms of Untreated Lyme Disease. Available online: https://www.cdc.gov/lyme/signs_symptoms/index.html (accessed on 1 September 2021).
- Bamm, V.V.; Ko, J.T.; Mainprize, I.L.; Sanderson, V.P.; Wills, M.K.B. Lyme Disease Frontiers: Reconciling Borrelia Biology and Clinical Conundrums. Pathogens 2019, 8, 299. [Google Scholar] [CrossRef] [Green Version]
- Wormser, G.P.; Dattwyler, R.; Shapiro, E.D.; Halperin, J.; Steere, A.C.; Klempner, M.S.; Krause, P.J.; Bakken, J.S.; Strle, F.; Stanek, G.; et al. The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 43, 1089–1134. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R. Efficacy of Double-Dose Dapsone Combination Therapy in the Treatment of Chronic Lyme Disease/Post-Treatment Lyme Disease Syndrome (PTLDS) and Associated Co-infections: A Report of Three Cases and Retrospective Chart Review. Antibiotics 2020, 9, 725. [Google Scholar] [CrossRef] [PubMed]
- Fallon, B.A.; Petkova, E.; Keilp, J.G.; Britton, C.B. A Reappraisal of the U.S. Clinical Trials of Post-Treatment Lyme Disease Syndrome. Open Neurol. J. 2012, 6, 79–87. [Google Scholar] [CrossRef]
- Klempner, M.S. Controlled Trials of Antibiotic Treatment in Patients with Post-Treatment Chronic Lyme Disease. Vector-Borne Zoonotic Dis. 2002, 2, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Gewurz, B.E.; Hill, D.; Marty, F.M.; Vannier, E.; Foppa, I.M.; Furman, R.R.; Neuhaus, E.; Skowron, G.; Gupta, S.; et al. Persistent and Relapsing Babesiosis in Immunocompromised Patients. Clin. Infect. Dis. 2008, 46, 370–376. [Google Scholar] [CrossRef] [Green Version]
- Horowitz, R.; Freeman, P.R. Healthy Fetal Outcomes Using A Novel Treatment For Maternal Lyme Disease And Babesiosis During Consecutive Pregnancies: A Case Study and Literature Review. Arch. Med Case Rep. 2020, 2, 1–19. [Google Scholar] [CrossRef]
- Křupka, M.; Zachová, K.; Weigl, E.; Raska, M. Prevention of Lyme Disease: Promising Research or Sisyphean Task? Arch. Immunol. Ther. Exp. 2011, 59, 261–275. [Google Scholar] [CrossRef]
- Cerar, D.; Cerar, T.; Ružić-Sabljić, E.; Wormser, G.P.; Strle, F. Subjective Symptoms after Treatment of Early Lyme Disease. Am. J. Med. 2010, 123, 79–86. [Google Scholar] [CrossRef]
- Aucott, J.N.; Crowder, L.; Kortte, K.B. Development of a foundation for a case definition of post-treatment Lyme disease syndrome. Int. J. Infect. Dis. 2013, 17, e443–e449. [Google Scholar] [CrossRef] [Green Version]
- Rudenko, N.; Golovchenko, M.; Kybicova, K.; Vancova, M. Metamorphoses of Lyme disease spirochetes: Phenomenon of Borrelia persisters. Parasites Vectors 2019, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.J. A Review of Antibiotic-Tolerant Persisters and Their Relevance to Posttreatment Lyme Disease Symptoms. Am. J. Med. 2020, 133, 429–431. [Google Scholar] [CrossRef]
- Shor, S.; Green, C.; Szantyr, B.; Phillips, S.; Liegner, K.; Burrascano, J.J.J.; Bransfield, R.; Maloney, E.L. Chronic Lyme Disease: An Evidence-Based Definition by the ILADS Working Group. Antibiotics 2019, 8, 269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lantos, P.M. Chronic Lyme Disease. Infect. Dis. Clin. N. Am. 2015, 29, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, J.; Bernasconi, E.; Heininger, U.; Abbas, M.; Nadal, D.; Strahm, C.; Erb, S.; Zimmerli, S.; Furrer, H.; Delaloye, J.; et al. Update of the Swiss guidelines on post-treatment Lyme disease syndrome. Swiss Med. Wkly. 2016, 146, w14353. [Google Scholar] [CrossRef] [Green Version]
- Marcum, L. LYME SCI: Tick-Borne Co-Infections Are the Rule, Not the Exception. Available online: https://www.lymedisease.org/lyme-sci-coinfections/ (accessed on 15 September 2021).
- Horowitz, R.I.; Freeman, P.R. Are mycobacterium drugs effective for treatment resistant Lyme disease, tick-borne co-infections, and autoimmune disease? JSM Arthritis 2016, 1, 1008. [Google Scholar]
- Grab, D.J.; Nyarko, E.; Barat, N.C.; Nikolskaia, O.V.; Dumler, J.S. Anaplasma phagocytophilum—Borrelia burgdorferi Coinfection Enhances Chemokine, Cytokine, and Matrix Metalloprotease Expression by Human Brain Microvascular Endothelial Cells. Clin. Vaccine Immunol. 2007, 14, 1420–1424. [Google Scholar] [CrossRef] [Green Version]
- Krause, P.J.; McKay, K.; Thompson, C.A.; Sikand, V.K.; Lentz, R.; Lepore, T.; Closter, L.; Christianson, D.; Telford, S.R.; Persing, D.; et al. Disease-Specific Diagnosis of Coinfecting Tickborne Zoonoses: Babesiosis, Human Granulocytic Ehrlichiosis, and Lyme Disease. Clin. Infect. Dis. 2002, 34, 1184–1191. [Google Scholar] [CrossRef] [Green Version]
- Dumler, J.S.; Choi, K.-S.; Garcia-Garcia, J.C.; Barat, N.S.; Scorpio, D.G.; Garyu, J.W.; Grab, D.J.; Bakken, J.S. Human Granulocytic Anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 2005, 11, 1828–1834. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef] [Green Version]
- Petrovec, M.; Furlan, S.L.; Zupanc, T.A.; Strle, F.; Brouqui, P.; Roux, V.; Dumler, J.S. Human disease in Europe caused by a granulocytic Ehrlichia species. J. Clin. Microbiol. 1997, 35, 1556–1559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvořáková Heroldová, M.; Dvořáčková, M. [Seroprevalence of Anaplasma phagocytophilum in patients with suspected Lyme borreliosis] In Czech. Epidemiol. Mikrobiol. Imunol. 2014, 63, 297–302. [Google Scholar]
- Boulouis, H.-J.; Chao-Chin, C.; Henn, J.B.; Kasten, R.W.; Chomel, B.B. Factors associated with the rapid emergence of zoonotic Bartonella infections. Veter Res. 2005, 36, 383–410. [Google Scholar] [CrossRef] [Green Version]
- Anderson, B.E.; Neuman, M.A. Bartonella spp. as emerging human pathogens. Clin. Microbiol. Rev. 1997, 10, 203–219. [Google Scholar] [CrossRef] [PubMed]
- Mozayeni, B.R.; Maggi, R.; Bradley, J.M.; Breitschwerdt, E.B. Rheumatological presentation of Bartonella koehlerae and Bartonella henselae bacteremias. Medicine 2018, 97, e0465. [Google Scholar] [CrossRef] [PubMed]
- Lobo, C.A.; Singh, M.; Rodriguez, M. Human babesiosis: Recent advances and future challenges. Curr. Opin. Hematol. 2020, 27, 399–405. [Google Scholar] [CrossRef]
- Parveen, N.; Bhanot, P. Babesia microti-Borrelia burgdorferi Coinfection. Pathogens 2019, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Lantos, P.M. Chronic Lyme disease: The controversies and the science. Expert Rev. Anti-Infect. Ther. 2011, 9, 787–797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lantos, P.M.; Wormser, G.P. Chronic Coinfections in Patients Diagnosed with Chronic Lyme Disease: A Systematic Review. Am. J. Med. 2014, 127, 1105–1110. [Google Scholar] [CrossRef]
- Stricker, R.B. Counterpoint: Long-Term Antibiotic Therapy Improves Persistent Symptoms Associated with Lyme Disease. Clin. Infect. Dis. 2007, 45, 149–157. [Google Scholar] [CrossRef]
- Horowitz, R.I.; Freeman, P.R. Precision medicine: Retrospective chart review and data analysis of 200 patients on dapsone combination therapy for chronic Lyme disease/post-treatment Lyme disease syndrome: Part 1. Int. J. Gen. Med. 2019, 12, 101–119. [Google Scholar] [CrossRef] [Green Version]
- Maurin, M.; Bakken, J.S.; Dumler, J.S. Antibiotic Susceptibilities of Anaplasma (Ehrlichia) phagocytophilum Strains from Various Geographic Areas in the United States. Antimicrob. Agents Chemother. 2003, 47, 413–415. [Google Scholar] [CrossRef] [Green Version]
- Matei, I.A.; Estrada-Peña, A.; Cutler, S.J.; Vayssier-Taussat, M.; Castro, L.V.; Potkonjak, A.; Zeller, H.; Mihalca, A.D. A review on the eco-epidemiology and clinical management of human granulocytic anaplasmosis and its agent in Europe. Parasites Vectors 2019, 12, 599–619. [Google Scholar] [CrossRef]
- Kříž, B.; Malý, M.; Balátová, P.; Kodym, P.; Kurzová, Z.; Daniel, M.; Kybicová, K. A serological study of antibodies to Anaplasma phagocytophilum and Borrelia burgdorferi sensu lato in the sera of healthy individuals collected two decades apart. Acta Parasitol. 2018, 63, 33–39. [Google Scholar] [CrossRef]
- Thortveit, E.T.; Aase, A.; Petersen, L.B.; Lorentzen, Å.R.; Mygland, Å.; Ljøstad, U. Human seroprevalence of antibodies to tick-borne microbes in southern Norway. Ticks Tick-Borne Dis. 2020, 11, 101410. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, K.; Skoog, E.; Jones, V.; Sandelin, L.L.; Björling, C.; Fridenström, E.; Edvinsson, M.; Mårtensson, A.; Olsen, B. A comprehensive clinical and laboratory evaluation of 224 patients with persistent symptoms attributed to presumed tick-bite exposure. PLoS ONE 2021, 16, e0247384. [Google Scholar] [CrossRef]
- Chmielewska-Badora, J.; Moniuszko, A.; Żukiewicz-Sobczak, W.; Zwolinski, J.; Piątek, J.; Pancewicz, S. Serological survey in persons occupationally exposed to tick-borne pathogens in cases of co-infections with Borrelia burgdorferi, Anaplasma phagocytophilum, Bartonella spp. and Babesia microti. Ann. Agric. Environ. Med. 2012, 19, 271–274. [Google Scholar] [PubMed]
- Chochlakis, D.; Papaeustathiou, A.; Minadakis, G.; Psaroulaki, A.; Tselentis, Y. A serosurvey of Anaplasma phagocytophilum in blood donors in Crete, Greece. Eur. J. Clin. Microbiol. Infect. Dis. 2008, 27, 473–475. [Google Scholar] [CrossRef]
- Wittesjö, B.; Bjöersdorff, A.; Eliasson, I.; Berglund, J. First long-term study of the seroresponse to the agent of human granulocytic ehrlichiosis among residents of a tick-endemic area of Sweden. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Łysakowska, M.E.; Brzezińska, O.; Szybka, M.; Konieczka, M.; Moskwa, S.; Brauncajs, M.; Makowska, J.; Pastuszak-Lewandoska, D.; Grzegorczyk, J. The seroprevalence of Bartonella spp. in the blood of patients with musculoskeletal complaints and blood donors, Poland: A pilot study. Clin. Rheumatol. 2019, 38, 2691–2698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zając, V.; Wójcik-Fatla, A.; Dutkiewicz, J.; Szymańska, J. Bartonella henselae in eastern Poland: The relationship between tick infection rates and the serological response of individuals occupationally exposed to tick bites. J. Vector Ecol. 2015, 40, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Sander, A.; Posselt, M.; Oberle, K.; Bredt, W. Seroprevalence of Antibodies to Bartonella henselae in Patients with Cat Scratch Disease and in Healthy Controls: Evaluation and Comparison of Two Commercial Serological Tests. Clin. Diagn. Lab. Immunol. 1998, 5, 486–490. [Google Scholar] [CrossRef] [Green Version]
- Maggi, R.G.; Mozayeni, B.R.; Pultorak, E.L.; Hegarty, B.C.; Bradley, J.M.; Correa, M.; Breitschwerdt, E.B. Bartonella spp. Bacteremia and Rheumatic Symptoms in Patients from Lyme Disease–endemic Region. Emerg. Infect. Dis. 2012, 18, 783–791. [Google Scholar] [CrossRef]
- La Scola, B.; Raoult, D. Serological cross-reactions between Bartonella quintana, Bartonella henselae, and Coxiella burnetii. J. Clin. Microbiol. 1996, 34, 2270–2274. [Google Scholar] [CrossRef] [Green Version]
- Maurin, M.; Eb, F.; Etienne, J.; Raoult, D. Serological cross-reactions between Bartonella and Chlamydia species: Implications for diagnosis. J. Clin. Microbiol. 1997, 35, 2283–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, M.; Verbakel, H.; Notermans, D.W.; Reimerink, J.H.J.; Peeters, M. Evaluation of sensitivity, specificity and cross-reactivity in Bartonella henselae serology. J. Med. Microbiol. 2010, 59, 743–745. [Google Scholar] [CrossRef] [Green Version]
- Maurin, M.; Rolain, J.-M.; Raoult, D. Comparison of In-House and Commercial Slides for Detection by Immunofluorescence of Immunoglobulins G and M against Bartonella henselae and Bartonella quintana. Clin. Vaccine Immunol. 2002, 9, 1004–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotté, V.; Bonnet, S.; Le Rhun, D.; Le Naour, E.; Chauvin, A.; Boulouis, H.-J.; Lecuelle, B.; Lilin, T.; Vayssier-Taussat, M. Transmission of Bartonella henselae by Ixodes ricinus. Emerg. Infect. Dis. 2008, 14, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Król, N.; Militzer, N.; Stöbe, E.; Nijhof, A.; Pfeffer, M.; Kempf, V.; Obiegala, A. Evaluating Transmission Paths for Three Different Bartonella spp. in Ixodes ricinus Ticks Using Artificial Feeding. Microorganisms 2021, 9, 901. [Google Scholar] [CrossRef] [PubMed]
- Schouls, L.M.; Van De Pol, I.; Rijpkema, S.G.T.; Schot, C.S. Detection and Identification of Ehrlichia, Borrelia burgdorferi Sensu Lato, and Bartonella Species in Dutch Ixodes ricinus Ticks. J. Clin. Microbiol. 1999, 37, 2215–2222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hercík, K.; Hášová, V.; Janeček, J.; Branny, P. Molecular evidence of DNA in ixodid ticks in Czechia. Folia Microbiol. 2007, 52, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Sanogo, Y.O.; Zeaiter, Z.; Caruso, G.; Merola, F.; Shpynov, S.; Brouqui, P.; Raoult, D. Bartonella henselae in Ixodes ricinus Ticks (Acari: Ixodida) Removed from Humans, Belluno Province, Italy. Emerg. Infect. Dis. 2003, 9, 329–332. [Google Scholar] [CrossRef]
- Eskow, E.; Rao, R.-V.S.; Mordechai, E. Concurrent Infection of the Central Nervous System by Borrelia burgdorferi and Bartonella henselae. Arch. Neurol. 2001, 58, 1357–1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halperin, J.J.; Wormser, G.P. Of fleas and ticks on cats and mice. Arch. Neurol. 2001, 58, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Telford, S.R.; Wormser, G.P. Bartonellaspp. Transmission by Ticks Not Established. Emerg. Infect. Dis. 2010, 16, 379–384. [Google Scholar] [CrossRef]
- Okaro, U.; George, S.; Anderson, B. What Is in a Cat Scratch? Growth of Bartonella henselae in a Biofilm. Microorganisms 2021, 9, 835. [Google Scholar] [CrossRef]
- Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J.; Diuk-Wasser, M.A.; Vannier, E.; Krause, P.J. Coinfection by Ixodes Tick-Borne Pathogens: Ecological, Epidemiological, and Clinical Consequences. Trends Parasitol. 2015, 32, 30–42. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Christie, J.; Köster, L.; Du, A.; Yao, C. Emerging Human Babesiosis with “Ground Zero” in North America. Microorganisms 2021, 9, 440. [Google Scholar] [CrossRef]
- Rudolf, I.; Golovchenko, M.; Šikutová, S.; Rudenko, N.; Grubhoffer, L.; Hubálek, Z. Babesia microti (Piroplasmida: Babesiidae) in nymphal Ixodes ricinus (Acari: Ixodidae) in the Czech Republic. Folia Parasitol. 2005, 52, 274–276. [Google Scholar] [CrossRef] [Green Version]
- Hildebrandt, A.; Gray, J.S.; Hunfeld, K.-P. Human Babesiosis in Europe: What clinicians need to know. Infection 2013, 41, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Nohynkova, E.; Kubek, J.; Mestankova, O.; Chalupka, P.; Hubalek, Z. A Case of Babesia microti Infection Imported to the Czech Republic from the USA. Cas. Lek. Ces. 2003, 142, 377–381. (In Czech) [Google Scholar]
- Lempereur, L.; Shiels, B.; Heyman, P.; Moreau, E.; Saegerman, C.; Losson, B.; Malandrin, L. A retrospective serological survey on human babesiosis in Belgium. Clin. Microbiol. Infect. 2015, 21, 96.e1–96.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, J.; Hunfeld, K.-P.; Persson, K.E.M. High seroprevalence of Babesia antibodies among Borrelia burgdorferi-infected humans in Sweden. Ticks Tick-Borne Dis. 2018, 10, 186–190. [Google Scholar] [CrossRef]
- Pianta, A.; Drouin, E.E.; Crowley, J.T.; Arvikar, S.; Strle, K.; Costello, C.E.; Steere, A.C. Annexin A2 is a target of autoimmune T and B cell responses associated with synovial fibroblast proliferation in patients with antibiotic-refractory Lyme arthritis. Clin. Immunol. 2015, 160, 336–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, J.T.; Drouin, E.E.; Pianta, A.; Strle, K.; Wang, Q.; Costello, C.; Steere, A.C. A Highly Expressed Human Protein, Apolipoprotein B-100, Serves as an Autoantigen in a Subgroup of Patients With Lyme Disease. J. Infect. Dis. 2015, 212, 1841–1850. [Google Scholar] [CrossRef] [Green Version]
- Drouin, E.E.; Seward, R.J.; Strle, K.; McHugh, G.; Katchar, K.; Londoño, D.; Yao, C.; Costello, C.; Steere, A.C. A novel human autoantigen, endothelial cell growth factor, is a target of T and B cell responses in patients with Lyme disease. Arthritis Rheum. 2012, 65, 186–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crowley, J.T.; Strle, K.; Drouin, E.E.; Pianta, A.; Arvikar, S.L.; Wang, Q.; Costello, C.E.; Steere, A.C. Matrix metalloproteinase-10 is a target of T and B cell responses that correlate with synovial pathology in patients with antibiotic-refractory Lyme arthritis. J. Autoimmun. 2016, 69, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Greco, T.P.; Conti-Kelly, A.M.; Greco, T.P. Antiphospholipid antibodies in patients with purported ‘chronic Lyme disease’. Lupus 2011, 20, 1372–1377. [Google Scholar] [CrossRef] [PubMed]
- Kratz, A.; Harding, M.W.; Craft, J.; Mackworth-Young, C.G.; Handschumacher, R.E. Autoantibodies against cyclophilin in systemic lupus erythematosus and Lyme disease. Clin. Exp. Immunol. 2008, 90, 422–427. [Google Scholar] [CrossRef]
- Chandra, A.; Wormser, G.P.; Klempner, M.S.; Trevino, R.P.; Crow, M.K.; Latov, N.; Alaedini, A. Anti-neural antibody reactivity in patients with a history of Lyme borreliosis and persistent symptoms. Brain Behav. Immun. 2010, 24, 1018–1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacek, E.; Fallon, B.A.; Chandra, A.; Crow, M.K.; Wormser, G.P.; Alaedini, A. Increased IFNα activity and differential antibody response in patients with a history of Lyme disease and persistent cognitive deficits. J. Neuroimmunol. 2012, 255, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Maccallini, P.; Bonin, S.; Trevisan, G. Autoimmunity against a glycolytic enzyme as a possible cause for persistent symptoms in Lyme disease. Med. Hypotheses 2018, 110, 1–8. [Google Scholar] [CrossRef]


| Number of Subject | 103 |
|---|---|
| Average Age (min.–max.) | 57.4 (6–90) years |
| Median of Age | 59 years |
| Females/Males | 46/57 |
| 0–20 years | 3 |
| 21–30 years | 8 |
| 31–40 years | 6 |
| 41–50 years | 19 |
| 51–60 years | 16 |
| 61–70 years | 21 |
| 71–80 years | 23 |
| 81+ years | 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sloupenska, K.; Dolezilkova, J.; Koubkova, B.; Hutyrova, B.; Racansky, M.; Horak, P.; Golovchenko, M.; Raska, M.; Rudenko, N.; Krupka, M. Seroprevalence of Antibodies against Tick-Borne Pathogens in Czech Patients with Suspected Post-Treatment Lyme Disease Syndrome. Microorganisms 2021, 9, 2217. https://doi.org/10.3390/microorganisms9112217
Sloupenska K, Dolezilkova J, Koubkova B, Hutyrova B, Racansky M, Horak P, Golovchenko M, Raska M, Rudenko N, Krupka M. Seroprevalence of Antibodies against Tick-Borne Pathogens in Czech Patients with Suspected Post-Treatment Lyme Disease Syndrome. Microorganisms. 2021; 9(11):2217. https://doi.org/10.3390/microorganisms9112217
Chicago/Turabian StyleSloupenska, Kristyna, Jana Dolezilkova, Barbora Koubkova, Beata Hutyrova, Mojmir Racansky, Pavel Horak, Maryna Golovchenko, Milan Raska, Natalie Rudenko, and Michal Krupka. 2021. "Seroprevalence of Antibodies against Tick-Borne Pathogens in Czech Patients with Suspected Post-Treatment Lyme Disease Syndrome" Microorganisms 9, no. 11: 2217. https://doi.org/10.3390/microorganisms9112217
APA StyleSloupenska, K., Dolezilkova, J., Koubkova, B., Hutyrova, B., Racansky, M., Horak, P., Golovchenko, M., Raska, M., Rudenko, N., & Krupka, M. (2021). Seroprevalence of Antibodies against Tick-Borne Pathogens in Czech Patients with Suspected Post-Treatment Lyme Disease Syndrome. Microorganisms, 9(11), 2217. https://doi.org/10.3390/microorganisms9112217







