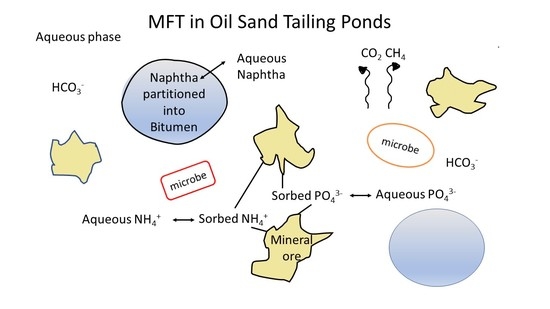
Does Addition of Phosphate and Ammonium Nutrients Affect Microbial Activity in Froth Treatment Affected Tailings?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Description of MFT, Its Collection and Storage
2.3. Microcosm Set Up
2.4. Gas Analysis
2.5. Pore Water Analysis
2.5.1. pH and Eh
2.5.2. Water Chemistry
2.6. Total Dried Solids
2.7. X-ray Diffraction
3. Results and Discussion
3.1. Characterization of MFT
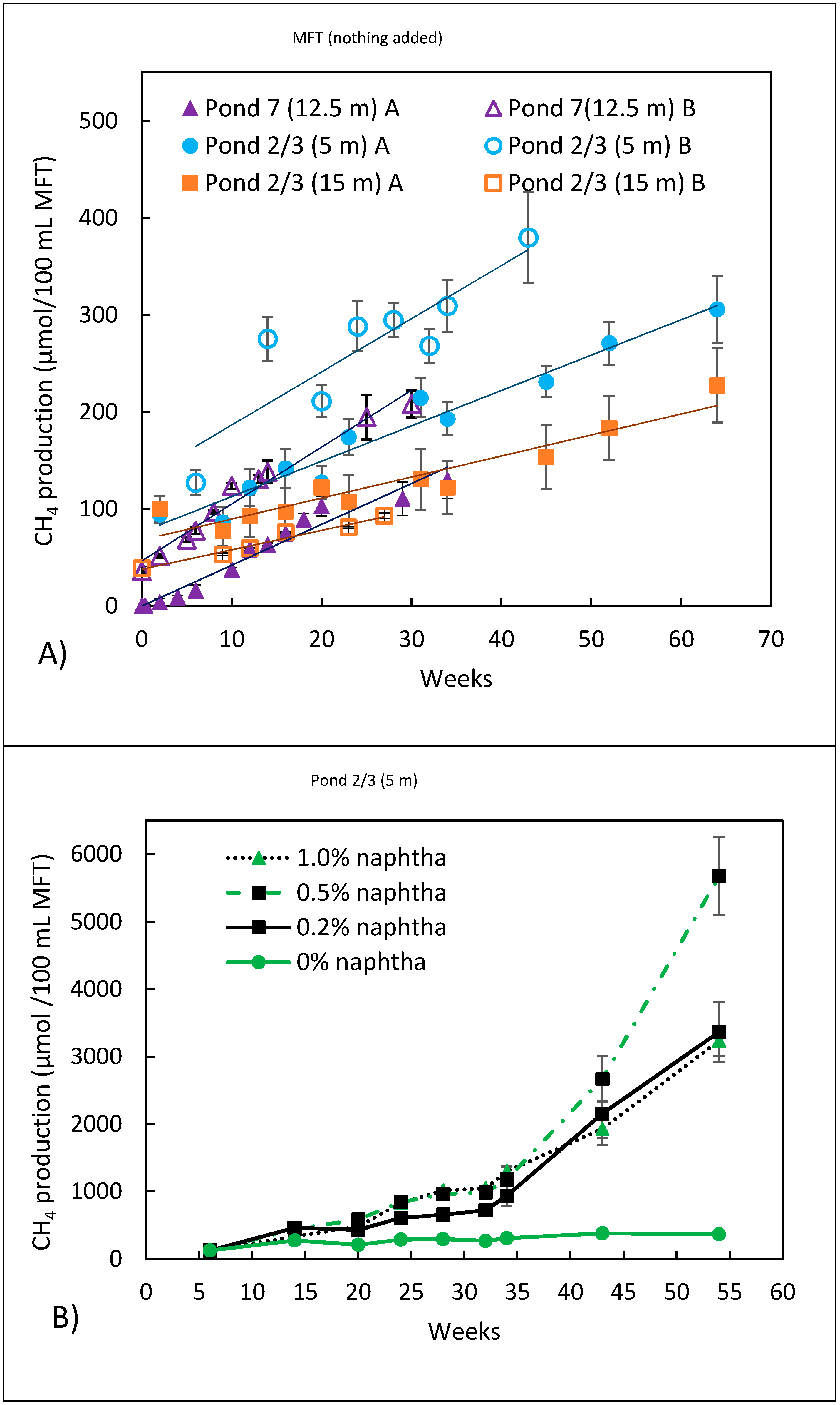
3.2. Methanogenic Metabolism
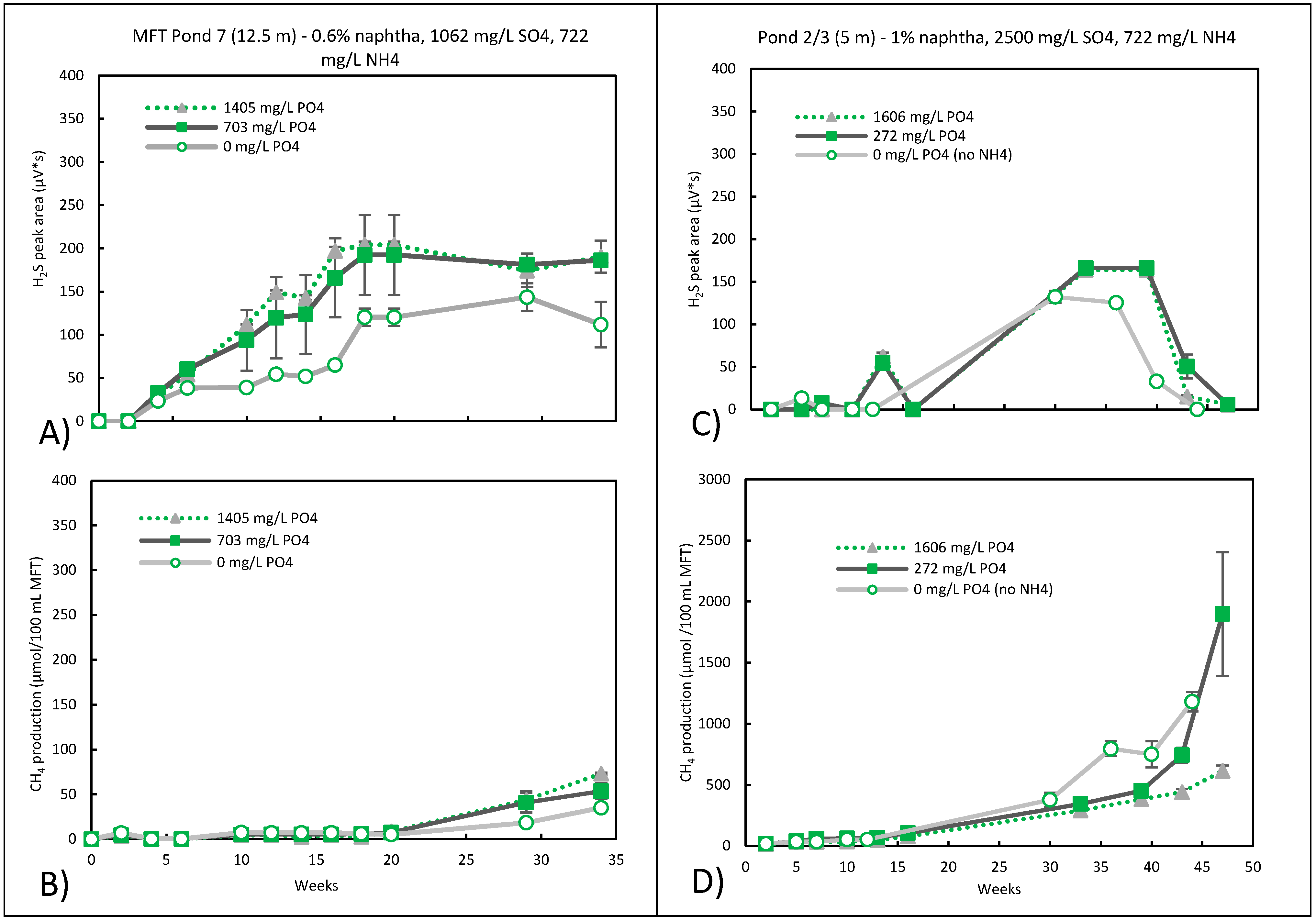
3.3. Sulfidogenic Metabolism
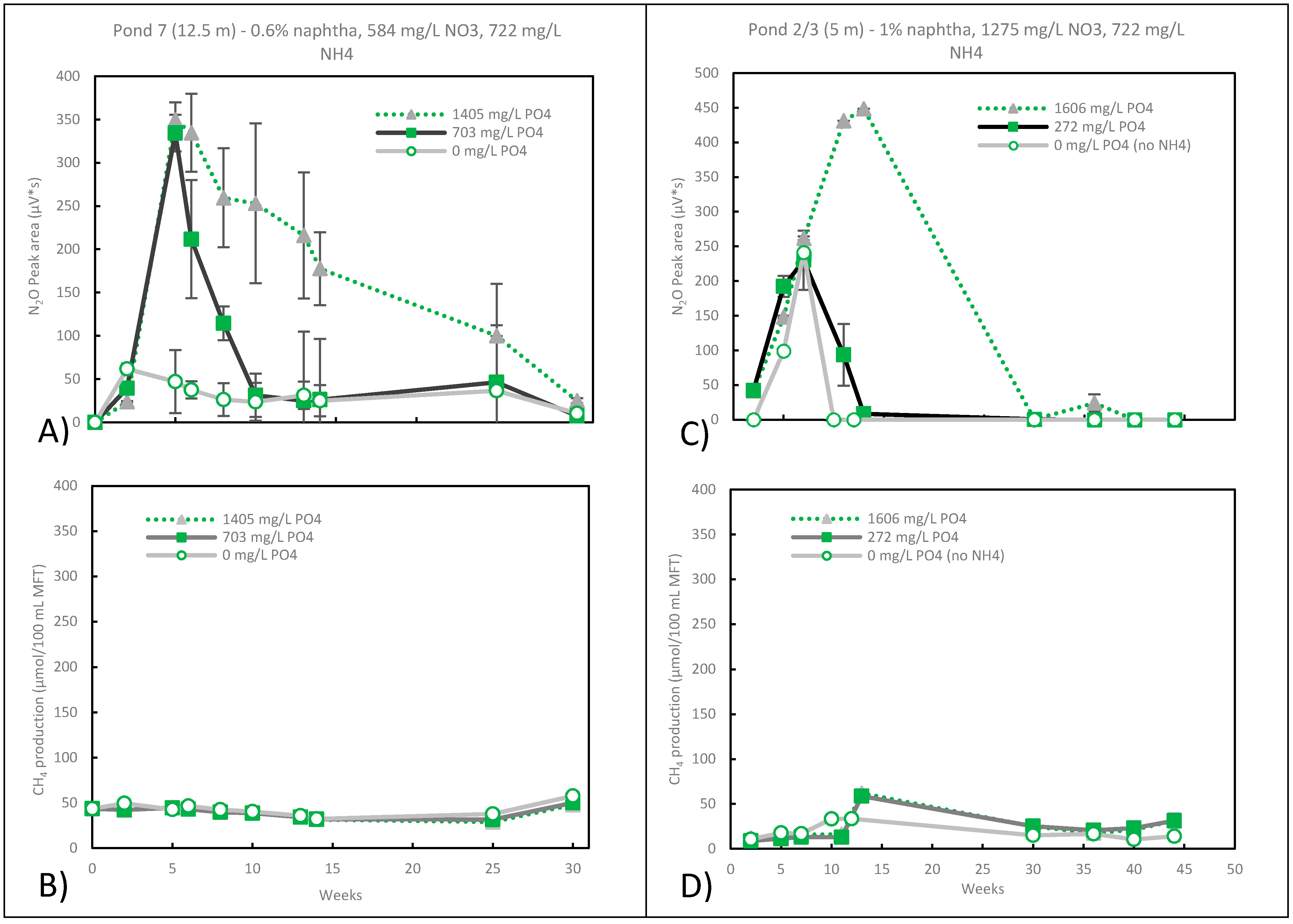
3.4. Nitrate Reduction
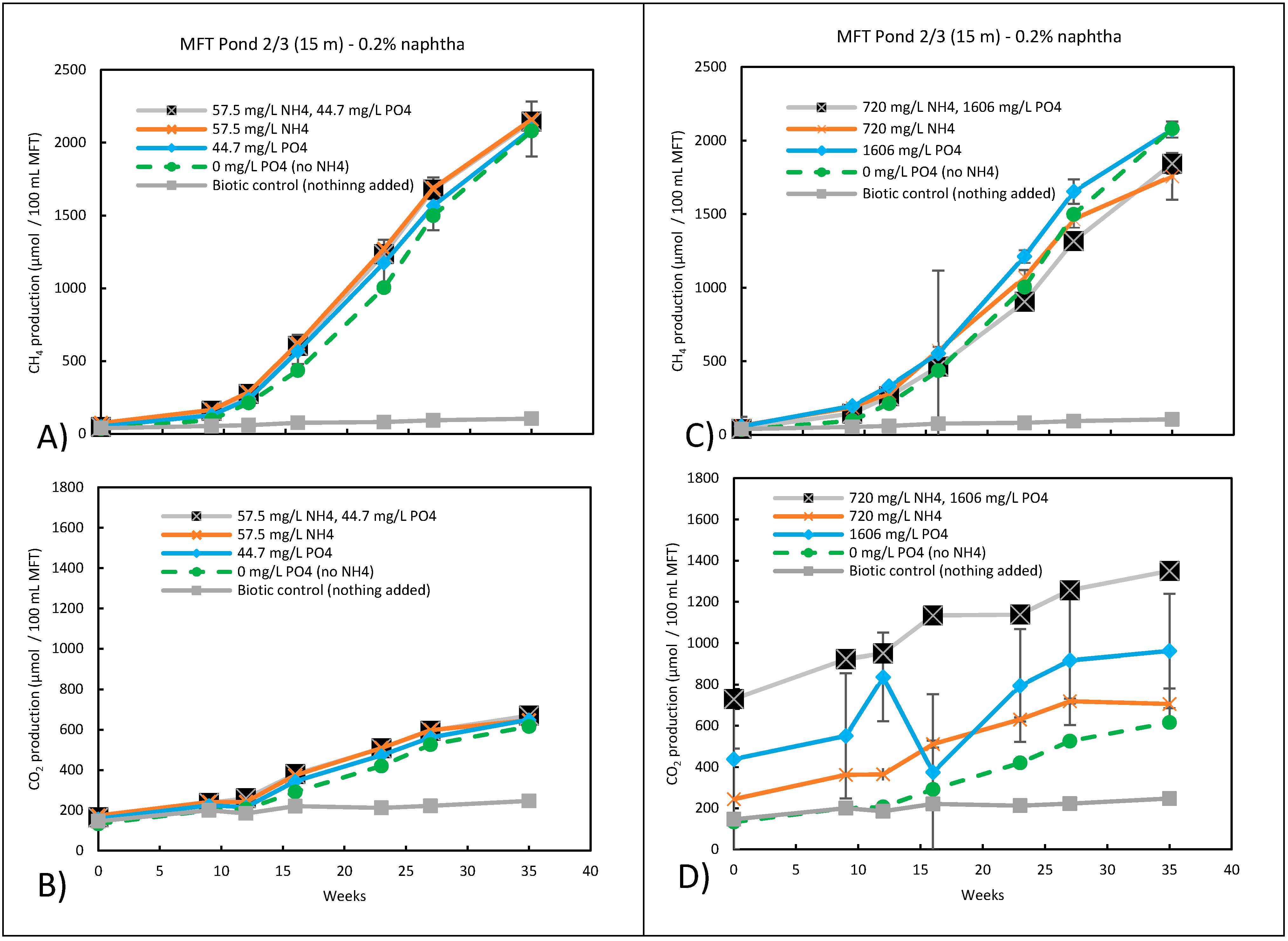
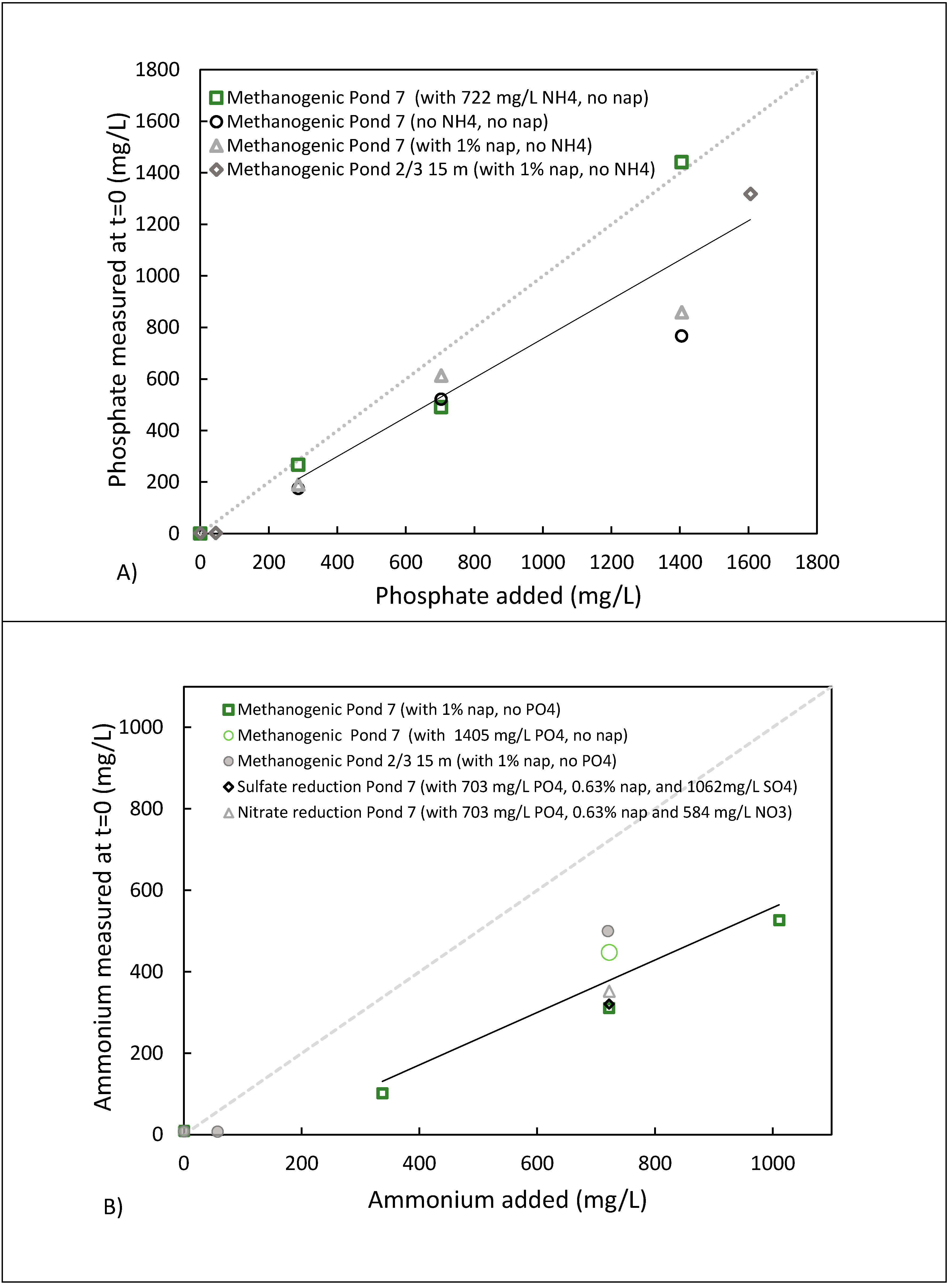
3.5. Potential Fate of Ammonium and Phosphate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crude Oil Reserves. Available online: https://countryeconomy.com/energy-and-environmen/crude-oil/reserves (accessed on 8 September 2021).
- IG. Available online: https://www.ig.com/en/trading-strategies/world-s-biggest-oil-producers-200722 (accessed on 8 September 2021).
- CAPP. Available online: https://www.capp.ca/oil/what-are-the-oil-sands/ (accessed on 6 September 2021).
- Suncor, E.H. Personal communication, August 2021.
- US EPA. Available online: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (accessed on 8 September 2021).
- Fedorak, P.M.; Hrudey, S.E. The effects of phenol and some alkyl phenolics on batch anaerobic methanogenesis. Water Res. 1984, 18, 361–367. [Google Scholar] [CrossRef]
- Fedorak, P.M.; Coy, D.L.; Salloum, M.J.; Dudas, M.J. Methanogenic potential of tailings samples from oil sands extraction plants. Can. J. Microbiol. 2002, 48, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Holowenko, F.M.; MacKinnon, M.D.; Fedorak, P.M. Methanogens and sulfate-reducing bacteria in oil sands fine tailings waste. Can. J. Microbiol. 2000, 46, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Fedorak, P.M.; Foght, J.M. Biodegradation of short-chain n-alkanes in oil sands tailings under methanogenic conditions. Environ. Sci. Technol. 2006, 40, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Fedorak, P.M.; MacKinnon, M.D.; Foght, J.M. Metabolism of BTEX and Naphtha Compounds to Methane in Oil Sands Tailings. Environ. Sci. Technol. 2007, 41, 2350–2356. [Google Scholar] [CrossRef]
- Siddique, T.; Penner, T.; Semple, K.; Foght, J.M. Anaerobic biodegradation of longer-Chain n -alkanes coupled to methane production in oil sands tailings. Environ. Sci. Technol. 2011, 45, 5892–5899. [Google Scholar] [CrossRef] [PubMed]
- Siddique, T.; Penner, T.; Klassen, J.; Nesbø, C.; Foght, J.M. Microbial communities involved in methane production from hydrocarbons in oil sands tailings. Environ. Sci. Technol. 2012, 46, 9802–9810. [Google Scholar] [CrossRef]
- Stasik, S.; Wendt-Potthoff, K. Interaction of microbial sulphate reduction and methanogenesis in oil sands tailings ponds. Chemosphere 2014, 103, 59–66. [Google Scholar] [CrossRef]
- Shahimin, M.M.F.; Siddique, T. Methanogenic biodegradation of paraffinic solvent hydrocarbons in two different oil sands tailings. Sci. Total Environ. 2017, 583, 115–122. [Google Scholar] [CrossRef]
- Collins, C.E.V.; Foght, J.M.; Siddique, T. Co-occurrence of methanogenesis and N2 fixation in oil sands tailings. Sci. Total Environ. 2016, 565, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Hungate, R.E. A roll tube method for cultivation of strict anaerobes. Method. Microbiol. 1969, 3B, 117–132. [Google Scholar]
- APHA. Standard Methods for the Examination of Water, 22nd ed.; American Public Health Assn: Washington, DC, USA, 2012. [Google Scholar]
- Symons, G.E.; Morey, B. The effect of drying time on the determination of solids in sewage and sewage sludges. Sewage Work J. 1941, 13, 936–939. [Google Scholar]
- Colleran, E.; Pender, S.; Philpott, U.; O’Flaherty, V.; Leahy, B. Full-scale and laboratory-scale anaerobic treatment of citric acid production wastewater. Biodegradation 1998, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Rinzema, A.; Lettinga, G. The effect of sulphide on the anaerobic degradation of propionate. Environ. Technol. Lett. 1988, 9, 83–88. [Google Scholar] [CrossRef]
- Visser, A.; Nozhevnikova, A.N.; Lettinga, G. Sulphide inhibition of methanogenic activity at various pH levels at 55 °C. J. Chem. Technol. Biotechnol. 1993, 57, 9–13. [Google Scholar] [CrossRef]
- Siddique, T.; Semple, K.; Li, C.; Foght, J.M. Methanogenic biodegradation of iso-alkanes and cycloalkanes during long-term incubation with oil sands tailings. Environ. Pollut. 2020, 258, 113768. [Google Scholar] [CrossRef]
- Small, C.C.; Cho, S.; Hashisho, Z.; Ulrich, A.C. Emissions from oil sands tailings ponds: Review of tailings pond parameters and emission estimates. J. Pet Sci. Eng. 2015, 127, 490–501. [Google Scholar] [CrossRef]
- Colosimo, F.; Thomas, R.; Lloyd, J.R.; Taylor, K.G.; Boothman, C.; Smith, A.D.; Lord, R.; Kalin, R.M. Biogenic methane in shale gas and coal bed methane: A review of current knowledge and gaps. Int. J. Coal Geol. 2016, 165, 106–120. [Google Scholar] [CrossRef] [Green Version]
- Laban, N.A.; Dao, A.; Semple, K.; Foght, J. Biodegradation of C7 and C8 iso-alkanes under methanogenic conditions: Methanogenic iso-alkane biodegradation. Environ. Microbiol. 2015, 17, 4898–4915. [Google Scholar] [CrossRef] [PubMed]
- Takashima, M.; Speece, R.R. Mineral nutrient requirements for high-rate methane fermentation of acetate at low SRT. Res. J. Water Pollut. Control Fed. 1989, 61, 1645–1650. [Google Scholar]
- Paulo, L.M.; Stams, A.J.M.; Sousa, D.Z. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Biotechnol. 2015, 14, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Luther, G.W.; Findlay, A.J.; MacDonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2011, 2, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, I.A.; Kockelkorn, D.; Ramos-Vera, W.H.; Say, R.F.; Zarzycki, J.; Hügler, M.; Alber, B.E.; Fuchs, G. Autotrophic carbon fixation in archaea. Nat. Rev. Microbiol. 2010, 8, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Padrón, E.; Bordenave, S.; Linn, S.; Bhaskar, I.M.; Dong, X.; Sensen, C.W.; Fournier, J.; Voordouw, G.; Gieg, L.M. Carbon and sulfur cycling by microbial communities in a gypsum-treated oil sands tailings pond. Environ. Sci. Technol. 2011, 45, 439–446. [Google Scholar] [CrossRef]
- Schulthess, R.; Kühni, M.; Gujer, W. Release of nitric and nitrous oxides from denitrifying activated sludge. Water Res. 1995, 29, 215–226. [Google Scholar] [CrossRef]
- Betlach, M.R.; Tiedje, J.M. Kinetic explanation for accumulation of nitrite, nitric oxide, and nitrous oxide during bacterial denitrification. Appl. Environ. Microbiol. 1981, 42, 1074–1108. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Pijuan, M.; Zeng, R.J.; Yuan, Z. Free nitrous acid inhibition on nitrous oxide reduction by a denitrifying-enhanced biological phosphorus removal sludge. Environ. Sci. Technol. 2008, 42, 8260–8265. [Google Scholar] [CrossRef] [PubMed]
- Klüber, H.D.; Conrad, R. Inhibitory effects of nitrate, nitrite, NO and N2O on methanogenesis by Methanosarcina barkeri and Methanobacterium bryantii. FEMS Microbiol. Ecol. 1998, 25, 331–339. [Google Scholar] [CrossRef]
- Banihani, Q.; Sierra-Alvarez, R.; Field, J.A. Nitrate and nitrite inhibition of methanogenesis during denitrification in granular biofilms and digested domestic sludges. Biodegradation 2009, 20, 801–812. [Google Scholar] [CrossRef]
- Coulon, F.; Whelan, M.J.; Paton, G.I.; Semple, K.T.; Villa, R.; Pollard, S.J.T. Multimedia fate of petroleum hydrocarbons in the soil: Oil matrix of constructed biopiles. Chemosphere 2010, 81, 1454–1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, M. Biodegradation and Bioremediation; Academic Press: New York, NY, USA, 1994; pp. 138–139. [Google Scholar]
- Stroud, J.L.; Paton, G.I.; Semple, K.T. Microbe-aliphatic hydrocarbon interactions in soil: Implications for biodegradation and bioremediation. J. Appl. Microbiol. 2007, 102, 1239–1253. [Google Scholar] [CrossRef]
- Nishizawa, M.; Miyazaki, J.; Makabe, A.; Koba, K.; Takai, K. Physiological and isotopic characteristics of nitrogen fixation by hyperthermophilic methanogens: Key insights into nitrogen anabolism of the microbial communities in Archean hydrothermal systems. Geochim. Cosmochim. Acta 2014, 138, 117–135. [Google Scholar] [CrossRef]
- Bae, H.-S.; Morrison, E.; Chanton, J.P.; Ogram, A. Methanogens are major contributors to nitrogen fixation in soils of the Florida Everglades. Appl. Environ. Microbiol. 2018, 84, e02222-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Offre, P.; Spang, A.; Schleper, C. Archaea in Biogeochemical Cycles. Annu. Rev. Microbiol. 2013, 67, 437–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gérard, F. Clay minerals, iron/aluminum oxides, and their contribution to. Geoderma 2016, 262, 213–226. [Google Scholar] [CrossRef]
- Gimsing, A.L.; Borggaard, O.K. Competitive adsorption and desorption of glyphosate and phosphate on clay silicates and oxides. Clay Miner. 2002, 37, 509–515. [Google Scholar] [CrossRef]
- Edzwald, J.K.; Toensing, D.C.; Leung, M.C.-Y.Y. Phosphate adsorption reactions with clay minerals. Environ. Sci. Technol. 1976, 10, 485–490. [Google Scholar] [CrossRef]
- He, L.M.; Zelazny, L.W.; Baligar, V.C.; Ritchey, K.D.; Martens, D.C. Ionic Strength Effects on Sulfate and Phosphate Adsorption on γ-Alumina and Kaolinite: Triple-Layer Model. Soil Sci. Soc. Am. J. 1997, 61, 784–793. [Google Scholar] [CrossRef]
- Moharami, S.; Jalali, M. Removal of phosphorus from aqueous solution by Iranian natural. Chem. Eng. J. 2013, 223, 328–339. [Google Scholar] [CrossRef]
- Ioannou, A.; Dimirkou, A. Phosphate adsorption on hematite, kaolinite, and kaolinite–hematite. J. Colloid Interface Sci. 1997, 192, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Tan, W.; Liu, F.; Zhao, W.; Weng, L. Surface properties and phosphate adsorption of binary systems containing goethite and kaolinite. Geoderma 2014, 213, 478–484. [Google Scholar] [CrossRef]
- El-Shafey, O.I.; Fathy, N.A.; El-Nabarawy, T.A. Sorption of ammonium ions onto natural and modified Egyptian kaolinites: Kinetic and equilibrium studies. Adv. Phys. Chem. 2014, 935854. [Google Scholar] [CrossRef] [Green Version]
- Alshameri, A.; He, H.; Zhu, J.; Xi, Y.; Zhu, R.; Ma, L.; Tao, Q. Adsorption of ammonium by different natural clay minerals: Characterization, kinetics, and adsorption isotherms. Appl. Clay Sci. 2018, 159, 83–93. [Google Scholar] [CrossRef]
- Jiang, C.; Jia, L.; Zhang, B.; He, Y.; Kirumba, G. Comparison of quartz sand, anthracite, shale and biological ceramsite for adsorptive removal of phosphorus from aqueous solution. J. Environ. Sci. 2014, 26, 466–477. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Nutrients removal from urban stormwater by different filter materials. Water Air Soil Pollut. 2014, 225, 1778. [Google Scholar] [CrossRef]
- Perrott, K.W.; Langdon, A.G.; Wilson, A.T. Sorption of anions by the cation exchange surface of muscovite. J. Colloid Interface Sci. 1974, 48, 10–19. [Google Scholar] [CrossRef]
- Freeman, J.S.; Rowell, D.L. The adsorption and precipitation of phosphate onto calcite. J. Soil Sci. 1981, 32, 75–84. [Google Scholar] [CrossRef]
- Geelhoed, J.S.; Hiemstra, T.; Van Riemsdijk, W.H. Phosphate and sulfate adsorption on goethite: Single anion and competitive adsorption. Geochim. Cosmochim. Acta 1997, 61, 2389–2396. [Google Scholar] [CrossRef]
- Lazaratou, C.; Lazaratou, C.V.; Vayenas, D.V.; Papoulis, D. The role of clays, clay minerals and clay-based materials for nitrate removal from water systems: A review. Appl. Clay Sci. 2020, 185, 105377. [Google Scholar] [CrossRef]
- Mazloomi, F.; Jalali, M. Adsorption of ammonium from simulated wastewater by montmorillonite nanoclay and natural vermicullite: Experimental study and simulation. Environ. Monitor Assess. 2017, 189, 415. [Google Scholar] [CrossRef] [PubMed]
- Muljadi, D.; Posner, A.M.; Quirk, J.P. The mechanism of phosphate adsorption by kaolinite, gibbsite, and pseudoboehmite. Part II. The location of adsorption sites. Eur. J. Soil Sci. 1966, 17, 230–237. [Google Scholar] [CrossRef]
- Farquhar, M.L.; Vaughan, D.J.; Hughes, C.R.; Charnock, J.M.; England, K.E.R. Experimental studies of the interaction of aqueous metal cations with mineral substrates: Lead, cadmium, and copper with perthitic feldspar, muscovite, and biotite. Geochim. Cosmochim. Acta 1997, 61, 3051–3064. [Google Scholar] [CrossRef]
| Parameter | Pond 2/3 at 5 m | Pond 2/3 at 15 m | Pond 7 at 12.5 m |
|---|---|---|---|
| PO43− (mg/L) | <3.1 | <3.1 | <3.1 |
| NH4+ (mg/L) | 20.8 ± 0.5 | 35.5 ± 0.7 | 6.4 ± 2.4 |
| SO42− (mg/L) | 20 | 100 | < 1.0 |
| NO3− (mg/L) | <0.5 | <0.5 | <0.5 |
| NO2− (mg/L) | <0.33 | <0.33 | <0.33 |
| Eh (mV) | −123 | −153 | −200 ± 40 |
| Naphthenic Acid (μg/L) a | 15,100 | 54,700 | 27,600 |
| pH | 8.41 ± 0.1 | 8.39 ± 0.1 | 8.11 ± 0.4 |
| Alkalinity, Bicarbonate (mg/L) a | 2100 | 1900 | 1900 |
| DOC (mg/L) a | 56 | 24 | 46 |
| Hardness (mg CaCO3/L) a | 240 | 180 | 160 |
| Total dried solids (g/L) | 743 ± 11.47 | 1000 ± 3.30 | 415 ± 4.77 |
| Unamended MFT (Nothing Added) | pH | Redox (mV) | CH4 Generation Rate (µmole/100 mL MFT/Day) | ||
|---|---|---|---|---|---|
| Initial | Final | Initial | Final | ||
| Pond 7 at 12.5 m | 7.89 ± 0.1 | 7.82 ± 0.1 | −136 ± 11 | −247 ± 4 | 0.72 ± 0.12 |
| Pond 2/3 at 5 m | 7.13 ± 0.3 | 7.84 ± 0.1 | −120 ± 2 | −129 ± 12 | 0.56 ± 0.04 |
| Pond 2/3 at 15 m | 7.39 * | NM | −155 * | NM | 0.30 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramsay, J.A.; de Lima e Silva, M.R.; Tawadrous, M.A.R.; Ramsay, B.A. Does Addition of Phosphate and Ammonium Nutrients Affect Microbial Activity in Froth Treatment Affected Tailings? Microorganisms 2021, 9, 2224. https://doi.org/10.3390/microorganisms9112224
Ramsay JA, de Lima e Silva MR, Tawadrous MAR, Ramsay BA. Does Addition of Phosphate and Ammonium Nutrients Affect Microbial Activity in Froth Treatment Affected Tailings? Microorganisms. 2021; 9(11):2224. https://doi.org/10.3390/microorganisms9112224
Chicago/Turabian StyleRamsay, Juliana A., Mara R. de Lima e Silva, Michael A. R. Tawadrous, and Bruce A. Ramsay. 2021. "Does Addition of Phosphate and Ammonium Nutrients Affect Microbial Activity in Froth Treatment Affected Tailings?" Microorganisms 9, no. 11: 2224. https://doi.org/10.3390/microorganisms9112224
APA StyleRamsay, J. A., de Lima e Silva, M. R., Tawadrous, M. A. R., & Ramsay, B. A. (2021). Does Addition of Phosphate and Ammonium Nutrients Affect Microbial Activity in Froth Treatment Affected Tailings? Microorganisms, 9(11), 2224. https://doi.org/10.3390/microorganisms9112224