Prediction of Insufficient Beta-Lactam Concentrations in Extracorporeal Membranous Oxygenation Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Extracorporeal Membrane Oxygenation
2.4. Beta-Lactam Antibiotics Treatment and TDM
2.5. Study Outcomes
2.6. Statistical Analysis
3. Results
3.1. Study Population
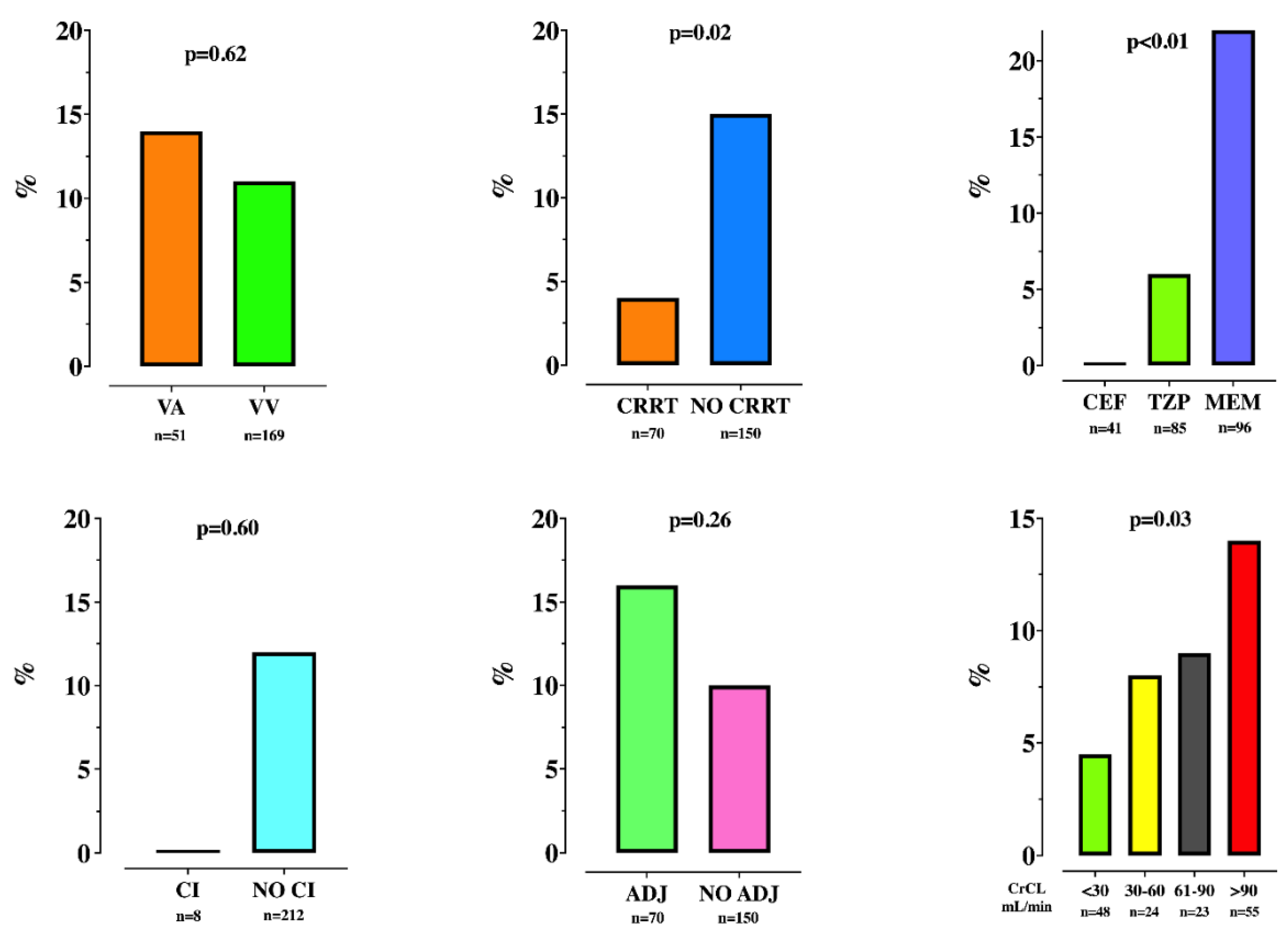
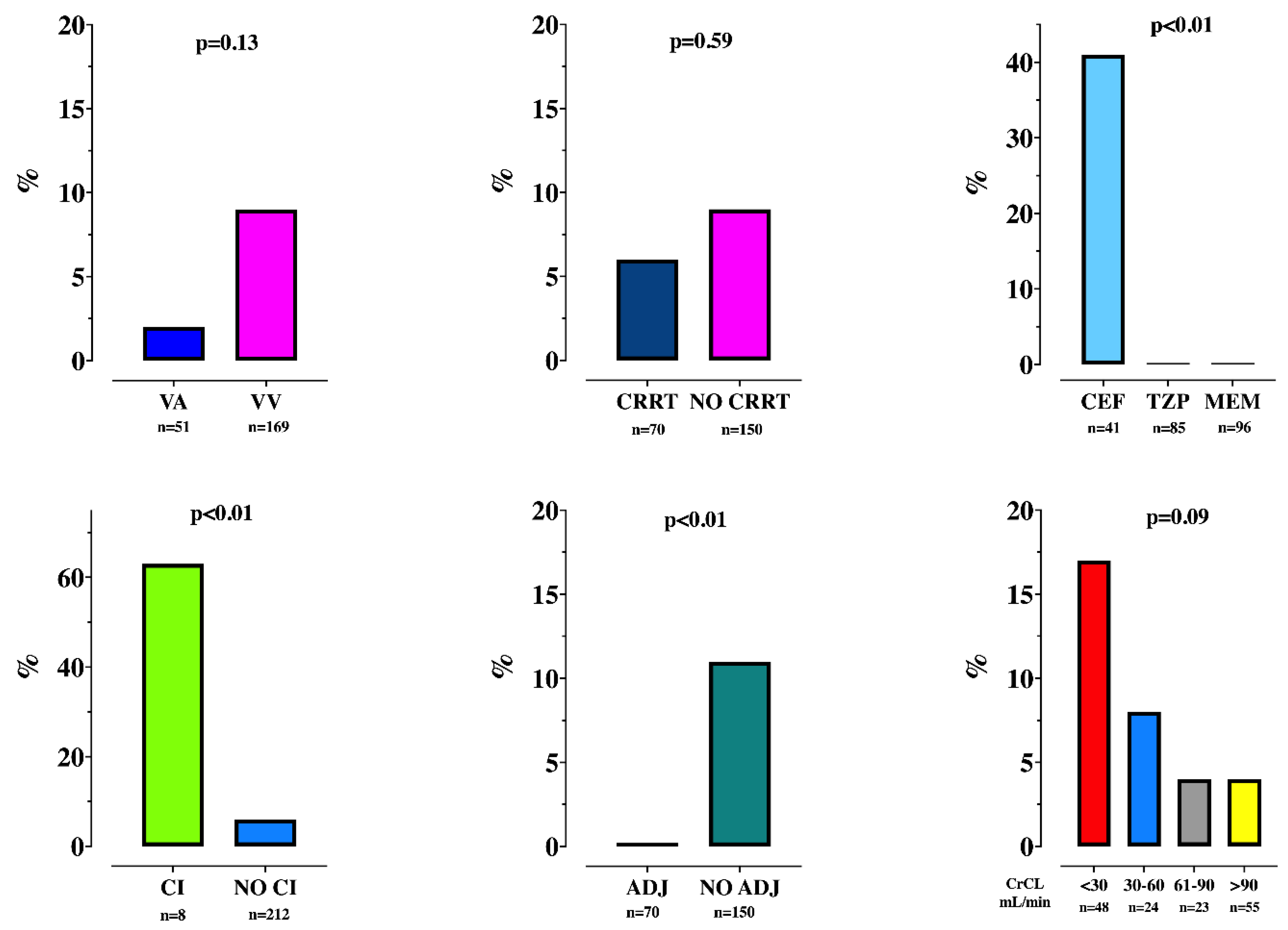
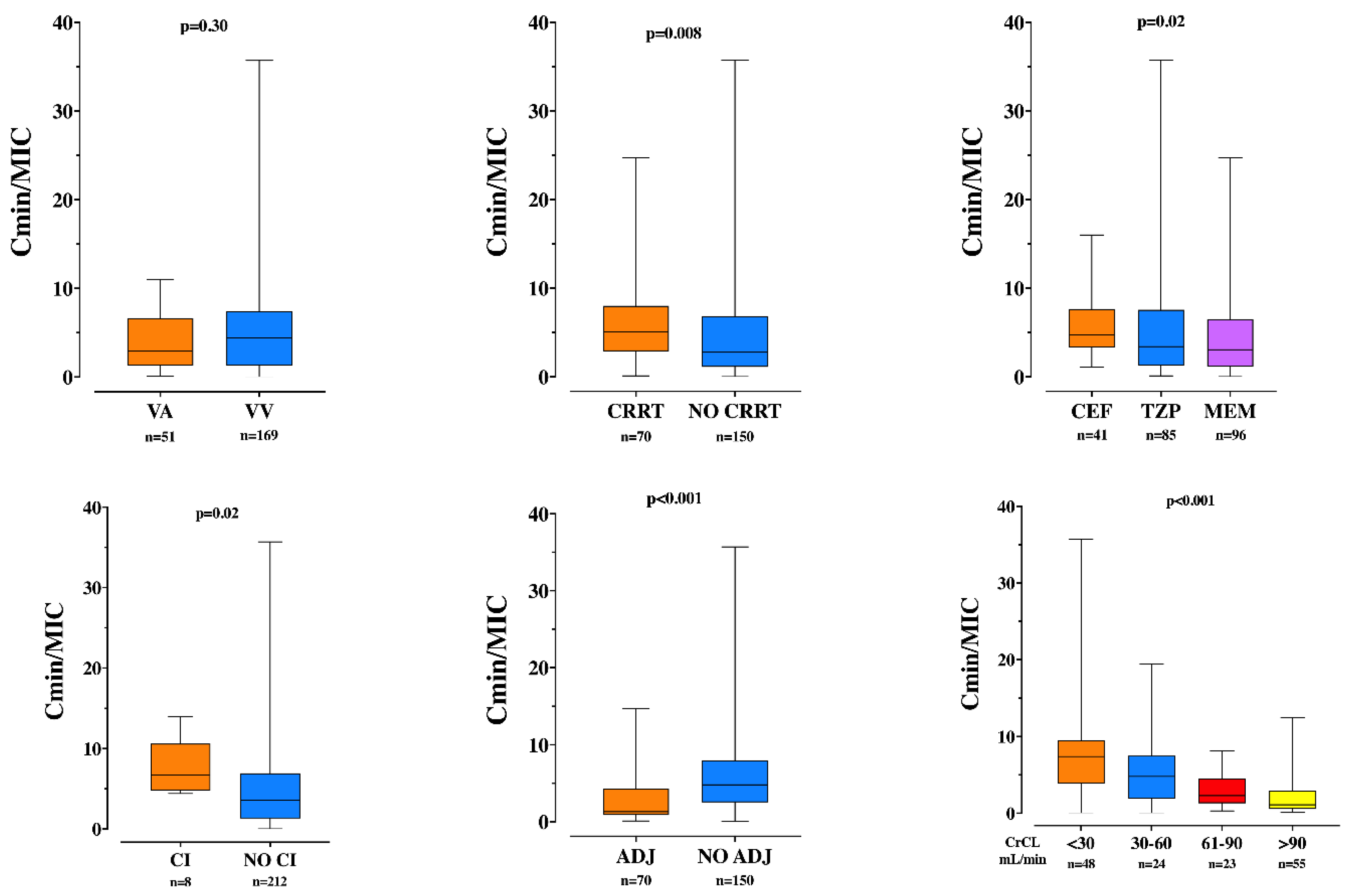
3.2. TDM and Insufficient Drug Concentrations
3.3. Secondary Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauer, C.M.; Yuh, D.D.; Bonde, P. Extracorporeal membrane oxygenation use has increased by 433% in adults in the United States from 2006 to 2011. ASAIO J. 2015, 61, 31–36. [Google Scholar] [CrossRef]
- Haneke, F.; Schildhauer, T.A.; Schlebes, A.D.; Strauch, J.T.; Swol, J. Infections and extracorporeal membrane oxygenation: Incidence, therapy, and outcome. ASAIO J. 2016, 62, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Annoni, F.; Grimaldi, D.; Taccone, F.S. Individualized antibiotic therapy in the treatment of severe infections. Expert Rev. Anti-Infect. Ther. 2020, 18, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Casu, G.S.; Hites, M.; Jacobs, F.; Cotton, F.; Wolff, F.; Beumier, M.; De Backer, D.; Vincent, J.L.; Taccone, F.S. Can changes in renal function predict variations in β-lactam concentrations in septic patients? Int. J. Antimicrob. Agents 2013, 42, 422–428. [Google Scholar] [CrossRef]
- Bouglé, A.; Dujardin, O.; Lepère, V.; Ait Hamou, N.; Vidal, C.; Lebreton, G.; Salem, J.E.; El-Helali, N.; Petijean, G.; Amour, J. Pharmecmo: Therapeutic drug monitoring and adequacy of current dosing regimens of antibiotics in patients on extracorporeal life support. Anaesth. Crit. Care Pain Med. 2019, 38, 493–497. [Google Scholar] [CrossRef]
- Donadello, K.; Antonucci, E.; Cristallini, S.; Roberts, J.A.; Beumier, M.; Scolletta, S.; Jacobs, F.; Rondelet, B.; de Backer, D.; Vincent, J.L.; et al. β-Lactam pharmacokinetics during extracorporeal membrane oxygenation therapy: A case-control study. Int. J. Antimicrob. Agents 2015, 45, 278–282. [Google Scholar] [CrossRef]
- Shekar, K.; Fraser, J.F.; Taccone, F.S.; Welch, S.; Wallis, S.C.; Mullany, D.V.; Lipman, J.; Roberts, J.A.; ASAP ECMO Study Investigators. The combined effects of extracorporeal membrane oxygenation and renal replacement therapy on meropenem pharmacokinetics: A matched cohort study. Crit. Care 2014, 18, 565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fillâtre, P.; Lemaitre, F.; Nesseler, N.; Schmidt, M.; Besset, S.; Launey, Y.; Maamar, A.; Daufresne, P.; Flecher, E.; Le Tulzo, Y.; et al. Impact of extracorporeal membrane oxygenation (ECMO) support on piperacillin exposure in septic patients: A case-control study. J. Antimicrob. Chemother. 2021, 76, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Beumier, M.; Casu, G.S.; Hites, M.; Wolff, F.; Cotton, F.; Vincent, J.L.; Jacobs, F.; Taccone, F.S. Elevated β-lactam concentrations associated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015, 81, 497–506. [Google Scholar]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A severity of disease classification system. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Vincent, J.L.; de Mendonça, A.; Cantraine, F.; Moreno, R.; Takala, J.; Suter, P.M.; Sprung, C.L.; Colardyn, F.; Blecher, S. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit. Care Med. 1998, 26, 1793–1800. [Google Scholar] [CrossRef]
- Pozzebon, S.; Blandino Ortiz, A.; Franchi, F.; Cristallini, S.; Belliato, M.; Lheureux, O.; Brasseur, A.; Vincent, J.L.; Scolletta, S.; Creteur, J.; et al. Cerebral near-infrared spectroscopy in adult patients undergoing veno-arterial extracorporeal membrane oxygenation. Neurocrit. Care 2018, 29, 94–104. [Google Scholar] [CrossRef]
- Delattre, I.K.; Musuamba, F.T.; Verbeeck, R.K.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wittebole, X.; Cumps, J.; Taccone, F.S.; Vincent, J.L.; et al. Empirical models for dosage optimization of four beta-lactams in critically ill septic patients based on therapeutic drug monitoring of amikacin. Clin. Biochem. 2010, 43, 589–598. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Alffenaar, J.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A position paper. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Taccone, F.S.; Laterre, P.F.; Dugernier, T.; Spapen, H.; Delattre, I.; Wittebole, X.; De Backer, D.; Layeux, B.; Wallemacq, P.; Vincent, J.L.; et al. Insufficient β-lactam concentrations in the early phase of severe sepsis and septic shock. Crit. Care 2010, 14, R126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hites, M.; Taccone, F.S.; Wolff, F.; Cotton, F.; Beumier, M.; De Backer, D.; Roisin, S.; Lorent, S.; Surin, R.; Seyler, L.; et al. Case-control study of drug monitoring of β-lactams in obese critically ill patients. Antimicrob. Agents Chemother. 2013, 57, 708–715. [Google Scholar] [CrossRef] [Green Version]
- Malfertheiner, M.V.; Broman, L.M.; Vercaemst, L.; Belliato, M.; Aliberti, A.; Di Nardo, M.; Swol, J.; Barrett, N.; Pappalardo, F.; Bělohlávek, J.; et al. Ex vivo models for research in extracorporeal membrane oxygenation: A systematic review of the literature. Perfusion 2020, 35 (Suppl. 1), 38–49. [Google Scholar] [CrossRef] [PubMed]
- Kühn, D.; Metz, C.; Seiler, F.; Wehrfritz, H.; Roth, S.; Alqudrah, M.; Becker, A.; Bracht, H.; Wagenpfeil, S.; Hoffmann, M.; et al. Antibiotic therapeutic drug monitoring in intensive care patients treated with different modalities of extracorporeal membrane oxygenation (ECMO) and renal replacement therapy: A prospective, observational single-center study. Crit. Care 2020, 24, 664. [Google Scholar] [CrossRef] [PubMed]
- Cheng, V.; Abdul-Aziz, M.H.; Burrows, F.; Buscher, H.; Cho, Y.J.; Corley, A.; Diehl, A.; Gilder, E.; Jakob, S.M.; Kim, H.S.; et al. Population pharmacokinetics of piperacillin and tazobactam in critically ill patients receiving extracorporeal membrane oxygenation (an ASAP ECMO study). Antimicrob. Agents Chemother. 2021. Epub ahead of print. [Google Scholar] [CrossRef]
- Gijsen, M.; Dreesen, E.; Annaert, P.; Nicolai, J.; Debaveye, Y.; Wauters, J.; Spriet, I. Meropenem pharmacokinetics and target attainment in critically Ill patients are not affected by extracorporeal membrane oxygenation: A matched cohort analysis. Microorganisms 2021, 9, 1310. [Google Scholar] [CrossRef]
- Hanberg, P.; Öbrink-Hansen, K.; Thorsted, A.; Bue, M.; Tøttrup, M.; Friberg, L.E.; Hardlei, T.F.; Søballe, K.; Gjedsted, J. Population pharmacokinetics of meropenem in plasma and subcutis from patients on extracorporeal membrane oxygenation treatment. Antimicrob. Agents Chemother. 2018, 62, e02390-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Ong, B.H.; Hoo, A.E.E.; Gao, F.; Chao, V.T.T.; Lim, C.H.; Tan, T.E.; Sin, K.Y.K. Prognostic factors for survival after extracorporeal membrane oxygenation for cardiogenic shock. ASAIO J. 2020, 66, 141–145. [Google Scholar] [CrossRef]
- Jacobs, A.; Taccone, F.S.; Roberts, J.A.; Jacobs, F.; Cotton, F.; Wolff, F.; Creteur, J.; Vincent, J.L.; Hites, M. β-Lactam dosage Regimens in septic patients with augmented renal clearance. Antimicrob. Agents Chemother. 2018, 62, e02534-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves-Pereira, J.; Silva, N.E.; Mateus, A.; Pinho, C.; Povoa, P. Assessment of pharmacokinetic changes of meropenem during therapy in septic critically ill patients. BMC Pharmacol. Toxicol. 2014, 15, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masich, A.M.; Heavner, M.S.; Gonzales, J.P.; Claeys, K.C. Pharmacokinetic/Pharmacodynamic considerations of beta-lactam antibiotics in adult critically Ill patients. Curr. Infect. Dis. Rep. 2018, 20, 9. [Google Scholar] [CrossRef] [PubMed]
| CrCL | >80 mL/min | 50–80 mL/min | 10–50 mL/min | <10 mL/min | CRRT |
|---|---|---|---|---|---|
| CAZ | 2 g q8h | 2 g q12h | 2 g q12h | 0.5 g daily | 2 g q8h |
| CEF | 2 g q8h | 2 g q12h | 2 g q12h | 0.5 g daily | 2 g q8h |
| PTAZ | 4 g q6h | 4 g q6h | 4 g q8h | 4 g q12h | 4 g q6h |
| MERO | 1 g q8h | 1 g q12h | 0.5 g q12h | 0.5 g daily | 1 g q8h |
| Overall (n = 110) | |
|---|---|
| Demographics | |
| Age, years | 53 [41–63] |
| Male gender, n (%) | 69 (63) |
| ICU length of stay, days | 19 [12–31] |
| ICU mortality, n (%) | 61 (55) |
| VA ECMO, n (%) | 33 (30) |
| Weight, kg | 75 [62–85] |
| BMI, kg/m2 | 25 [22–28] |
| Comorbidities | |
| Cancer, n (%) | 13 (12) |
| COPD, n (%) | 15 (14) |
| Diabetes, n (%) | 26 (24) |
| Heart disease, n (%) | 36 (33) |
| Chronic kidney disease, n (%) | 17 (15) |
| Chronic dialysis, n (%) | 11 (10) |
| Liver cirrhosis, n (%) | 6 (5) |
| HIV, n (%) | 1 (1) |
| Solid organ transplantation, n (%) | 23 (21) |
| Other immunosuppression, n (%) | 34 (31) |
| Blood sample tests on admission | |
| White cell count, n/mm3 | 13,350 [9225–19,300] |
| Hematocrit, % | 31 [28–37] |
| Platelets, n/mm3 | 175,000 [104,500–272,500] |
| Sodium, mEq/L | 139 [136–143] |
| Potassium, mEq/L | 4.1 [3.8–4.7] |
| Bicarbonate, mEq/L | 25 [21–30] |
| Creatinine, mg/dL | 1 [0.7–1.8] |
| C-reactive protein, mg/L | 130 [63–210] |
| Total bilirubin, mg/dL | 1.0 [0.4–1.2] |
| Parameters on the first day of ICU | |
| Urine output, mL/day | 1188 [605–1742] |
| Glasgow Coma Score | 3 [3–11] |
| Temperature, °C | 37 [36.4–37.7] |
| Heart rate, bpm | 105 [85–120] |
| Mean arterial pressure, mmHg | 73 [67–82] |
| Respiratory rate, rpm | 25 [16–31] |
| pH | 7 [7.3–7.4] |
| PaO2, mmHg | 76 [62–100] |
| PaCO2, mmHg | 42 [36–49] |
| FiO2, % | 0.6 [0.5–0.8] |
| Lactate, mmol/L | 2.0 [1.2–2.5] |
| Mechanical ventilation, n (%) | 110 (100) |
| APACHE II score | 24 [17–29] |
| Characteristics of infections | |
| Infections | |
| Respiratory tract | 78 (71) |
| Urinary tract | 1 (1) |
| Abdominal | 10 (9) |
| Skin | 3 (3) |
| Catheter | 4 (3) |
| Primary bacteremia | 1 (1) |
| Mediastinitis | 2 (2) |
| Unknown | 11 (10) |
| Positive blood cultures, n (%) | 37 (34) |
| Identified micro-organisms | |
| No pathogen | 19 (17) |
| Methicillin-Susceptible-Staphylococcus Aureus | 3 (3) |
| Methicillin-Resistant-Staphylococcus Aureus | 5 (5) |
| Pseudomonas aeruginosa | 24 (22) |
| Enterobacter spp. | 8 (7) |
| Staphylococcus epidermidis | 4 (3) |
| Enterococcus spp. | 5 (5) |
| Acinetobacter spp. | 1 (1) |
| Citrobacter spp. | 2 (2) |
| Klebsiella pneumoniae | 11 (10) |
| E. Coli | 8 (7) |
| Streptococcus pneumoniae | 2 (2) |
| Others | 19 (17) |
| All (n = 222) | Sufficient (n = 196) | Insufficient (n = 26) | p Values | |
|---|---|---|---|---|
| Antibiotics | <0.01 | |||
| Cefepime-Ceftazidime | 41 (19) | 41 (21) | 0 (0) | |
| Piperacilline/Tazobactam | 85 (38) | 80 (41) | 5 (19) | |
| Meropenem | 96 (43) | 75 (38) | 21 (81) | |
| ICU admission to antibiotics, days | 9 [3–15] | 9 [4–16] | 8 [3–13] | 0.34 |
| Start antibiotics to TDM, days | 3 [1–6] | 3 [1–6] | 2 [1–4] | 0.04 |
| Single antibiotic dose, g | 2 [1–4] | 2 [1–4] | 1 [1, 2] | <0.01 |
| Intervals between doses, hours | 0.08 | |||
| 6 | 72 (32) | 67 (34) | 5 (19) | |
| 8 | 119 (54) | 99 (51) | 20 (77) | |
| 12 | 23 (10) | 22 (11) | 1 (4) | |
| 24 | 8 (4) | 8 (4) | 0 (0) | |
| Cmin | 18.0 [6.0–55.0] | 22.0 [9.0–61.0] | 1.7 [1.0–2.0] | <0.01 |
| Cmin/MIC | 8.6 [2.9–26.9] | 11 [4.65–30.55] | 0.7 [0.35–1.00] | <0.01 |
| Toxic levels, n (%) | 17 (8) | 17 (9) | - | 0.23 |
| Continuous infusion, n (%) | 8 (4) | 8 (4) | - | 0.6 |
| Adequate regimen, n (%) | 70 (32) | 59 (30) | 11 (42) | 0.26 |
| Day of Antibiotic Measurement | ||||
| Plasmatic creatinine, mg/dL | 1.00 [0.60–1.80] | 1.1 [0.60–1.89] | 0.6 [0.46–1.0] | <0.01 |
| CrCL, mL/min | 31 [6–97] | 24 [5–77] | 90 [34–175] | <0.01 |
| Mechanical ventilation, n (%) | 216 (97) | 190 (97) | 26 (100) | 1 |
| Fluid balance, mL/Day | 925 [−300 to 2300] | 925 [−321 to 2287] | 818 [217–3100] | 0.29 |
| Vasopressors, n (%) | 177 (80) | 155 (79) | 22 (85) | 0.61 |
| Platelets, n/mm3 | 94,500 [50,000–158,000] | 93,500 [49,000–156,500] | 106,500 [68,000–186,000] | 0.18 |
| Bilirubin, mg/dL | 0.91 [0.49–2.30] | 0.92 [0.47–2.55] | 0.90 [0.65–1.7] | 0.99 |
| Inotropes, n (%) | 43 (90) | 39 (20) | 4 (15) | 0.79 |
| Glasgow Coma Scale, n | 3 [3–10] | 3 [3–10] | 4 [3–10] | 0.54 |
| SOFA, n | 12 [10–15] | 13 [10–15] | 11 [9–13] | 0.04 |
| CRRT, n (%) | 70 (32) | 67 (34) | 3 (12) | 0.02 |
| VA ECMO, n (%) | 51 (23) | 44 (22) | 7 (27) | 0.62 |
| ECMO flow, L/min | 4.0 [3.6–4.7] | 4.0 [3.5–4.7] | 4 [3.6–4.3] | 0.52 |
| ECMO gas flow, L/min | 6 [5–9] | 7 [5–9] | 6 [4–8] | 0.44 |
| Lactate, mmol/L | 1.6 [1–2.4] | 1.6 [1.0–2.5] | 1.5 [1.1–2.1] | 0.99 |
| Bleeding with transfusion, n (%) | 80 (36) | 74 (38) | 6 (23) | 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polain, A.; Gorham, J.; Romeo, I.; Belliato, M.; Peluso, L.; Partipilo, F.; Njimi, H.; Brasseur, A.; Jacobs, F.; Creteur, J.; et al. Prediction of Insufficient Beta-Lactam Concentrations in Extracorporeal Membranous Oxygenation Patients. Microorganisms 2021, 9, 2219. https://doi.org/10.3390/microorganisms9112219
Polain A, Gorham J, Romeo I, Belliato M, Peluso L, Partipilo F, Njimi H, Brasseur A, Jacobs F, Creteur J, et al. Prediction of Insufficient Beta-Lactam Concentrations in Extracorporeal Membranous Oxygenation Patients. Microorganisms. 2021; 9(11):2219. https://doi.org/10.3390/microorganisms9112219
Chicago/Turabian StylePolain, Amandine, Julie Gorham, Immacolata Romeo, Mirko Belliato, Lorenzo Peluso, Francesco Partipilo, Hassane Njimi, Alexandre Brasseur, Frederique Jacobs, Jacques Creteur, and et al. 2021. "Prediction of Insufficient Beta-Lactam Concentrations in Extracorporeal Membranous Oxygenation Patients" Microorganisms 9, no. 11: 2219. https://doi.org/10.3390/microorganisms9112219
APA StylePolain, A., Gorham, J., Romeo, I., Belliato, M., Peluso, L., Partipilo, F., Njimi, H., Brasseur, A., Jacobs, F., Creteur, J., Hites, M., & Taccone, F. S. (2021). Prediction of Insufficient Beta-Lactam Concentrations in Extracorporeal Membranous Oxygenation Patients. Microorganisms, 9(11), 2219. https://doi.org/10.3390/microorganisms9112219







