Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review
Abstract
1. Introduction
2. Environmental Impacts of Glyphosate
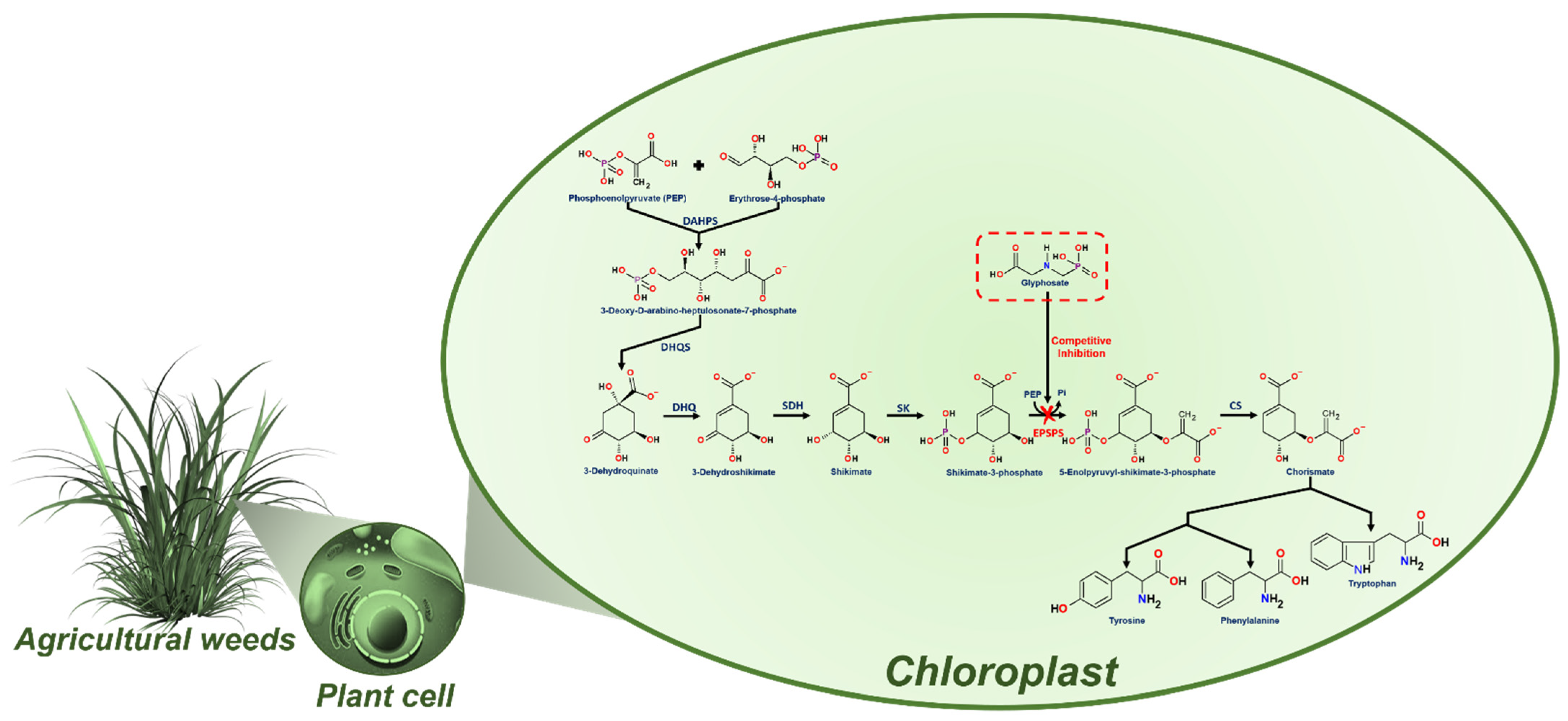
3. Glyphosate Human Health Threats
4. Environmental Risk Assessment through Glyphosate and Its Metabolites Detection
5. Need for Pollution Prevention and Treatment
6. Physicochemical Treatments for Glyphosate Remediation
7. Glyphosate Biodegradation Alternatives
7.1. Bacterial Degradation of Glyphosate
7.2. Fungal Degradation of Glyphosate
7.3. Algae Degradation of Glyphosate
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tarazona, J.V.; Tiramani, M.; Reich, H.; Pfeil, R.; Istace, F.; Crivellente, F. Glyphosate toxicity and carcinogenicity: A review of the scientific basis of the European Union assessment and its differences with IARC. Arch. Toxicol. 2017, 91, 2723–2743. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ. Health. 2016, 15, 19. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O. The history and current status of glyphosate. Pest. Manag. Sci. 2018, 74, 5. [Google Scholar] [CrossRef] [PubMed]
- Botten, N.; Wood, L.J.; Werner, J.R. Glyphosate remains in forest plant tissues for a decade or more. For. Ecol. Manag. 2021, 493, 119259. [Google Scholar] [CrossRef]
- Cuhra, M.; Bøhn, T.; Cuhra, P. Glyphosate: Too much of a good thing? Front. Environ. Sci. 2016, 4, 1–28. [Google Scholar] [CrossRef]
- Zabalza, A.; Orcaray, L.; Fernández-Escalada, M.; Zulet-González, M.; Royuela, M. The pattern of shikimate pathway and phenylpropanoids after inhibition by glyphosate or quinate feeding in pea roots. Pestic. Biochem. Physiol. 2017, 141, 96–102. [Google Scholar] [CrossRef]
- Boocock, M.R.; Coggins, J.R. Kinetics of 5-enolpyruvylshikimate-3-phosphate synthase inhibition by glyphosate. FEBS Lett. 1983, 154, 127–133. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- Fritschi, L.; McLaughlin, J.; Sergi, C.M.; Calaf, G.M.; Le Curieux, F.; Zeise, L. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet. Oncol. 2015, 16, 490–491. [Google Scholar] [CrossRef]
- Duke, S.O.; Powles, S.B. Glyphosate: A once-in-a-century herbicide. Pest. Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Dill, G.M.; Sammons, R.D.; Feng, P.C.C.; Kohn, F.; Kretzmer, K.; Mehrsheikh, A.; Bleeke, M.; Honegger, J.L.; Farmer, D.; Wright, D.; et al. Glyphosate: Discovery, development, applications and properties. In Glyphosate Resistance in Crops and Weeds: History, Development, and Management; Wiley: Hoboken, NJ, USA, 2010; pp. 1–33. [Google Scholar]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Seralini, G.E. Potential toxic effects of glyphosate and its commercial formulations below regulatory limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.; Habibi, H.R.; Hontela, A. Impacts of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef]
- Martens, M.A.; Bleeke, M.S.; Leopold, V.A.; Farmer, D.R. Toxicology and human health risk assessment of polyethoxylated tallow amine surfactant used in glyphosate formulations. Regul. Toxicol. Pharmacol. 2019, 107, 104347. [Google Scholar] [CrossRef]
- Mesnage, R.; Benbrook, C.; Antoniou, M.N. Insight into the confusion over surfactant co-formulants in glyphosate-based herbicides. Food Chem. Toxicol. 2019, 128, 137–145. [Google Scholar] [CrossRef]
- Mertens, M.; Höss, S.; Neumann, G.; Afzal, J.; Reichenbecher, W. Glyphosate, a chelating agent relevant for ecological risk assessment? Environ. Sci. Pollut. Res. 2018, 25, 5298–5317. [Google Scholar] [CrossRef]
- Golt, A.R.; Wood, L.J. Glyphosate-Based Herbicides Alter the Reproductive Morphology of Rosa acicularis (Prickly Rose). Front. Plant. Sci. 2021, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Borggaard, O.K.; Gimsing, A.L. Fate of Glyphosate in Soil and the Possibility of Leaching to Ground and Surface Waters: A Review. Pest. Manag. Sci. 2008, 64, 441–456. [Google Scholar] [CrossRef] [PubMed]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Ruiz-Toledo, J.; Castro, R.; Rivero-Pérez, N.; Bello-Mendoza, R.; Sánchez, D. Occurrence of Glyphosate in Water Bodies Derived from Intensive Agriculture in a Tropical Region of Southern Mexico. Bull. Environ. Contam. Toxicol. 2014, 93, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Bedmar, F.; Puricelli, M.; Marino, D.; Aparicio, V.C.; Wunderlin, D.; Miglioranza, K.S. Glyphosate runoff and its occurrence in rainwater and subsurface soil in the nearby area of agricultural fields in Argentina. Chemosphere 2019, 225, 906–914. [Google Scholar] [CrossRef]
- Bonansea, R.; Filippi, I.; Wunderlin, D.; Marino, D.; Ame, M. The fate of glyphosate and AMPA in a freshwater endorheic basin: An ecotoxicological risk assessment. Toxics 2018, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Instituto Regional de Estudios en Sustancias Tóxicas (IRET). (2015). Base de Datos de Ingredientes Activos Importados en Centroamérica. Universidad Nacional. Costa Rica. Available online: http://www.plaguicidasdecentroamerica.info/index.php/base-de-datos/ingredientes-activos/306-glifosato (accessed on 1 October 2021).
- Cuhra, M. Review of GMO safety assessment studies: Glyphosate residues in Roundup Ready crops is an ignored issue. Environ. Sci. Eur. 2015, 27, 20. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Leontievsky, A.A. Glyphosate: Safety risks, biodegradation, and bioremediation. In Current Environmental Issues and Challenges; Springer: Berlin/Heidelberg, Germany, 2014; ordrecht; pp. 183–195. [Google Scholar]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G., Jr. Environmental and health effects of glyphosate. Sci. Total Environ. 2018, 616, 255–268. [Google Scholar] [CrossRef]
- Travaglia, C.; Masciarelli, O.; Fortuna, J.; Marchetti, G.; Cardozo, P.; Lucero, M.; Zorza, E.; Luna, V.; Reinoso, H. Towards sustainable maize production: Glyphosate detoxification by Azospirillum sp. and Pseudomonas sp. Crop. Prot. 2015, 77, 102–109. [Google Scholar] [CrossRef]
- Cassigneul, A.; Benoit, P.; Bergheaud, V.; Dumeny, V.; Etiévant, V.; Goubard, Y.; Maylin, A.; Justes, E.; Alletto, L. Fate of glyphosate and degradates in cover crop residues and underlying soil: A laboratory study. Sci. Total Environ. 2016, 545, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Sidoli, P.; Baran, N.; Angulo-Jaramillo, R. Glyphosate and AMPA adsorption in soils: Laboratory experiments and pedotransfer rules. Environ. Sci. Pollut. Res. 2016, 23, 5733–5742. [Google Scholar] [CrossRef]
- Okada, E.; Costa, J.L.; Bedmar, F. Adsorption and mobility of glyphosate in different soils under no-till and conventional tillage. Geoderma 2016, 263, 78–85. [Google Scholar] [CrossRef]
- Sihtmäe, M.; Blinova, I.; Kunnis-Beres, K.; Kanarbik, L.; Heinlaan, M.; Kahru, A. Ecotoxicological effects of different glyphosate formulations. Appl. Soil Ecol. 2013, 72, 215–224. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A. Analysis of the glyphosate herbicide in water, soil and food using derivatising agents. Environ. Chem. Lett. 2017, 15, 85–100. [Google Scholar] [CrossRef]
- Maqueda, C.; Undabeytia, T.; Villaverde, J.; Morillo, E. Behaviour of glyphosate in a reservoir and the surrounding agricultural soils. Sci. Total Environ. 2017, 593, 787–795. [Google Scholar] [CrossRef]
- Van Stempvoort, D.R.; Spoelstra, J.; Senger, N.D.; Brown, S.J.; Post, R.; Struger, J. Glyphosate residues in rural groundwater, Nottawasaga River watershed, Ontario, Canada. Pest Manag. Sci. 2016, 72, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Rendón-von Osten, J.; Dzul-Caamal, R. Glyphosate residues in groundwater, drinking water and urine of subsistence farmers from intensive agriculture localities: A survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, F.; Bento, C.P.M.; Xue, S.; Gai, L.; van Dam, R.; Mol, H.; Ritsema, C.J.; Geissen, V. Short-term transport of glyphosate with erosion in Chinese loess soil-a flume experiment. Sci. Total Environ. 2015, 512, 406–414. [Google Scholar] [CrossRef]
- Alonso, L.L.; Demetrio, P.M.; Etchegoyen, M.A.; Marino, D. Glyphosate and atrazine in rainfall and soils in agroproductive areas of the pampas region in Argentina. Sci. Total Environ. 2018, 645, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.; Montanarella, L.; Jones, A.; Fernandez-Ugalde, O.; Mol, H.G.J.; Ritsema, C.J.; Geissen, V. Distribution of glyphosate and aminomethylphosphonic acid (AMPA) in agricultural topsoils of the European Union. Sci. Total Environ. 2018, 621, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.C.; Simcik, M.F.; Capel, P. Occurrence and fate of the herbicide glyphosate and its degradate aminomethylphosphonic acid in the atmosphere. Environ. Toxicol. Chem. 2011, 30, 548–555. [Google Scholar] [CrossRef]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2014, 85, 385–390. [Google Scholar] [CrossRef]
- WHO (World Health Organization) Glyphosate and AMPA in Drinking-Water. 2005. Available online: http://www.who.int/water_sanitation_health/dwq/chemicals/glyphosateampa290605.pdf (accessed on 7 September 2021).
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of agrochemicals on soil microbiota and management: A review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Sterren, M.; Uhrich, W.; Benintende, S. Residualidad de glifosato en suelos de Entre Ríos y su efecto sobre los microorganismos del suelo. Ecología Austral. 2016, 26, 46–255. [Google Scholar] [CrossRef]
- Helander, M.; Saloniemi, I.; Omacini, M.; Druille, M.; Salminen, J.P.; Saikkonen, K. Glyphosate decreases mycorrhizal colonization and affects plant-soil feedback. Sci. Total Environ. 2018, 642, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Vázquez, M.B.; Moreno, M.V.; Amodeo, M.R.; Bianchinotti, M.V. Effects of glyphosate on soil fungal communities: A field study. Rev. Argent. Microbiol. 2021. [Google Scholar] [CrossRef]
- Correia, F.V.; Moreira, J.C. Effects of glyphosate and 2, 4-D on earthworms (Eisenia foetida) in laboratory tests. Bull. Environ. Contam. Toxicol. 2010, 85, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Piola, L.; Fuchs, J.; Oneto, M.L.; Basack, S.; Kesten, E.; Casabe, N. Comparative toxicity of two glyphosate-based formulations to Eisenia andrei under laboratory conditions. Chemosphere 2013, 91, 545–551. [Google Scholar] [CrossRef] [PubMed]
- García-Torre, T.; Giufre, L.; Romaniuk, R.; Rios, R.P.; Pagano, E.A. Exposure assessment to glyphosate of two species of annelids. Bull. Environ. Contam. Toxicol. 2014, 93, 209–214. [Google Scholar] [CrossRef]
- Hagner, M.; Mikola, J.; Saloniemi, I.; Saikkonen, K.; Helander, M. Effects of a glyphosate-based herbicide on soil animal trophic groups and associated ecosystem functioning in a northern agricultural field. Sci. Rep. 2019, 9, 8540. [Google Scholar] [CrossRef]
- Gomes, M.P.; Manaćh, L.; Sarah, G.; Henault-Ethier, L.; Labrecque, M.; Lucotte, M.; Juneau, P. Glyphosate-dependent inhibition of photosynthesis in willow. Front. Plant Sci. 2017, 8, 207. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; Finckh, M.R. Plant diseases and management approaches in organic farming systems. Annu. Rev. Phytopathol. 2016, 54, 25–54. [Google Scholar] [CrossRef]
- Li, M.H.; Ruan, L.Y.; Zhou, J.W.; Fu, Y.H.; Jiang, L.; Zhao, H.; Wang, J.S. Metabolic profiling of goldfish (Carassius auratis) after long-term glyphosate based herbicide exposure. Aquat. Toxicol. 2017, 188, 159–169. [Google Scholar] [CrossRef]
- Moreno, N.C.; Sofia, S.H.; Martinez, C.B.R. Genotoxic effects of the herbicide Roundup Transorb® and its active ingredient glyphosate on the fish Prochilodus lineatus. Eviron. Toxicol. Pharmacol. 2014, 37, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Caramello, C.S.; Jorge, M.J.; Jorge, N.L.; Jorge, L.C. Evaluation of herbicide glyphosate effects in the fish Prochilodus lineatus using chromosome aberration test. Rev. Vet. 2017, 28, 65–68. [Google Scholar] [CrossRef]
- Kreutz, L.C.; Gil Barcellos, L.J.; Marteninghe, E.; Davidos Santos, E.; Zanatta, R. Exposure to sublethal concentration of glyphosate or atrazine-based herbicides alters the phagocytic function and increases the susceptibility of silver catfish fingerlings (Rhamdia quelen) to Aeromonas hydrophila challenge. Fish Shellfish. Immunol. 2010, 29, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Kreutz, L.C.; Gil Barcellos, L.J.; Valle, S.F.; Silva, T.O.; Anziliero, D.; Davidos Santos, E.; Pivato, M.; Zanatta, R. Altered hematological and immunological parameters in silver catfish (Rhamdia quelen) following short term exposure to sublethal concentration of glyphosate. Fish Shellfish. Immunol. 2011, 30, 51–57. [Google Scholar] [CrossRef]
- Balbuena, M.S.; Tison, L.; Hahn, M.L.; Greggers, U.; Menzel, R.; Farina, W.M. Effects of sublethal doses of glyphosate on honeybee navigation. J. Exp. Biol. 2015, 218, 2799–2805. [Google Scholar] [CrossRef] [PubMed]
- Samsel, A.; Seneff, S. Glyphosate’s suppression of cytochrome P450 enzymes and amino acid biosynthesis by the gut microbiome: Pathways to modern diseases. Entroy 2013, 15, 1416–1463. [Google Scholar] [CrossRef]
- Gandhi, K.; Khan, S.; Patrikar, M.; Markad, A.; Kumar, N.; Choudhari, A.; Sagar, P.; Indurkar, S. Exposure risk and environmental impacts of glyphosate: Highlights on the toxicity of herbicide co-formulants. Environ. Chall. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Gillezeau, C.; Van Gerwen, M.; Shaffer, R.; Rana, I.; Zhang, L.; Sheppard, L.; Taioli, E. The evidence of human exposure to glyphosate: A review. Environ. Health 2019, 18, 2. [Google Scholar] [CrossRef]
- WHO Europe. Results of Joint FAO/WHO Meeting on Pesticide Residues (JMPR). 2016. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/food-safety/news/news/2016/05/results-of-joint-faowho-meeting-on-pesticide-residues-jmpr (accessed on 1 October 2021).
- Duke, S.O. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef]
- Mann, R.M.; Bidwell, J.R. The toxicity of glyphosate and several glyphosate formulations to four species of southwestern Australian frogs. Arch. Environ. Contam. Toxicol. 1999, 36, 193–199. [Google Scholar] [CrossRef]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef]
- Gress, S.; Lemoine, S.; Séralini, G.E.; Puddu, P.E. Glyphosate-based herbicides potently affect cardiovascular system in mammals: Review of the literature. Cardiovasc. Toxicol. 2015, 15, 117–126. [Google Scholar] [CrossRef]
- IARC. Glyphosate. In Some Organophosphate Insecticides and Herbicides: Diazinon, Glyphosate, Malathion, Parathion, Tetrachlorvinphos. IARC Working Group, 3–10 March 2015; World Health Organization (WHO), International Agency for Research on Cancer (IARC) (IARC Monographs on the Evaluation of Carcinogen Risks to Humans): Lyon, France, 2015; Volume 112, pp. 1–92. Available online: http://monographs.iarc.fr/ENG/Monographs/vol112/index.php (accessed on 1 October 2021).
- Bai, S.H.; Ogbourne, S.M. Glyphosate: Environmental contamination, toxicity and potential risks to human health via food contamination. Environ. Sci. Pollut. Res. 2016, 23, 18988–19001. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Aguilar, O.; Montero-Montoya, R. Glifosato y los cultivos transgénicos en México. In Los plaguicidas altamente peligrosos en México; Bejarano-González, F., Ed.; Red de Acción sobre Plaguicidas y Alternativas en México, A.C. (RAPAM): Estado de México, México, 2015; pp. 153–166. [Google Scholar]
- Samsel, A.; Seneff, S. Glyphosate, pathways to modern diseases II: Celiac sprue and gluten intolerance. Interdiscip. Toxicol. 2013, 6, 159–184. [Google Scholar] [CrossRef]
- Schinasi, L.; Leon, M.E. Non-Hodgkin lymphoma and occupational exposure to agricultural pesticide chemical groups and active ingredients: A systematic review and meta-analysis. Int. J. Environ. Res. Public. Health. 2014, 11, 4449–4527. [Google Scholar] [CrossRef]
- Henneberger, P.K.; Liang, X.; London, S.J.; Umbach, D.M.; Sandler, D.P.; Hoppin, J.A. Exacerbation of symptoms in agricultural pesticide applicators with asthma. Int. Arch. Occup. Environ. Health 2014, 87, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Zouaoui, K.; Dulaurent, S.; Gaulier, J.M.; Moesch, C.; Lachatre, G. Determination of glyphosate and AMPA in blood and urine from humans: About cases of acute intoxication. Forensic. Sci. Int. 2013, 226, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Beecham, J.E.; Seneff, S. The possible link between autism and glyphosate acting as glycine mimetic—A review of evidence from the literature with analysis. J. Mol. Genet. Med. 2015, 9, 1000197. [Google Scholar] [CrossRef]
- Young, F.; Ho, D.; Glynn, D.; Edwards, V. Endocrine disruption and cytotoxicity of glyphosate and roundup in human JAr cells in vitro. Integr. Pharm. Toxicol. Gentocicol. 2015, 1, 12–19. [Google Scholar] [CrossRef][Green Version]
- Peillex, C.; Pelletier, M. The impact and toxicity of glyphosate and glyphosate-based herbicides on health and immunity. J. Immunotoxicol. 2020, 17, 163–174. [Google Scholar] [CrossRef]
- Weisenburger, D.D. A review and update with perspective of evidence that the herbicide glyphosate (Roundup) is a Cause of non-Hodgkin lymphoma. Clin. Lymphoma Myeloma Leuk. 2021, 21, 621–630. [Google Scholar] [CrossRef]
- Venugopal, K.; Suresh, C.; Vishwanath, H.; Lingaraja, M.; Bharath Raj, M.Y. Glyphosate: Surfactant herbicide poisoning—Is it mild? Med. J. DY. Patil. Univ. 2015, 8, 816–818. [Google Scholar] [CrossRef]
- Melo, K.; De Nucci, G.; Trape, A.; Jacobucci, S.; Garlipp, C.; Rosa, P. Brief review analytical methods for the determination of glyphosate. MOJ Toxicol. 2018, 4, 39–42. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Wani, A.B.; Dhanjal, D.S.; Romero, R.; Singh, J. Glyphosate uptake, translocation, resistance emergence in crops, analytical monitoring, toxicity, and degradation: A review. Environ. Chem. 2020, 18, 663–702. [Google Scholar] [CrossRef]
- Ansari, M.; Sedighi-Khavida, S.; Hatami, B. Toxicity, Biodegradability and Detection Methods of Glyphosate; the Most Used Herbicide: A Systematic Review. J. Environ. Health Sustain. Dev. 2019, 4, 731–743. [Google Scholar] [CrossRef]
- Arkan, T.; Molnár-Perl, I. The role of derivatization techniques in the analysis of glyphosate and aminomethyl-phosphonic acid by chromatography. Microchem. J. 2015, 121, 99–106. [Google Scholar] [CrossRef]
- Kawai, S.; Uno, B.; Tomita, M. Determination of glyphosate and its major metabolite aminomethyl phosphonic acid by high-performance liquid chromatography alter derivatization with p-toluenesulphonyl chloride. J. Chromatogr. A 1991, 540, 411–415. [Google Scholar] [CrossRef]
- Sancho, J.V.; Hernández, F.; López, F.J.; Hognedoom, E.A.; Dijkman, E. Rapid determination of glufosinate, glyphosate, and aminomethylphosphonic acid in environmental water samples using precolumn fluorogenic labeling and coupled-column liquid chromatography. J. Chromatogr. A 1996, 737, 75–83. [Google Scholar] [CrossRef]
- Nedelkoska, T.V.; Low, G.K.C. High-performance liquid chromatographic determination of glyphosate in water and plant material after pre-column derivatisation with 9-fluorenylmethyl chloroformate. Anal Chim. Acta 2004, 511, 45–153. [Google Scholar] [CrossRef]
- Fang, F.; Wei, R.; Liu, X. Novel pre-column derivatisation reagent for glyphosate by high-performance liquid chromatography and ultraviolet detection. Int. J. Environ. Anal. Chem. 2014, 94, 661–667. [Google Scholar] [CrossRef]
- De Almeida, L.; Chigome, S.; Torto, N.; Frost, C.; Pletschke, B. A novel colorimetric sensor strip for the detection of glyphosate in water. Sens. Actuators B Chem. 2015, 206, 357–363. [Google Scholar] [CrossRef]
- Goodwin, L.; Hanna, M.; Startin, J.R.; Keely, B.J.; Goodall, D.M. Isotachophoretic separation of glyphosate, glufosinate, AMPA and MPP with contactless conductivity detection. Analyst 2002, 127, 204–206. [Google Scholar] [CrossRef]
- You, J.; Koropchak, J.A. Condensation nucleation light scattering detection with ion chromatography for direct determination of glyphosate and its metabolite in water. J. Chromatogr. A 2003, 989, 231–238. [Google Scholar] [CrossRef]
- Guo, Z.X.; Cai, Q.; Yang, Z. Ion chromatography/inductively coupled plasma mass spectrometry for simultaneous determination of glyphosate, glufosinate, fosamine and ethephon at nanogram levels in water. Rapid Commun. Mass Spectrom. 2007, 21, 1606–1612. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, F.; Lin, Y.X.; Deng, N.; Bazhin, N.; Glebov, E. Photodegradation of glyphosate in the ferrioxalate system. J. Hazard. Mater. 2007, 148, 360–365. [Google Scholar] [CrossRef]
- Songa, E.A.; Somerset, V.S.; Waryo, T.; Baker, P.G.L.; Iwuoha, E.I. Amperometric nanobiosensor for quantitative determination of glyphosate and glufosinate residues in corn samples. Pure Appl. Chem. 2009, 81, 123–139. [Google Scholar] [CrossRef]
- Sanchís, J.; Kantiani, L.; Llorca, M.; Rubio, F.; Ginebreda, A.; Fraile, J.; Garrido, T.; Farré, M. Determination of Glyphosate in Groundwater Samples Using an Ultrasensitive Immunoassay and Confirmation by On-Line Solid-Phase Extraction Followed by Liquid Chromatography Coupled to Tandem Mass Spectrometry. Anal. Bioanal. Chem. 2012, 402, 2335–2345. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.Y.; Hong, T.P.; Whang, C.W. A simple and rapid screening method for glyphosate in water using fow-injection with electrochemiluminescence detection. Anal. Methods 2013, 5, 6186–6191. [Google Scholar] [CrossRef]
- Mörtl, M.; Németh, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Krüger, M.; Schledorn, P.; Schrödl, W.; Hoppe, H.W.; Lutz, W.; Shehata, A.A. Detection of glyphosate residues in animals and humans. J. Environ. Anal. Toxicol. 2014, 4, 1–5. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, Z.; Hao, J.; Yang, W.; Tang, J. A simple label free colorimetric method for glyphosate detection based on the inhibition of peroxidase-like activity of Cu (II). Sens. Actuators B Chem. 2016, 228, 410–415. [Google Scholar] [CrossRef]
- Wang, S.; Seiwert, B.; Kästner, M.; Miltner, A.; Schäffer, A.; Reemtsma, T.; Yang, Q.; Nowak, K.M. (Bio)degradation of glyphosate in water-sediment microcosms-A stable isotope co-labeling approach. Water. Res. 2016, 99, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Koskinen, W.C.; Marek, L.J.; Hall, K.E. Analysis of glyphosate and aminomethylphosphonic acid in water, plant materials and soil. Pest. Manag. Sci. 2016, 72, 423–432. [Google Scholar] [CrossRef]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate detection: Methods, needs and challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- ATSDR. Toxicological Profile of Glyphosate; Agency for Toxic Substances and Disease Registry, the Public Health Service, or the U.S. Department of Health and Human Services: Atlanta, GA, USA, 2020. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp214.pdf (accessed on 1 October 2021).
- Antier, C.; Kudsk, P.; Reboud, X.; Ulber, L.; Baret, P.V.; Messéan, A. Glyphosate use in the European agricultural sector and a framework for its further monitoring. Sustainability 2020, 12, 5682. [Google Scholar] [CrossRef]
- Richmond, M.E. Glyphosate: A review of its global use, environmental impact, and potential health effects on humans and other species. J. Environ. Stud. Sci. 2018, 8, 416–434. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Gill, J.P.K.; Datta, S.; Singh, S.; Dhaka, V.; Kapoor, D.; Wani, A.B.; Dhanjal, D.S.; Kumar, M.; et al. Herbicide glyphosate: Toxicity and microbial degradation. Int. J. Environ. Res. Public Health 2020, 17, 7519. [Google Scholar] [CrossRef]
- Pérez, G.L.; Vera, M.S.; Miranda, L. Effects of Herbicide Glyphosate and Glyphosate-Based Formulations on Aquatic Ecosystems. In Herbicides and Environment; Kortekamp, A., Ed.; InTech Publications: Rijeka, Croatia, 2011. [Google Scholar]
- Espinoza-Montero, P.J.; Vega-Verduga, C.; Alulema-Pullupaxi, P.; Fernández, L.; Paz, J.L. Technologies employed in the treatment of water contaminated with glyphosate: A Review. Molecules 2020, 25, 5550. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Soric, A.; Boutin, O. Treatment technologies and degradation pathways of glyphosate: A critical review. Sci. Total Environ. 2020, 742, 140559. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Yang, Q.; Evans, D.G.; Forano, C.; Duan, X. Study on adsorption of glyphosate (N-phosphonomethyl glycine) pesticide on Mg Al-layered double hydroxides in aqueous solution. J. Hazard. Mater. 2005, 125, 89–95. [Google Scholar] [CrossRef]
- Nourouzi, M.M.; Chuah, T.G.; Choong, T.S.Y. Adsorption of glyphosate onto activated carbon derived from waste newspaper. Desalination. Water. Treat. 2010, 24, 321–326. [Google Scholar] [CrossRef]
- Salman, J.M.; Abid, F.M.; Muhammed, A.A. Batch study for pesticide glyphosate adsorption onto palm oil fronds activated carbon. Asian. J. Chem. 2012, 24, 5646–5648. [Google Scholar]
- Zavareh, S.; Farrokhzad, Z.; Darvishi, F. Modification of zeolite 4A for use as an adsorbent for glyphosate and as an antibacterial agent for water. Ecotoxicol. Environ. Saf. 2018, 155, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Herath, I.; Kumarathilaka, P.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.A.; Vithanage, M. Mechanistic modeling of glyphosate interaction with rice husk derived engineered biochar. Microporous Mesoporous Mater. 2016, 225, 280–288. [Google Scholar] [CrossRef]
- Jia, D.M.; Li, C.H.; Li, A.M. Effective removal of glyphosate from water by double valent nano-sized hydroxyl iron oxide. RSC Adv. 2017, 7, 24430–24437. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, C.; Wen, Y.; Wang, Y.; Yang, Y. Adsorption performance and mechanism of magnetic reduced graphene oxide in glyphosate contaminated water. Environ. Sci. Pollut. Res. 2018, 25, 21036–21048. [Google Scholar] [CrossRef] [PubMed]
- Rissouli, L.; Benicha, M.; Chafik, T.; Chabbi, M. Decontamination of water polluted with pesticide using biopolymers: Adsorption of glyphosate by chitin and chitosan. J. Mater. Environ. Sci. 2017, 8, 4544–4549. [Google Scholar] [CrossRef]
- Xiao, G.; Meng, Q. D151 resin preloaded with Fe3+ as a salt resistant adsorbent for glyphosate from water in the presence 16% NaCl. Ecotoxicol. Environ. Saf. 2020, 190, 110140. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Xie, M.; Ni, F.; Xu, Y.H. Nanofiltration process of glyphosate simulated wastewater. Water Sci. Technol. 2012, 65, 816–822. [Google Scholar] [CrossRef]
- Yuan, J.; Duan, J.; Saint, C.P.; Mulcahy, D. Removal of glyphosate and aminomethylphosphonic acid from synthetic water by nanofiltration. Environ. Technol. 2018, 39, 1384–1392. [Google Scholar] [CrossRef]
- Hosseini, N.; Toosi, M.R. Removal of 2,4-D, glyphosate, trifluralin, and butachlor herbicides from water by polysulfone membranes mixed by graphene oxide/TiO nanocomposite: Study of filtration and batch adsorption. J. Environ. Health Sci. Eng. 2019, 17, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Xu, L. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Villamar-Ayala, C.A.; Carrera-Cevallos, J.V.; Vasquez-Medrano, R.; Espinoza-Montero, P.J. Fate, eco-toxicological characteristics, and treatment processes applied to water polluted with glyphosate: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1476–1514. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.H.; Dong, H.; Zhao, L.; Wang, D.X.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total. Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Souza, D.R.; Trovó, A.G.; Filho, A.R.N.; Silva, M.A.A.; Machado, A.E.H. Degradation of the commercial herbicide glyphosate by photo-Fenton process: Evaluation of kinetic parameters and toxicity. J. Braz. Chem. Soc. 2013, 24, 1451–1460. [Google Scholar] [CrossRef]
- Rubí-Juárez, H.; Cotillas, S.; Sáez, C.; Cañizares, P.; Barrera-Díaz, C.; Rodrigo, M.A. Removal of herbicide glyphosate by conductive-diamond electrochemical oxidation. Appl. Catal. B Environ. 2016, 188, 305–312. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Doan, T.L.; Le, T.S.; Nguyen, H.C. Electrochemical degradation and mineralization of glyphosate herbicide. Environ. Technol. 2017, 38, 2939–2948. [Google Scholar] [CrossRef]
- Comninellis, C.; Chen, G. Electrochemistry for the Environment; Springer: New York, NY, USA, 2010. [Google Scholar]
- Lan, H.; Jiao, Z.; Zhao, X.; He, W.; Wang, A.; Liu, H.; Liu, R.; Qu, J. Removal of glyphosate from water by electrochemically assisted MnO2 oxidation process. Sep. Purif. Technol. 2013, 117, 30–34. [Google Scholar] [CrossRef]
- Farinos, R.M.; Ruotolo, L.A.M. Comparison of the electrooxidation performance of three-dimensional RVC/PbO2 and boron-doped diamond electrodes. Electrochim. Acta 2017, 224, 32–39. [Google Scholar] [CrossRef]
- Kukurina, O.; Elemesova, Z.; Syskina, A. Mineralization of organophosphorous pesticides by electro-generated oxidants. Procedia Chem. 2014, 10, 209–216. [Google Scholar] [CrossRef]
- Sánchez-Montes, I.; Pérez, J.F.; Sáez, C.; Rodrigo, M.A.; Cañizares, P.; Aquino, J.M. Assessing the performance of electrochemical oxidation using DSA® and BDD anodes in the presence of UVC light. Chemosphere 2020, 238, 124575. [Google Scholar] [CrossRef]
- Assalin, M.R.; De Moraes, S.G.; Queiroz, S.C.N.; Ferracini, V.L.; Duran, N. Studies on degradation of glyphosate by several oxidative chemical processes: Ozonation, photolysis and heterogeneous photocatalysis. J. Environ. Sci. Health. Part B Pestic. Food Contam. Agric. Wastes 2009, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, J.; Camm, R.; Hall, T. Removal and degradation of Glyphosate in water treatment: A review. J. Water Supply Res. Technol. AQUA 2013, 62, 395–408. [Google Scholar] [CrossRef]
- Xing, B.; Chen, H.; Zhang, X. Efficient degradation of organic phosphorus in glyphosate wastewater by catalytic wet oxidation using modified activated carbon as a catalyst. Environ. Technol. 2018, 39, 749–758. [Google Scholar] [CrossRef]
- Yang, H.; Dick, W.A.; McCoy, E.L.; Phelan, P.L.; Grewal, P.S. Field evaluation of a new biphasic rain garden for stormwater flow management and pollutant removal. Ecol. Eng. 2013, 54, 22–31. [Google Scholar] [CrossRef]
- Zhang, K.; Deletic, A.; Page, D.; McCarthy, D.T. Surrogates for herbicide removal in stormwater biofilters. Water Res. 2015, 81, 64–71. [Google Scholar] [CrossRef]
- Rossi, F.; Carles, L.; Donnadieu, F.; Batisson, I.; Artigas, J. Glyphosate-degrading behavior of five bacterial strains isolated from stream biofilms. J. Hazard Mater. 2021, 420, 126651. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Ermakova, I.T.; Ivanova, E.V.; Epiktetov, D.O.; Leontievsky, A.A. Microbial degradation of glyphosate herbicides. Appl. Biochem. Microbiol. 2015, 51, 188–195. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent advances in glyphosate biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.M.; Chen, Y.; Li, R.Y.; Yuan, X.Q.; Liu, C.M.; Li, B.; Wan, Y. Pathway and rate-limiting step of glyphosate degradation by Aspergillus oryzae A-F02. Prep. Biochem. Biotechnol. 2017, 47, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Wijekoon, N.; Yapa, N. Assessment of plant growth promoting rhizobacteria (PGPR) on potential biodegradation of glyphosate in contaminated soil and aquifers. Groundw. Sustain. Dev. 2018, 7, 465–469. [Google Scholar]
- Manogaran, M.; Ahmad, S.A.; Yasid, N.A.; Yakasai, H.M.; Shukor, M.Y. Characterisation of the simultaneous molybdenum reduction and glyphosate degradation by Burkholderia vietnamiensis AQ5-12 and Burkholderia sp. AQ5-13. 3 Biotech 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Iqbal, S.; Anwar, S.; Jabeen, H. Identification and analysis of 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) gene from glyphosate-resistant Ochrobactrum intermedium Sq20. Pest Manag. Sci. 2018, 74, 1184–1196. [Google Scholar] [CrossRef]
- Hove-Jensen, B.; Zechel, D.L.; Jochimsen, B. Utilization of glyphosate as phosphate source: Biochemistry and genetics of bacterial carbon-phosphorus lyase. Microbiol. Mol. Biol. Rev. 2014, 78, 176–197. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, P.; Joshi, T.; Bhatt, K.; Zhang, W.; Huang, Y.; Chen, S. Binding interaction of glyphosate with glyphosate oxidoreductase and C–P lyase: Molecular docking and molecular dynamics simulation studies. J. Hazar. Mater. 2021, 409, 124927. [Google Scholar] [CrossRef] [PubMed]
- Kishore, G.M.; Jacob, G.S. Degradation of glyphosate by Pseudomonas sp. PG2982 via a sarcosine intermediate. J. Biol. Chem. 1987, 262, 12164–12168. [Google Scholar] [CrossRef]
- Pipke, R.; Amrhein, N.; Jacob, G.S.; Schaefer, J.; Kishore, G.M. Metabolism of glyphosate in an Arthrobacter sp. GLP-1. Eur. J. Biochem. 1987, 165, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Balthazor, T.M.; Hallas, L.E. Glyphosate-degrading microorganisms from industrial activated sludge. Appl. Environ. Microbiol. 1986, 51, 432–434. [Google Scholar] [CrossRef]
- Sviridov, A.V.; Shushkova, T.V.; Zelenkova, N.F.; Vinokurova, N.G.; Morgunov, I.G.; Ermakova, I.T.; Leontievsky, A.A. Distribution of glyphosate and methylphosphonate catabolism systems in soil bacteria Ochrobactrum anthropi and Achromobacter sp. Appl. Microbiol. Biotechnol. 2012, 93, 787–796. [Google Scholar] [CrossRef]
- Masotti, F.; Garavaglia, B.S.; Piazza, A.; Burdisso, P.; Altabe, S.; Gottig, N.; Ottado, J. Bacterial isolates from Argentine Pampas and their ability to degrade glyphosate. Sci. Total Environ. 2021, 774, 145761. [Google Scholar] [CrossRef] [PubMed]
- Mousa, N.K.; Ali, A.J.; Hussein, M. Bacillus Megaterium Biodegradation Glyphosate; IntechOpen: Rijeka, Croatia, 2021; pp. 1–9. [Google Scholar] [CrossRef]
- Elarabi, N.I.; Abdelhadi, A.A.; Ahmed, R.H.; Saleh, I.; Arif, I.A.; Osman, G.; Ahmed, D.S. Bacillus aryabhattai FACU: A promising bacterial strain capable of manipulate the glyphosate herbicide residues. Saudi J. Biol. Sci. 2020, 27, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Firdous, S.; Iqbal, S.; Anwar, S. Optimization and modeling of glyphosate biodegradation by a novel Comamonas odontotermitis P2 through response surface methodology. Pedosphere 2020, 30, 618–627. [Google Scholar] [CrossRef]
- Góngora-Echeverría, V.R.; García-Escalante, R.; Rojas-Herrera, R.; Giácoman-Vallejos, G.; Ponce-Caballero, C. Pesticide bioremediation in liquid media using a microbial consortium and bacteria-pure strains isolated from a biomixture used in agricultural areas. Ecotox. Environ. Saf. 2020, 200, 110734. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Cortés, A.G.; Martinez-Ledezma, C.; López-Chuken, U.J.; Kaushik, G.; Nimesh, S.; Villarreal-Chiu, J.F. Polyphosphate recovery by a native Bacillus cereus strain as a direct effect of glyphosate uptake. ISME J. 2019, 13, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, M.; Melo, C.; Jiménez, E.; Dussán, J. Glyphosate bioremediation through the sarcosine oxidase pathway mediated by Lysinibacillus sphaericus in soils cultivated with potatoes. Agriculture 2019, 9, 217. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Singh, J. Kinetic study of the biodegradation of glyphosate by indigenous soil bacterial isolates in presence of humic acid, Fe (III) and Cu (II) ions. J. Environ. Chem. Eng. 2019, 7, 103098. [Google Scholar] [CrossRef]
- Ezaka, E.; Akintokun, A.K.; Akintokun, P.O.; Taiwo, L.B.; Uthman, A.C.O.; Oyedele, O.A.; Aluko, O.I. Glyphosate degradation by two plant growth promoting bacteria (PGPB) isolated from rhizosphere of maize. Microb. Resea. J. Inter. 2018, 26, 1–11. [Google Scholar] [CrossRef]
- Chauhan, M.P.; Singh, N.K.; Chaudhary, A.K.; Shalini, R. Characterization of rhizobium isolates from Sesbania rhizosphere and their role in bioremediation of glyphosate and Monocrotophos. Int. J. Appl. Nat. Sci. 2017, 6, 11–22. [Google Scholar]
- Ermakova, I.T.; Shushkova, T.V.; Sviridov, A.V.; Zelenkova, N.F.; Vinokurova, N.G.; Baskunov, B.P.; Leontievsky, A.A. Organophosphonates utilization by soil strains of Ochrobactrum anthropi and Achromobacter sp. Arch. Microb. 2017, 199, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.O.; Bezerra, A.F.M.; Honorato, L.R.S.; Cortêz, A.C.A.; Souza, J.V.B.; Souza, E.S. Amazonian soil fungi are efficient degraders of glyphosate herbicide; novel isolates of Penicillium, Aspergillus, and Trichoderma. Braz. J. Biol. 2021, 83, e242830. [Google Scholar] [CrossRef] [PubMed]
- Njoku, K.L.; Eludini, P.O.; Adesuyi, A.A.; Ude, E.O.; Oyelami, A.O. Physiological and molecular characterization of active fungi in pesticides contaminated soils for degradation of glyphosate. Res. Square 2020, 1–14. [Google Scholar] [CrossRef]
- Swe, T.M.; Nandar, W.; Ei, H.H.; Win, N.N.; Swe, K.K.; kyaw Ko, T.; Win, T.T. Bio-removal efficiency of glyphosate by using indigenous laccase producing fungi. Int. J. Res. Appl. Sci. Biotechnol. 2020, 7, 249–256. [Google Scholar] [CrossRef]
- Carranza, C.S.; Regnicoli, J.P.; Aluffi, M.E.; Benito, N.; Chiacchiera, S.M.; Barberis, C.L.; Magnoli, C.E. Glyphosate in vitro removal and tolerance by Aspergillus oryzae in soil microcosms. Int. J. Environ. Sci. Technol. 2019, 16, 7673–7682. [Google Scholar] [CrossRef]
- Salman, J.M.; Abdul-Adel, E. Potential use of cyanophyta species Oscillatoria limnetica in bioremediation of organophosphorus herbicide glyphosate. Meso. Environ. J. 2015, 1, 15–26. [Google Scholar]
- Carranza, C.S.; Barberis, C.L.; Chiacchiera, S.M.; Magnoli, C.E. Assessment of growth of Aspergillus spp. from agricultural soils in the presence of glyphosate. Rev. Argent. Microbiol. 2017, 49, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Kulikova, N.A.; Zhelezova, A.D.; Filippova, O.I.; Plyushchenko, I.V.; Rodin, I.A. The Degradation of Glyphosate and Its Effect on the Microbial Community of Agro-Sod–Podzolic Soil under Short-Term Model Experiment Conditions. Mosc. Univ. Soil Sci.Bull. 2020, 75, 138–145. [Google Scholar] [CrossRef]
- Mesnage, R.; Oestreicher, N.; Poirier, F.; Nicolas, V.; Boursier, C.; Vélot, C. Transcriptome profiling of the fungus Aspergillus nidulans exposed to a commercial glyphosate-based herbicide under conditions of apparent herbicide tolerance. Environ. Res. 2020, 182, 109116. [Google Scholar] [CrossRef]
- Guo, J.; Song, X.; Zheng, C.; Sun, S.; Zhuang, B.; Tao, B. Transcriptome analysis and identification of candidate genes involved in glyphosate resistance in the fungus Fusarium verticillioides. J. Environ. Sci. Health. Part B 2021, 56, 658–669. [Google Scholar] [CrossRef]

| Species | Concentration (mg/L) | Degradation (%) | System | Degradation Pathway § | Reference |
|---|---|---|---|---|---|
| Bacteria | |||||
| Achromobacter denitrificans SOS5 | 253 | 56 | In vitro | glyphosate oxidoreductase | [152] |
| Achromobacter insolitus SOR2 | 47 | glyphosate oxidoreductase | |||
| Achromobacter xylosoxidans SOS3 | 37 | glyphosate oxidoreductase | |||
| Agrobacterium tumefaciens CHLDO | 40 | glyphosate oxidoreductase and C–P lyase | |||
| Ochrobactrum haematophilum SR | 41 | glyphosate oxidoreductase | |||
| Bacillus megaterium | 5–25 | 70–71 | In vitro | NR | [153] |
| Acidovorax sp. CNI26 | 22.31 | NR | In vitro | C–P lyase | [139] |
| Agrobacterium tumefaciens CNI28 | glyphosate oxidoreductase and C–P lyase | ||||
| Ensifer sp. CNI115 | glyphosate oxidoreductase | ||||
| Novosphingobium sp. CNI35 | glyphosate oxidoreductase and C–P lyase | ||||
| Ochrobactrum pituitosum CNI52 | glyphosate oxidoreductase and C–P lyase | ||||
| Bacillus aryabhattai FACU | 50, 100, 150, 200 and 250 | NR | In vitro | glyphosate oxidoreductase | [154] |
| Comamonas odontotermitis P2 | 500 | 90 | In vitro | glyphosate oxidoreductase and C–P lyase | [155] |
| Ochrobactrum sp. | 50 | 60 | In vitro | NR | [156] |
| Pseudomonas citronellolis | |||||
| Bacillus cereus | 169 | 38 | In vitro | C–P lyase | [157] |
| Lysinibacillus sphaericus | 679 g/Kg * | 79 | In situ | C–P lyase | [158] |
| Bacillus subtilis | 250 | 90 | In vitro | glyphosate oxidoreductase and C–P lyase | [159] |
| Rhizobium leguminosarum | 88 | ||||
| Streptomyces sp. | 89 | ||||
| Bacillus cereus | 3, 100, 7200 and 14,400 | 86, 73 and 57 | In situ | glyphosate oxidoreductase and C–P lyase | [160] |
| Pseudomonas aeruginosa | 76, 85 and 47 | ||||
| Ochrobactrum intermedium Sq20 | 500 | 100 | In vitro | C–P lyase | [145] |
| Burkholderia sp. AQ5-13 | 50 | 91 | In vitro | NR | [144] |
| Burkholderia vietnamiensis AQ5-12 | 74 | ||||
| Ensifer sp. AC01b | 5072 | 44 | In vitro | NR | [161] |
| Rhizobium sp. SCAUS14 | 41 | ||||
| Sinorhizobium saheli OP3-1 | 39 | ||||
| Achromobacter sp. MPK 7A | 500 | 60 | In vitro | C–P lyase | [162] |
| Fungi | |||||
| Aspergillus 2B112 | 500 | 60 | In vitro | NR | [163] |
| Penicillium 4A21 | 26 | glyphosate oxidoreductase and C–P lyase | |||
| Trichoderma | 8 | NR | |||
| Aspergillus flavus EFB01 | 100 | 19.9 | In vitro | glyphosate oxidoreductase | [164] |
| Aspergillus flavus JN-YG-3-5 | 85.6 | ||||
| Aspergillus fumigatus FJAT-31052 | 84.7 | ||||
| Aspergillus niger APBSDSF96 | 84.8 | ||||
| Penicillium simplicissimum SNB-VECD11G | 84.7 | ||||
| Trichoderma gamsii P2-18 | 84.2 | ||||
| Trichoderma harzianum MT871998 | 200, 400, 600, 800 and 1000 | 78.1 | In vitro | NR | [165] |
| Aspergillus oryzae AM1 | 1690 | 57 | In vitro | glyphosate oxidoreductase | [166] |
| Aspergillus oryzae A-F02 | 500 | 66.9 | In vitro | glyphosate oxidoreductase and C–P lyase | [142] |
| Algae | |||||
| Oscillatoria limnetica | 5 | 85 | In vitro | NR | [167] |
| 10 | 38 | ||||
| 15 | 27 | ||||
| 20 | 75 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castrejón-Godínez, M.L.; Tovar-Sánchez, E.; Valencia-Cuevas, L.; Rosas-Ramírez, M.E.; Rodríguez, A.; Mussali-Galante, P. Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms 2021, 9, 2322. https://doi.org/10.3390/microorganisms9112322
Castrejón-Godínez ML, Tovar-Sánchez E, Valencia-Cuevas L, Rosas-Ramírez ME, Rodríguez A, Mussali-Galante P. Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms. 2021; 9(11):2322. https://doi.org/10.3390/microorganisms9112322
Chicago/Turabian StyleCastrejón-Godínez, María Luisa, Efraín Tovar-Sánchez, Leticia Valencia-Cuevas, Marcos Eduardo Rosas-Ramírez, Alexis Rodríguez, and Patricia Mussali-Galante. 2021. "Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review" Microorganisms 9, no. 11: 2322. https://doi.org/10.3390/microorganisms9112322
APA StyleCastrejón-Godínez, M. L., Tovar-Sánchez, E., Valencia-Cuevas, L., Rosas-Ramírez, M. E., Rodríguez, A., & Mussali-Galante, P. (2021). Glyphosate Pollution Treatment and Microbial Degradation Alternatives, a Review. Microorganisms, 9(11), 2322. https://doi.org/10.3390/microorganisms9112322










