Correlation between mazEF Toxin-Antitoxin System Expression and Methicillin Susceptibility in Staphylococcus aureus and Its Relation to Biofilm-Formation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Testing for Methicillin Sensitivity
2.2. Antimicrobial Susceptibility Testing for MRSA and MSSA Isolates Using Kirby-Bauer Disk Diffusion Method
2.3. Screening for Biofilm-Formation
2.4. Expression of mazEF Toxin-Antitoxin Genes in MSSA and MRSA
2.5. Statistics
3. Results
3.1. Antimicrobial Susceptibility Pattern
3.2. Biofilm-Formation and Its Relation to Methicillin Resistance
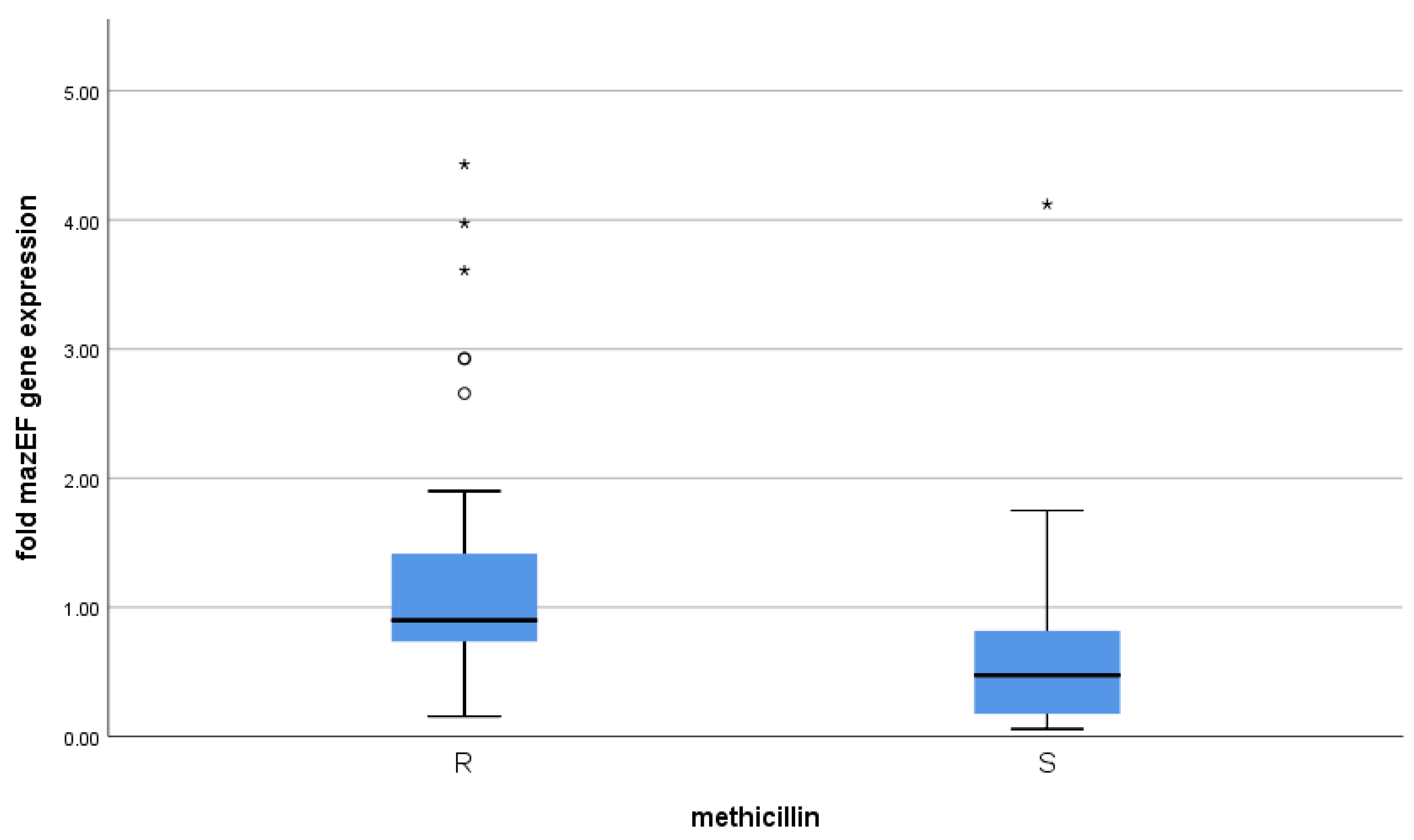
3.3. Correlation between mazEF Toxin-Antitoxin Gene Expression and Methicillin Resistance
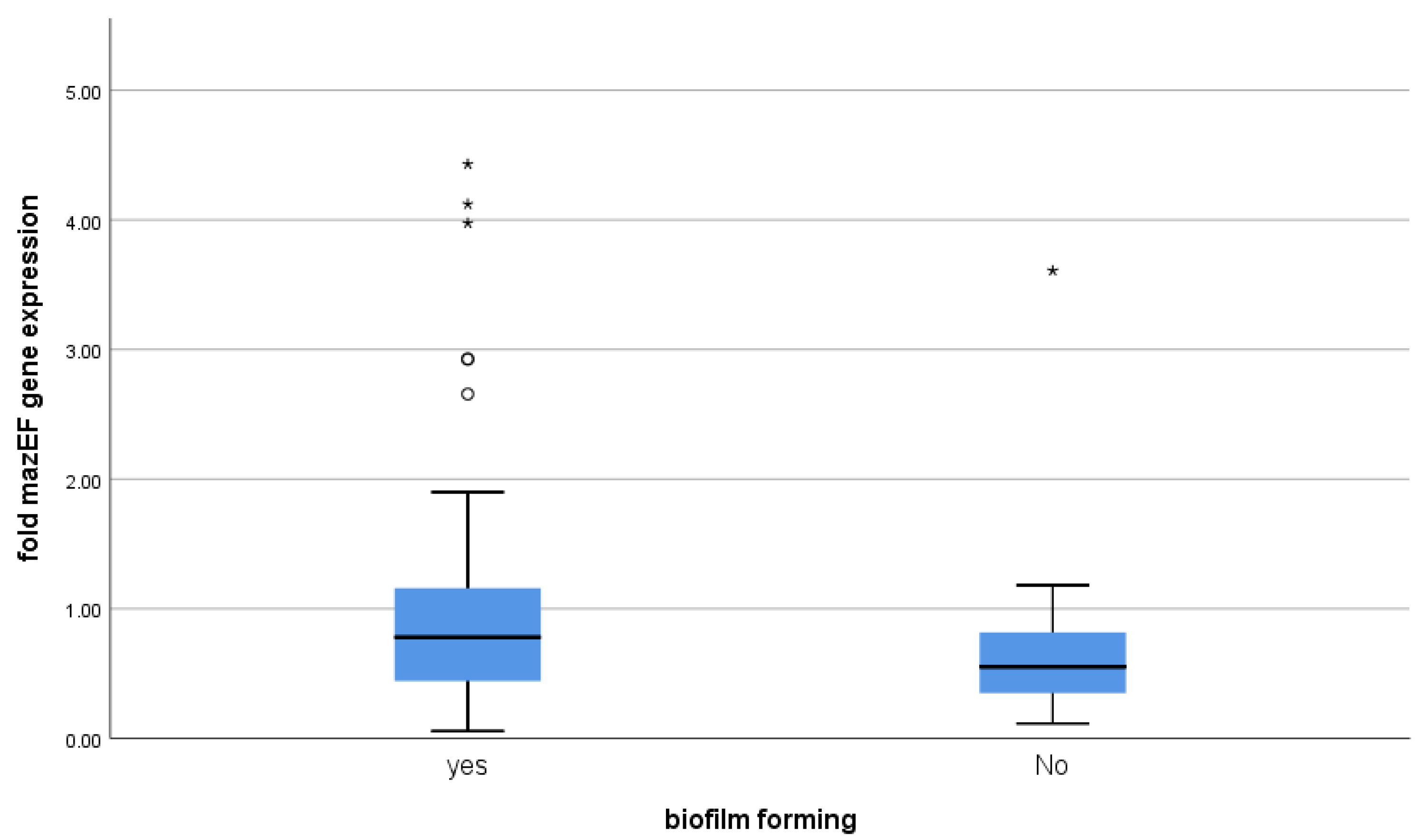
3.4. Correlation between mazEF Toxin-Antitoxin Gene Expression and Biofilm-Formation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, A.S.; De Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 18033. [Google Scholar] [CrossRef]
- Pantosti, A.; Sanchini, A.; Monaco, M. Mechanisms of antibiotic resistance in Staphylococcus aureus. Future Microbiol. 2007, 2, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Briggs, T.; Blunn, G.; Hislop, S.; Ramalhete, R.; Bagley, C.; McKenna, D.; Coathup, M. Antimicrobial photodynamic therapy-a promising treatment for prosthetic joint infections. Lasers Med. Sci. 2017, 33, 523–532. [Google Scholar] [CrossRef] [Green Version]
- O’Gara, J.P. Ica and beyond: Biofilm mechanisms and regulation in Staphylococcus epidermidis and Staphylococcus aureus. FEMS Microbiol Lett. 2007, 270, 179–188. [Google Scholar] [CrossRef] [Green Version]
- Page, R.; Peti, W. Toxin-antitoxin systems in bacterial growth arrest and persistence. Nat. Chem. Biol. 2016, 12, 208–214. [Google Scholar] [CrossRef]
- Fozo, E.; Makarova, K.S.; Shabalina, S.A.; Yutin, N.; Koonin, E.V.; Storz, G. Abundance of type I toxin–antitoxin systems in bacteria: Searches for new candidates and discovery of novel families. Nucleic Acids Res. 2010, 38, 3743–3759. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yao, J.; Sun, Y.-C.; Wood, T.K. Type VII Toxin/Antitoxin Classification System for Antitoxins that Enzymatically Neutralize Toxins. Trends Microbiol. 2020, 29, 388–393. [Google Scholar] [CrossRef]
- Aizenman, E.; Engelberg-Kulka, H.; Glaser, G. An Escherichia coli chromosomal “addiction module” regulated by guanosine [corrected] 3′,5′-bispyrophosphate: A model for programmed bacterial cell death. Proc. Natl. Acad. Sci. USA 1996, 93, 6059–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, C.F.; Bertram, R. Toxin-antitoxin systems of Staphylococcus aureus. Toxins 2016, 8, 140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.J.; Hergenrother, P.J. Artificial activation of toxin–antitoxin systems as an antibacterial strategy. Trends Microbiol. 2012, 20, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Clinical and Laboratory Standards Institute. Zone Diameter And Minimal Inhibitory Concentration Interpretive Standards for S. aureus; M100-S-25; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Piechota, M.; Kot, B.; Frankowska-Maciejewska, A.; Grużewska, A.; Woźniak-Kosek, A. Biofilm Formation by Methicillin-Resistant and Methicillin-Sensitive Staphylococcus aureus Strains from Hospitalized Patients in Poland. BioMed Res. Int. 2018, 2018, 4657396. [Google Scholar] [CrossRef] [Green Version]
- Cafiso, V.; Bertuccio, T.; Santagati, M.; Demelio, V.; Spina, D.; Nicoletti, G.; Stefani, S. agr-Genotyping and transcriptional analysis of biofilm-producing Staphylococcus aureus. FEMS Immunol. Med. Microbiol. 2007, 51, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, J.J.; Halvorsen, E.M.; Dwyer, E.M.; DiFazio, R.M.; Hergenrother, P.J. Toxin-antitoxin (TA) systems are prevalent and transcribed in clinical isolates of Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus. FEMS Microbiol. Lett. 2011, 322, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monday, S.; Bohach, G. Use of Multiplex PCR To Detect Classical and Newly Described Pyrogenic Toxin Genes in Staphylo-coccal isolates. J. Clin. Microbiol. 1999, 3, 3411–3414. [Google Scholar] [CrossRef] [Green Version]
- Coskun, U.S.S.; Cicek, A.C.; Kilinc, C.; Guckan, R.; Dagcioglu, Y.; Demir, O.; Sandalli, C. Effect of mazEF, higBA and relBE toxin-antitoxin systems on antibiotic resistance in Pseudomonas aeruginosa and Staphylococcus isolates. Malawi Med. J. 2018, 30, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.H. Biostatistics 102: Quantitative data--parametric & non-parametric tests. Singap. Med. J. 2003, 44, 391–396. [Google Scholar]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-resistant Staphylococcus aureus (MRSA): Antibiotic-resistance and the biofilm phenotype. Med. Chem. Comm. 2019, 10, 1231–1241. [Google Scholar] [CrossRef]
- Harms, A.; Brodersen, D.E.; Mitarai, N.; Gerdes, K. Toxins, Targets, and Triggers: An Overview of Toxin-Antitoxin Biology. Mol. Cell 2018, 70, 768–784. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, N.J. The Resistance of Staphylococcus Species to Different Antibiotics in Al-Kharj City. J. Pharm. Res. Int. 2020, 35–41. [Google Scholar] [CrossRef]
- Gurung, R.R.; Maharjan, P.; Chhetri, G.G. Antibiotic resistance pattern of Staphylococcus aureus with reference to MRSA isolates from pediatric patients. Futur. Sci. 2020, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Weiss, L.; Lansell, A.; Figueroa, J.; Suchdev, P.S.; Kirpalani, A. Declining Prevalence of Methicillin-Resistant Staphylococcus aureus Septic Arthritis and Osteomyelitis in Children: Implications for Treatment. Antibiotics 2020, 9, 101. [Google Scholar] [CrossRef]
- Abu Haneen, Z.S.; Al-Hamadany, W.S. Relation between biofilm formation ability and antibiotics resistance in Staphylococcus aureus from suppurative post-operation infections. Ann. Tro.p Med. Public Health 2019, 22, S379. [Google Scholar]
- Kadkhoda, H.; Ghalavand, Z.; Nikmanesh, B.; Kodori, M.; Houri, H.; Maleki, D.T.; Bavandpour, A.K.; Eslami, G. Characterization of biofilm formation and virulence factors of Staphylococcus aureus isolates from paediatric patients in Tehran, Iran. Iran J. Basic Med. Sci. 2020, 23, 1–8. [Google Scholar]
- Ma, D.; Mandell, J.B.; Donegan, N.P.; Cheung, A.L.; Ma, W.; Rothenberger, S.; Shanks, R.M.Q.; Richardson, A.R.; Urish, K.L. The Toxin-Antitoxin MazEF Drives Staphylococcus aureus Biofilm Formation, Antibiotic Tolerance, and Chronic Infection. mBio 2019, 10, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, C.F.; Mechler, L.; Nolle, N.; Krismer, B.; Zelder, M.-E.; Götz, F.; Bertram, R. The MazEF Toxin-Antitoxin System Alters the β-Lactam Susceptibility of Staphylococcus aureus. PLoS ONE 2015, 10, e0126118. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Tamber, S.; Memmi, G.; Donegan, N.P.; Cheung, A.L. Overexpression of MazFSa in Staphylococcus aureus Induces Bacterio-stasis by Selectively Targeting mRNAs for Cleavage. J. Bacteriol. 2009, 191, 2051–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, S.; Ghafourian, S.; Kalani, M.T.; Jalilian, F.A.; Hemati, S.; Sadeghifard, N. Association Between Toxin-Antitoxin Systems and Biofilm Formation. Jundishapur J. Microbiol. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Lemos, J.A.; Brown, T.A.; Abranches, J., Jr.; Burne, R.A. Characteristics of Streptococcus mutans strains lacking the MazEF and RelBE toxin-antitoxin modules. FEMS Microbiol. Lett. 2005, 253, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Kato, F.; Yabuno, Y.; Yamaguchi, Y.; Sugai, M.; Inouye, M. Deletion of mazF increases Staphylococcus aureus biofilm formation in an ica-dependent manner. Pathog. Dis. 2017, 75. [Google Scholar] [CrossRef]


| MRSA | MSSA | p-Value | |||
|---|---|---|---|---|---|
| Count | % | Count | % | ||
| Biofilm-forming isolates | 47 | 94.0% | 40 | 80.0% | 0.037 * |
| Non-biofilm-forming isolates | 3 | 6.0% | 10 | 20.0% | |
| Methicillin | |||||||
|---|---|---|---|---|---|---|---|
| R | S | p Value | |||||
| Median | 1st Quartile | 3rd Quartile | Median | 1st Quartile | 3rd Quartile | ||
| Fold mazEF gene expression | 0.90 | 0.73 | 1.42 | 0.47 | 0.17 | 0.82 | <0.001 * |
| Biofilm Forming | |||||||
|---|---|---|---|---|---|---|---|
| Yes | No | p Value | |||||
| Median | 1st Quartile | 3rd Quartile | Median | 1st Quartile | 3rd Quartile | ||
| fold mazEF gene expression | 0.78 | 0.42 | 1.18 | 0.55 | 0.35 | 0.82 | 0.136 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El rahman, A.; El kholy, Y.; Shash, R.Y. Correlation between mazEF Toxin-Antitoxin System Expression and Methicillin Susceptibility in Staphylococcus aureus and Its Relation to Biofilm-Formation. Microorganisms 2021, 9, 2274. https://doi.org/10.3390/microorganisms9112274
Abd El rahman A, El kholy Y, Shash RY. Correlation between mazEF Toxin-Antitoxin System Expression and Methicillin Susceptibility in Staphylococcus aureus and Its Relation to Biofilm-Formation. Microorganisms. 2021; 9(11):2274. https://doi.org/10.3390/microorganisms9112274
Chicago/Turabian StyleAbd El rahman, Aya, Yasmine El kholy, and Rania Y. Shash. 2021. "Correlation between mazEF Toxin-Antitoxin System Expression and Methicillin Susceptibility in Staphylococcus aureus and Its Relation to Biofilm-Formation" Microorganisms 9, no. 11: 2274. https://doi.org/10.3390/microorganisms9112274
APA StyleAbd El rahman, A., El kholy, Y., & Shash, R. Y. (2021). Correlation between mazEF Toxin-Antitoxin System Expression and Methicillin Susceptibility in Staphylococcus aureus and Its Relation to Biofilm-Formation. Microorganisms, 9(11), 2274. https://doi.org/10.3390/microorganisms9112274






