Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. Parasites
2.4. In Vivo Infection and Sample Collection
2.5. Quantification of Tissue Parasites
2.6. Histological Analysis
2.7. In Vitro Preparation of Primary Murine Cells
2.7.1. Astrocytes
2.7.2. Microglia
2.7.3. Peritoneal Macrophages
2.8. RNA-Seq Analysis
2.9. Identification of Differentially Expressed Genes (DEGs) in Which Upregulation during T. Gondii Infection Was Impaired by CXCR3-Deficiency
2.10. Functional Enrichment Analyses of DEGs in Which Upregulation during T. gondii Infection Was Impaired by CXCR3-Deficiency
2.11. Cytokine ELISA
2.12. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.13. Statistical Analysis
3. Results
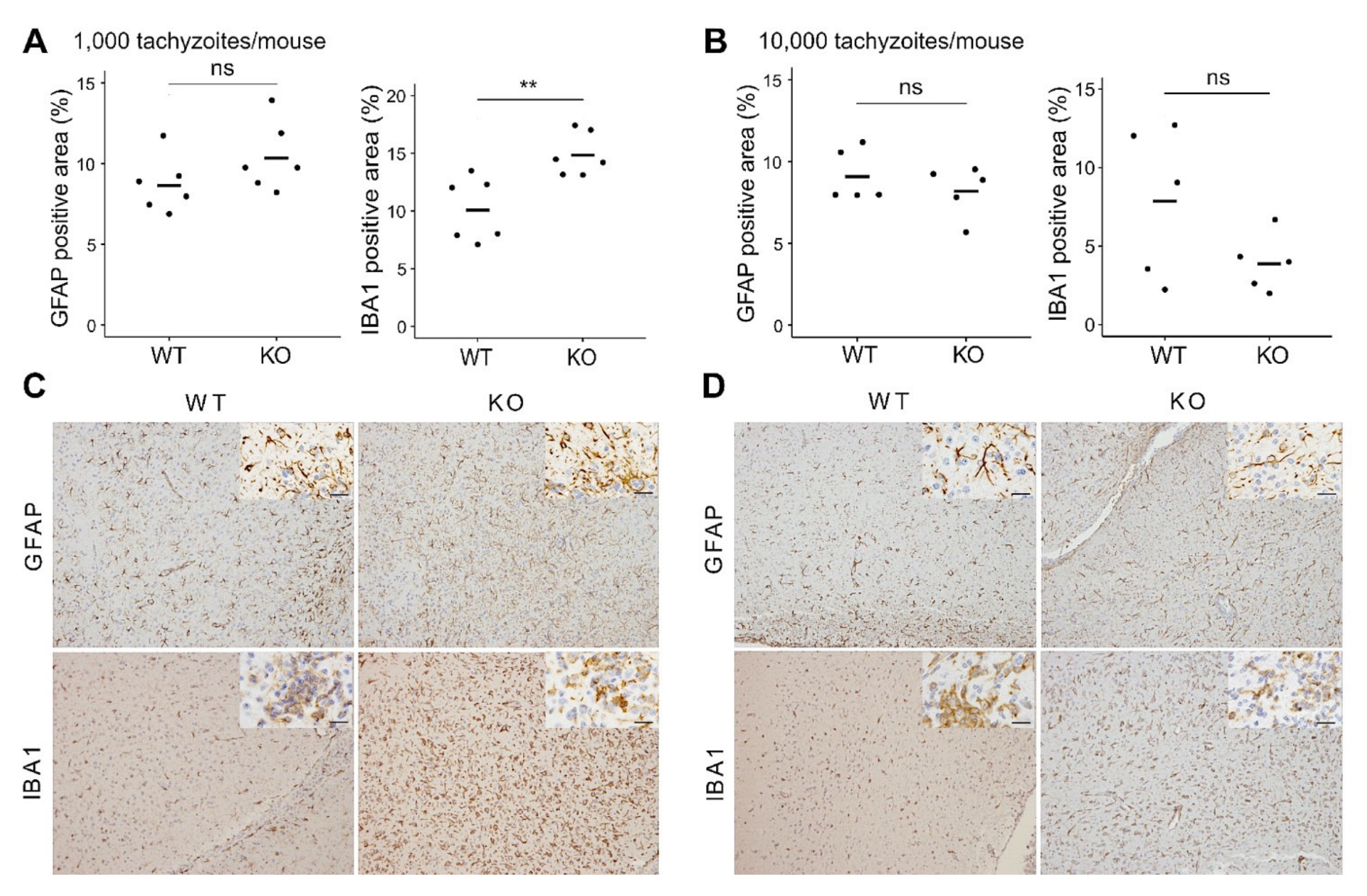
3.1. In Vivo Effects of CXCR3-Deficiency during T. gondii Infection
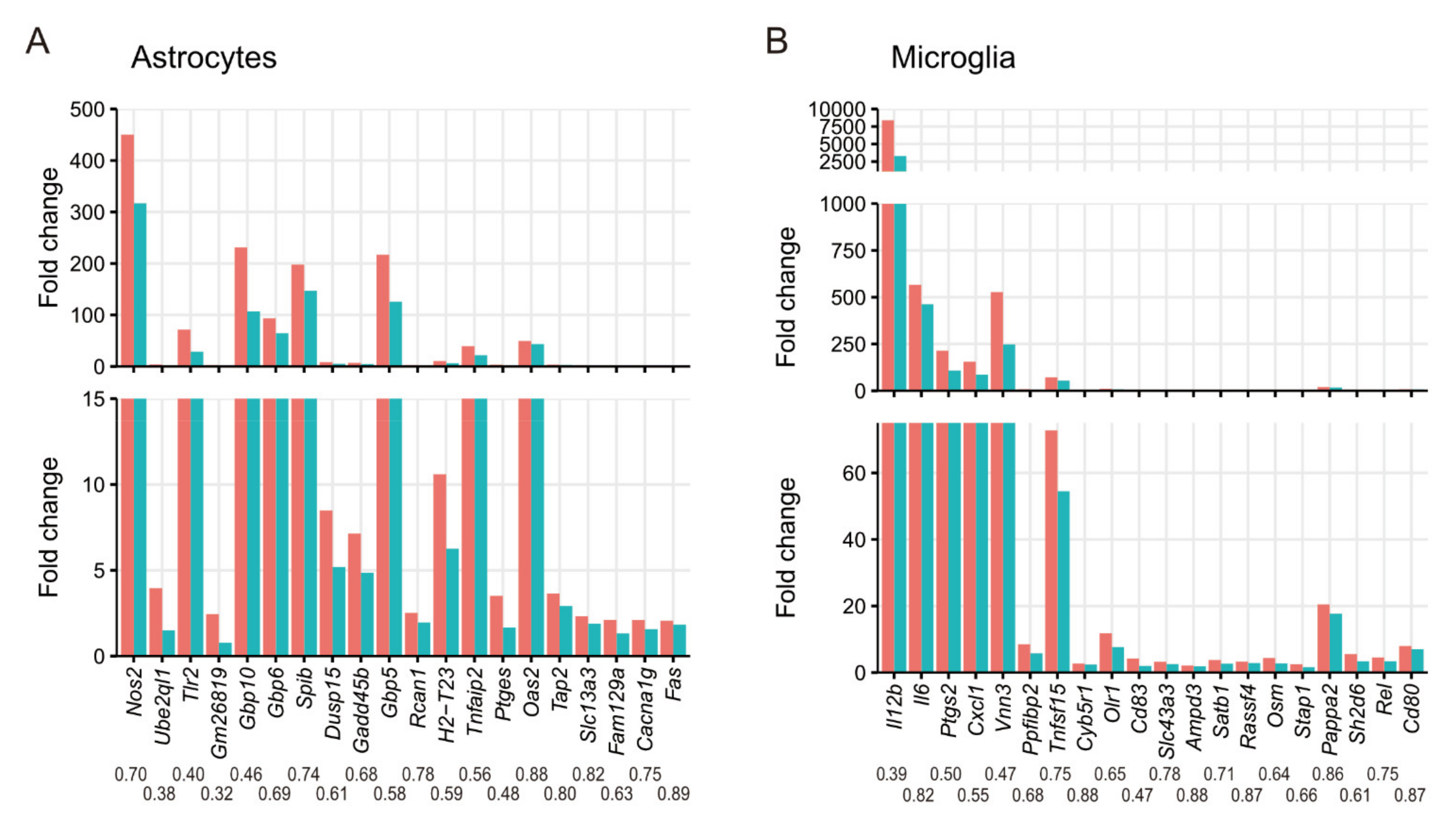
3.2. Effects of CXCR3-Deficiency on Gene Expression in Primary Glial Cells during T. gondii Infection
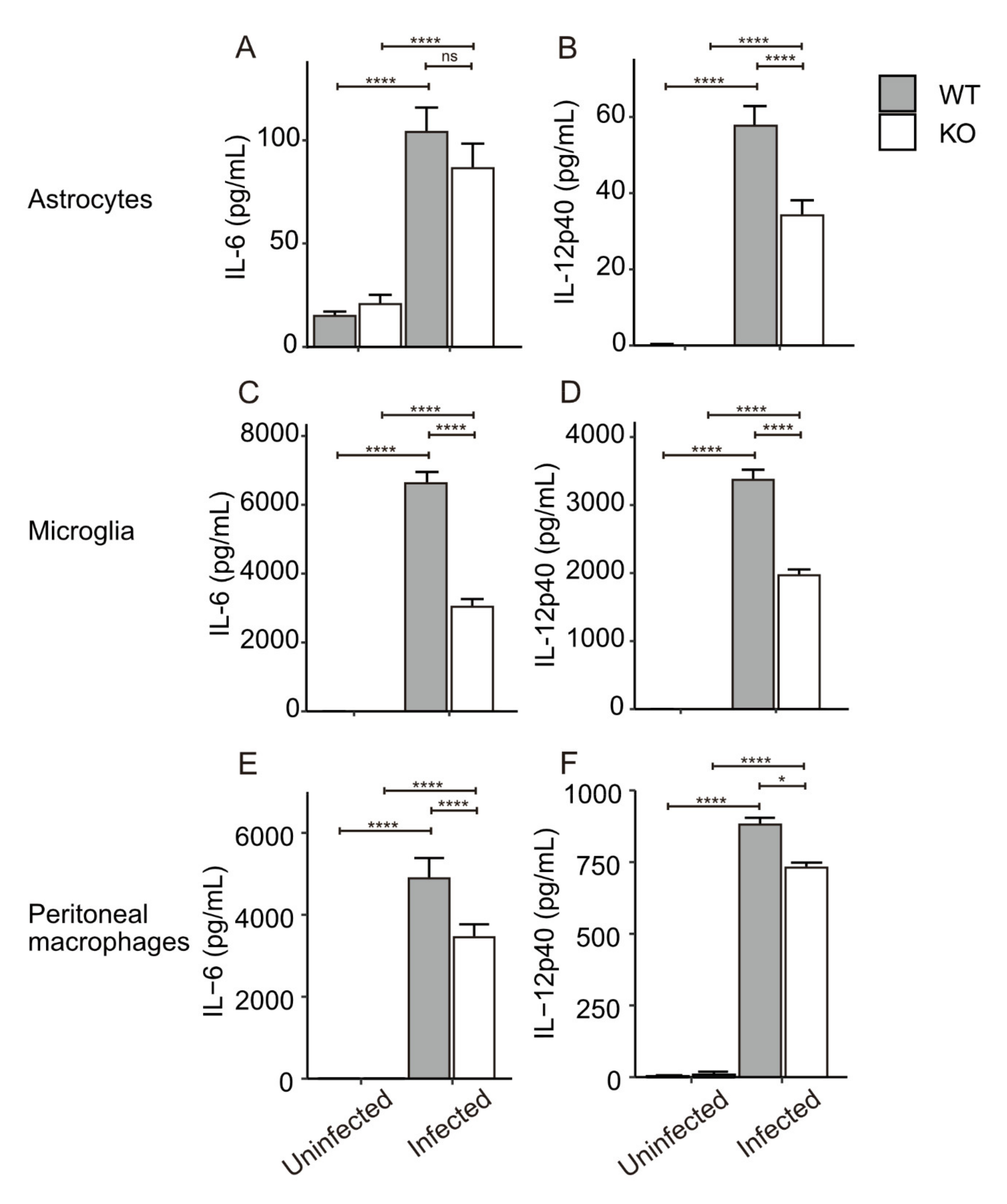
3.3. In Vitro Cytokine Production from Primary Glial Cells and Macrophages
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jones, J.L.; Dubey, J.P. Foodborne Toxoplasmosis. Clin. Infect. Dis. 2012, 55, 845–851. [Google Scholar] [CrossRef]
- Montoya, J.; Liesenfeld, O. Toxoplasmosis. Lancet 2004, 363, 1965–1976. [Google Scholar] [CrossRef]
- Jones, J.; Lopez, A.; Wilson, M. Congenital Toxoplasmosis. Am. Fam. Physician 2003, 67, 2131–2138. [Google Scholar]
- Basavaraju, A. Toxoplasmosis in HIV Infection: An Overview. Trop. Parasitol. 2016, 6, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Joiner, K.A.; Dubremetz, J.F. Toxoplasma Gondii: A Protozoan for the Nineties. Infect. Immun. 1993, 61, 1169–1172. [Google Scholar] [CrossRef]
- Blackwell, T.S.; Christman, J.W. The Role of Nuclear Factor-Kappa B in Cytokine Gene Regulation. Am. J. Respir. Cell Mol. Biol. 1997, 17, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Shapira, S.; Speirs, K.; Gerstein, A.; Caamano, J.; Hunter, C.A. Suppression of NF-ΚB Activation by Infection with Toxoplasma Gondii. J. Infect. Dis. 2002, 185, S66–S72. [Google Scholar] [CrossRef] [PubMed]
- Rosowski, E.E.; Lu, D.; Julien, L.; Rodda, L.; Gaiser, R.A.; Jensen, K.D.C.; Saeij, J.P.J. Strain-Specific Activation of the NF-ΚB Pathway by GRA15, a Novel Toxoplasma Gondii Dense Granule Protein. J. Exp. Med. 2011, 208, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Aliberti, J. Host Persistence: Exploitation of Anti-Inflammatory Pathways by Toxoplasma Gondii. Nat. Rev. Immunol. 2005, 5, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yarovinsky, F. Innate Immunity to Toxoplasma Gondii Infection. Nat. Rev. Immunol. 2014, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Lüder, C.G.K.; Giraldo-Velásquez, M.; Sendtner, M.; Gross, U. Toxoplasma gondii in Primary Rat CNS Cells: Differential Contribution of Neurons, Astrocytes, and Microglial Cells for the Intracerebral Development and Stage Differentiation. Exp. Parasitol. 1999, 93, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.M.; Tuladhar, S.; Dietrich, H.K.; Nguyen, E.; MacDonald, W.R.; Trivedi, T.; Devineni, A.; Koshy, A.A. Neurons Are the Primary Target Cell for the Brain-Tropic Intracellular Parasite Toxoplasma Gondii. PLoS Pathog. 2016, 12, e1005447. [Google Scholar] [CrossRef] [PubMed]
- Hatten, M.E.; Liem, R.K.H.; Shelanski, M.L.; Mason, C.A. Astroglia in CNS Injury. Glia 1991, 4, 233–243. [Google Scholar] [CrossRef]
- Araque, A.; Carmignoto, G.; Haydon, P.G. Dynamic Signaling Between Astrocytes and Neurons. Annu. Rev. Physiol. 2001, 63, 795–813. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ochoa, C.O.; Lagunas-Martínez, A.; Belkind-Gerson, J.; Correa, D. Toxoplasma Gondii Invasion and Replication in Astrocyte Primary Cultures and Astrocytoma Cell Lines: Systematic Review of the Literature. Parasitol. Res. 2012, 110, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- Hidano, S.; Randall, L.M.; Dawson, L.; Dietrich, H.K.; Konradt, C.; Klover, P.J.; John, B.; Harris, T.H.; Fang, Q.; Turek, B.; et al. STAT1 Signaling in Astrocytes Is Essential for Control of Infection in the Central Nervous System. mBio 2016, 7, e01881-16. [Google Scholar] [CrossRef]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of Microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Kreutzberg, G.W. Microglia: A Sensor for Pathological Events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Dellacasa-Lindberg, I.; Fuks, J.M.; Arrighi, R.B.G.; Lambert, H.; Wallin, R.P.A.; Chambers, B.J.; Barragan, A. Migratory Activation of Primary Cortical Microglia upon Infection with Toxoplasma Gondii. Infect. Immun. 2011, 79, 3046–3052. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishimura, M.; Ihara, F.; Yamagishi, J.; Suzuki, Y.; Nishikawa, Y. Transcriptome Analysis of Mouse Brain Infected with Toxoplasma Gondii. Infect. Immun. 2013, 81, 3609–3619. [Google Scholar] [CrossRef]
- Cole, K.E.; Strick, C.A.; Paradis, T.J.; Ogborne, K.T.; Loetscher, M.; Gladue, R.P.; Lin, W.; Boyd, J.G.; Moser, B.; Wood, D.E.; et al. Interferon-Inducible T Cell Alpha Chemoattractant (I-TAC): A Novel Non-ELR CXC Chemokine with Potent Activity on Activated T Cells through Selective High Affinity Binding to CXCR3. J. Exp. Med. 1998, 187, 2009–2021. [Google Scholar] [CrossRef]
- Heise, C.E.; Pahuja, A.; Hudson, S.C.; Mistry, M.S.; Putnam, A.L.; Gross, M.M.; Gottlieb, P.A.; Wade, W.S.; Kiankarimi, M.; Schwarz, D.; et al. Pharmacological Characterization of CXC Chemokine Receptor 3 Ligands and a Small Molecule Antagonist. J. Pharmacol. Exp. Ther. 2005, 313, 1263–1271. [Google Scholar] [CrossRef]
- Loetscher, M.; Loetscher, P.; Brass, N.; Meese, E.; Moser, B. Lymphocyte-Specific Chemokine Receptor CXCR3: Regulation, Chemokine Binding and Gene Localization. Eur. J. Immunol. 1998, 28, 3696–3705. [Google Scholar] [CrossRef]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal CXCL10 Directs CD8+ T-Cell Recruitment and Control of West Nile Virus Encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef] [PubMed]
- Campanella, G.S.V.; Tager, A.M.; El Khoury, J.K.; Thomas, S.Y.; Abrazinski, T.A.; Manice, L.A.; Colvin, R.A.; Luster, A.D. Chemokine Receptor CXCR3 and Its Ligands CXCL9 and CXCL10 Are Required for the Development of Murine Cerebral Malaria. Proc. Natl. Acad. Sci. USA 2008, 105, 4814–4819. [Google Scholar] [CrossRef]
- Cohen, S.B.; Maurer, K.J.; Egan, C.E.; Oghumu, S.; Satoskar, A.R.; Denkers, E.Y. CXCR3-Dependent CD4+ T Cells Are Required to Activate Inflammatory Monocytes for Defense against Intestinal Infection. PLoS Pathog. 2013, 9, e1003706. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A.; MacLean, J.A.; Lee, F.S.; Casciotti, L.; DeHaan, E.; Schwartzman, J.D.; Luster, A.D. IP-10 Is Critical for Effector T Cell Trafficking and Host Survival in Toxoplasma Gondii Infection. Immunity 2000, 12, 483–494. [Google Scholar] [CrossRef]
- Norose, K.; Kikumura, A.; Luster, A.D.; Hunter, C.A.; Harris, T.H. CXCL10 Is Required to Maintain T-Cell Populations and to Control Parasite Replication during Chronic Ocular Toxoplasmosis. Invest. Ophthalmol. Vis. Sci. 2011, 52, 389–398. [Google Scholar] [CrossRef]
- Harris, T.H.; Banigan, E.J.; Christian, D.A.; Konradt, C.; Wojno, E.D.T.; Norose, K.; Wilson, E.H.; John, B.; Weninger, W.; Luster, A.D.; et al. Generalized Lévy Walks and the Role of Chemokines in Migration of Effector CD8+ T Cells. Nature 2012, 486, 545–548. [Google Scholar] [CrossRef]
- Biber, K.; Dijkstra, I.; Trebst, C.; De Groot, C.J.A.; Ransohoff, R.M.; Boddeke, H.W.G.M. Functional Expression of CXCR3 in Cultured Mouse and Human Astrocytes and Microglia. Neuroscience 2002, 112, 487–497. [Google Scholar] [CrossRef]
- Xia, M.Q.; Bacskai, B.J.; Knowles, R.B.; Qin, S.X.; Hyman, B.T. Expression of the Chemokine Receptor CXCR3 on Neurons and the Elevated Expression of Its Ligand IP-10 in Reactive Astrocytes: In Vitro ERK1/2 Activation and Role in Alzheimer’s Disease. J. Neuroimmunol. 2000, 108, 227–235. [Google Scholar] [CrossRef]
- Ambrosini, E.; Aloisi, F. Chemokines and Glial Cells: A Complex Network in the Central Nervous System. Neurochem. Res. 2004, 29, 1017–1038. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Kameyama, K.; Rasul, N.H.; Xuan, X.; Nishikawa, Y. Development of an Immunochromatographic Assay Based on Dense Granule Protein 7 for Serological Detection of Toxoplasma Gondii Infection. Clin. Vaccine Immunol. 2013, 20, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Umeda, K.; Tanaka, S.; Ihara, F.; Yamagishi, J.; Suzuki, Y.; Nishikawa, Y. Transcriptional Profiling of Toll-like Receptor 2-Deficient Primary Murine Brain Cells during Toxoplasma Gondii Infection. PLoS ONE 2017, 12, e0187703. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.G.; Nitzgen, B.; Germann, T.; Degitz, K.; Däubener, W.; Hadding, U. Differentiation Driven by Granulocyte-Macrophage Colony-Stimulating Factor Endows Microglia with Interferon-Gamma-Independent Antigen Presentation Function. J. Neuroimmunol. 1993, 42, 87–95. [Google Scholar] [CrossRef]
- Kobayashi, K.; Umeda, K.; Ihara, F.; Tanaka, S.; Yamagishi, J.; Suzuki, Y.; Nishikawa, Y. Transcriptome Analysis of the Effect of C-C Chemokine Receptor 5 Deficiency on Cell Response to Toxoplasma Gondii in Brain Cells. BMC Genom. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- McCarthy, D.J.; Chen, Y.; Smyth, G.K. Differential Expression Analysis of Multifactor RNA-Seq Experiments with Respect to Biological Variation. Nucleic Acids Res. 2012, 40, 4288–4297. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. EdgeR: A Bioconductor Package for Differential Expression Analysis of Digital Gene Expression Data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Sun, J.; Shimizu, K.; Kadota, K. Evaluation of Methods for Differential Expression Analysis on Multi-Group RNA-Seq Count Data. BMC Bioinform. 2015, 16, 360. [Google Scholar] [CrossRef] [PubMed]
- Young, M.D.; Wakefield, M.J.; Smyth, G.K.; Oshlack, A. Gene Ontology Analysis for RNA-Seq: Accounting for Selection Bias. Genome Biol. 2010, 11, R14. [Google Scholar] [CrossRef]
- Carlson, M. Org. Mm. Eg. Db: Genome Wide Annotation for Mouse. 2019. Available online: https://bioconductor.org/packages/release/data/annotation/html/org.Mm.eg.db.html (accessed on 2 October 2019).
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. ClusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Bonfá, G.; Benevides, L.; Souza, M.d.C.; Fonseca, D.M.; Mineo, T.W.P.; Rossi, M.A.; Silva, N.M.; Silva, J.S.; de Barros Cardoso, C.R. CCR5 Controls Immune and Metabolic Functions during Toxoplasma Gondii Infection. PLoS ONE 2014, 9, e104736. [Google Scholar] [CrossRef]
- Xie, F.; Xiao, P.; Chen, D.; Xu, L.; Zhang, B. MiRDeepFinder: A MiRNA Analysis Tool for Deep Sequencing of Plant Small RNAs. Plant Mol. Biol. 2012, 80, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Aviles, H.; Stiles, J.; O’Donnell, P.; Orshal, J.; Leid, J.; Sonnenfeld, G.; Monroy, F. Kinetics of Systemic Cytokine and Brain Chemokine Gene Expression in Murine Toxoplasma Infection. J. Parasitol. 2008, 94, 1282–1288. [Google Scholar] [CrossRef]
- Ferreira, C.P.; Cariste, L.d.M.; Noronha, I.H.; Durso, D.F.; Lannes-Vieira, J.; Bortoluci, K.R.; Ribeiro, D.A.; Golenbock, D.; Gazzinelli, R.T.; Vasconcelos, J.R.C. de CXCR3 Chemokine Receptor Contributes to Specific CD8+ T Cell Activation by PDC during Infection with Intracellular Pathogens. PLoS Negl. Trop. Dis. 2020, 14, e0008414. [Google Scholar] [CrossRef]
- Vasquez, R.E.; Soong, L. CXCL10/Gamma Interferon-Inducible Protein 10-Mediated Protection against Leishmania Amazonensis Infection in Mice. Infect. Immun. 2006, 74, 6769–6777. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vasquez, R.E.; Xin, L.; Soong, L. Effects of CXCL10 on Dendritic Cell and CD4+ T-Cell Functions during Leishmania Amazonensis Infection. Infect. Immun. 2008, 76, 161–169. [Google Scholar] [CrossRef]
- Rappert, A.; Bechmann, I.; Pivneva, T.; Mahlo, J.; Biber, K.; Nolte, C.; Kovac, A.D.; Gerard, C.; Boddeke, H.W.G.M.; Nitsch, R.; et al. CXCR3-Dependent Microglial Recruitment Is Essential for Dendrite Loss after Brain Lesion. J. Neurosci. 2004, 24, 8500–8509. [Google Scholar] [CrossRef] [PubMed]
- Aksoy, M.O.; Yang, Y.; Ji, R.; Reddy, P.J.; Shahabuddin, S.; Litvin, J.; Rogers, T.J.; Kelsen, S.G. CXCR3 Surface Expression in Human Airway Epithelial Cells: Cell Cycle Dependence and Effect on Cell Proliferation. Am. J. Physiol. Lung Cell Mol. Physiol. 2006, 290, L909–L918. [Google Scholar] [CrossRef]
- Silva, N.M.; Vieira, J.C.M.; Carneiro, C.M.; Tafuri, W.L. Toxoplasma gondii: The Role of IFN-Gamma, TNFRp55 and INOS in Inflammatory Changes during Infection. Exp. Parasitol. 2009, 123, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.R.d.A.; Wang, Z.T.; Sibley, L.D.; DaMatta, R.A. Inhibition of Nitric Oxide Production in Activated Macrophages Caused by Toxoplasma Gondii Infection Occurs by Distinct Mechanisms in Different Mouse Macrophage Cell Lines. Front. Microbiol. 2018, 9, 1936. [Google Scholar] [CrossRef]
- Butcher, B.A.; Fox, B.A.; Rommereim, L.M.; Kim, S.G.; Maurer, K.J.; Yarovinsky, F.; Herbert, D.R.; Bzik, D.J.; Denkers, E.Y. Toxoplasma gondii Rhoptry Kinase ROP16 Activates STAT3 and STAT6 Resulting in Cytokine Inhibition and Arginase-1-Dependent Growth Control. PLoS Pathog. 2011, 7, e1002236. [Google Scholar] [CrossRef]
- Yamamoto, M.; Okuyama, M.; Ma, J.S.; Kimura, T.; Kamiyama, N.; Saiga, H.; Ohshima, J.; Sasai, M.; Kayama, H.; Okamoto, T.; et al. A Cluster of Interferon-γ-Inducible P65 GTPases Plays a Critical Role in Host Defense against Toxoplasma Gondii. Immunity 2012, 37, 302–313. [Google Scholar] [CrossRef]
- Shenoy, A.R.; Wellington, D.A.; Kumar, P.; Kassa, H.; Booth, C.J.; Cresswell, P.; MacMicking, J.D. GBP5 Promotes NLRP3 Inflammasome Assembly and Immunity in Mammals. Science 2012, 336, 481–485. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Hizukuri, Y.; Yamashiro, K.; Makita, N.; Ohnishi, K.; Takeya, M.; Komohara, Y.; Hayashi, Y. Guanylate-Binding Protein 5 Is a Marker of Interferon-γ-Induced Classically Activated Macrophages. Clin. Transl. Immunol. 2016, 5, e111. [Google Scholar] [CrossRef]
- Matta, S.K.; Patten, K.; Wang, Q.; Kim, B.-H.; MacMicking, J.D.; Sibley, L.D. NADPH Oxidase and Guanylate Binding Protein 5 Restrict Survival of Avirulent Type III Strains of Toxoplasma Gondii in Naive Macrophages. mBio 2018, 9, e01393-18. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Hoshino, K.; Akira, S. Cutting Edge: TLR2-Deficient and MyD88-Deficient Mice Are Highly Susceptible to Staphylococcus Aureus Infection. J. Immunol. 2000, 165, 5392–5396. [Google Scholar] [CrossRef] [PubMed]
- Thoma-Uszynski, S.; Stenger, S.; Takeuchi, O.; Ochoa, M.T.; Engele, M.; Sieling, P.A.; Barnes, P.F.; Rollinghoff, M.; Bolcskei, P.L.; Wagner, M.; et al. Induction of Direct Antimicrobial Activity through Mammalian Toll-like Receptors. Science 2001, 291, 1544–1547. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, I.; Hoshino, K.; Sugiyama, T.; Yamazaki, C.; Yano, T.; Iizuka, A.; Hemmi, H.; Tanaka, T.; Saito, M.; Sugiyama, M.; et al. Spi-B Is Critical for Plasmacytoid Dendritic Cell Function and Development. Blood 2012, 120, 4733–4743. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.S.; Stumhofer, J.S.; Passos, S.; Ernst, M.; Hunter, C.A. IL-6 Mediates the Susceptibility of Gp130 Hypermorphs to Toxoplasma Gondii. J. Immunol. 2011, 187, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Jebbari, H.; Roberts, C.W.; Ferguson, D.J.; Bluethmann, H.; Alexander, J. A Protective Role for IL-6 during Early Infection with Toxoplasma Gondii. Parasite Immunol. 1998, 20, 231–239. [Google Scholar] [CrossRef]
- Yap, G.; Pesin, M.; Sher, A. Cutting Edge: IL-12 Is Required for the Maintenance of IFN-Gamma Production in T Cells Mediating Chronic Resistance to the Intracellular Pathogen, Toxoplasma Gondii. J. Immunol. 2000, 165, 628–631. [Google Scholar] [CrossRef] [PubMed]
- Sher, A.; Collazzo, C.; Scanga, C.; Jankovic, D.; Yap, G.; Aliberti, J. Induction and Regulation of IL-12-Dependent Host Resistance to Toxoplasma Gondii. Immunol. Res. 2003, 27, 521–527. [Google Scholar] [CrossRef]
- Peng, B.-W.; Lin, J.; Zhang, T. Toxoplasma gondii Induces Prostaglandin E2 Synthesis in Macrophages via Signal Pathways for Calcium-Dependent Arachidonic Acid Production and PKC-Dependent Induction of Cyclooxygenase-2. Parasitol. Res. 2008, 102, 1043–1050. [Google Scholar] [CrossRef]
- Pereira, A.C.A.; Silva, R.J.; Franco, P.S.; de Oliveira Gomes, A.; Souza, G.; Milian, I.C.B.; Ribeiro, M.; Rosini, A.M.; Guirelli, P.M.; Ramos, E.L.P.; et al. Cyclooxygenase (COX)-2 Inhibitors Reduce Toxoplasma Gondii Infection and Upregulate the Pro-Inflammatory Immune Response in Calomys Callosus Rodents and Human Monocyte Cell Line. Front. Microbiol. 2019, 10, 225. [Google Scholar] [CrossRef]
- Ha, M.; Jeong, H.; Roh, J.S.; Lee, B.; Lee, D.; Han, M.-E.; Oh, S.-O.; Sohn, D.H.; Kim, Y.H. VNN3 Is a Potential Novel Biomarker for Predicting Prognosis in Clear Cell Renal Cell Carcinoma. Anim. Cells Syst. 2019, 23, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.A.; Bredon, N.; Ohmori, Y.; Tannenbaum, C.S. IFN-Gamma and IFN-Beta Independently Stimulate the Expression of Lipopolysaccharide-Inducible Genes in Murine Peritoneal Macrophages. J. Immunol. 1989, 142, 2325–2331. [Google Scholar]
- Der, S.D.; Zhou, A.; Williams, B.R.G.; Silverman, R.H. Identification of Genes Differentially Regulated by Interferon α, β, or γ Using Oligonucleotide Arrays. PNAS 1998, 95, 15623–15628. [Google Scholar] [CrossRef] [PubMed]
- Schneider, W.M.; Chevillotte, M.D.; Rice, C.M. Interferon-Stimulated Genes: A Complex Web of Host Defenses. Annu. Rev. Immunol. 2014, 32, 513–545. [Google Scholar] [CrossRef] [PubMed]
- Smit, M.J.; Verdijk, P.; van der Raaij-Helmer, E.M.H.; Navis, M.; Hensbergen, P.J.; Leurs, R.; Tensen, C.P. CXCR3-Mediated Chemotaxis of Human T Cells Is Regulated by a Gi- and Phospholipase C-Dependent Pathway and Not via Activation of MEK/P44/P42 MAPK nor Akt/PI-3 Kinase. Blood 2003, 102, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Shahabuddin, S.; Ji, R.; Wang, P.; Brailoiu, E.; Dun, N.; Yang, Y.; Aksoy, M.O.; Kelsen, S.G. CXCR3 Chemokine Receptor-Induced Chemotaxis in Human Airway Epithelial Cells: Role of P38 MAPK and PI3K Signaling Pathways. Am. J. Physiol. - Cell Physiol. 2006, 291, C34–C39. [Google Scholar] [CrossRef] [PubMed]
- Willox, I.; Mirkina, I.; Westwick, J.; Ward, S.G. Evidence for PI3K-Dependent CXCR3 Agonist-Induced Degranulation of Human Cord Blood-Derived Mast Cells. Mol. Immunol. 2010, 47, 2367–2377. [Google Scholar] [CrossRef]
- Yin, M.; Shen, Z.; Yang, L.; Zheng, W.; Song, H. Protective Effects of CXCR3/HO-1 Gene-modified BMMSCs on Damaged Intestinal Epithelial Cells: Role of the P38-MAPK Signaling Pathway. Int. J. Mol. Med. 2019, 43, 2086–2102. [Google Scholar] [CrossRef] [PubMed]
- Mun, H.-S.; Aosai, F.; Norose, K.; Chen, M.; Piao, L.-X.; Takeuchi, O.; Akira, S.; Ishikura, H.; Yano, A. TLR2 as an Essential Molecule for Protective Immunity against Toxoplasma Gondii Infection. Int. Immunol. 2003, 15, 1081–1087. [Google Scholar] [CrossRef] [PubMed]


| Cell Type | Accession NO. | # DEGs | # Reference Genes | GO Term | FDR |
|---|---|---|---|---|---|
| Astrocyte | GO:0019882 | 10 | 96 | antigen processing and presentation | 0 |
| GO:0002376 | 42 | 2306 | immune system process | 1.8 × 10−17 | |
| GO:0006952 | 32 | 1432 | defense response | 7.0 × 10−15 | |
| GO:0006955 | 31 | 1316 | immune response | 8.5 × 10−15 | |
| GO:0045087 | 24 | 714 | innate immune response | 4.1 × 10−14 | |
| Microglia | GO:0006954 | 18 | 647 | inflammatory response | 3.6 × 10−7 |
| GO:0001819 | 14 | 402 | positive regulation of cytokine production | 3.5 × 10−6 | |
| GO:0006955 | 22 | 1316 | immune response | 1.0 × 10−5 | |
| GO:0001817 | 16 | 635 | regulation of cytokine production | 1.0 × 10−5 | |
| GO:0001816 | 16 | 707 | cytokine production | 4.1 × 10−5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umeda, K.; Goto, Y.; Watanabe, K.; Ushio, N.; Fereig, R.M.; Ihara, F.; Tanaka, S.; Suzuki, Y.; Nishikawa, Y. Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection. Microorganisms 2021, 9, 2340. https://doi.org/10.3390/microorganisms9112340
Umeda K, Goto Y, Watanabe K, Ushio N, Fereig RM, Ihara F, Tanaka S, Suzuki Y, Nishikawa Y. Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection. Microorganisms. 2021; 9(11):2340. https://doi.org/10.3390/microorganisms9112340
Chicago/Turabian StyleUmeda, Kousuke, Youta Goto, Kenichi Watanabe, Nanako Ushio, Ragab M. Fereig, Fumiaki Ihara, Sachi Tanaka, Yutaka Suzuki, and Yoshifumi Nishikawa. 2021. "Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection" Microorganisms 9, no. 11: 2340. https://doi.org/10.3390/microorganisms9112340
APA StyleUmeda, K., Goto, Y., Watanabe, K., Ushio, N., Fereig, R. M., Ihara, F., Tanaka, S., Suzuki, Y., & Nishikawa, Y. (2021). Transcriptomic Analysis of the Effects of Chemokine Receptor CXCR3 Deficiency on Immune Responses in the Mouse Brain during Toxoplasma gondii Infection. Microorganisms, 9(11), 2340. https://doi.org/10.3390/microorganisms9112340







